Objective: Antidepressant medication is efficacious in the treatment of depression, but not all patients improve with antidepressant medication alone. Despite this treatment gap, limited evidence regarding the effectiveness of supplementing psychotherapy for pharmacotherapy-resistant depression is available. Therefore, we investigated the effectiveness of supplementing usual medication management (treatment as usual [TAU]) with cognitive-behavioral therapy (CBT) in patients with pharmacotherapy-resistant depression seeking psychiatric specialty care.
Methods: A 16-week assessor-masked randomized controlled trial with a 12-month follow-up was conducted in 1 university hospital and 1 psychiatric hospital from September 2008 to December 2014. Outpatients aged 20-65 years with pharmacotherapy-resistant depression (taking antidepressant medications for ≥ 8 weeks, 17-item GRID-Hamilton Depression Rating Scale [GRID-HDRS17] score ≥ 16, Maudsley Staging Method for treatment-resistant depression score ≥ 3, and DSM-IV criteria for major depressive disorder) were randomly assigned (1:1) to CBT combined with TAU or to TAU alone. The primary outcome was the alleviation of depressive symptoms, as measured by change in the total GRID-HDRS17 score from baseline to 16 weeks; primary analysis was done on an intention-to-treat basis.
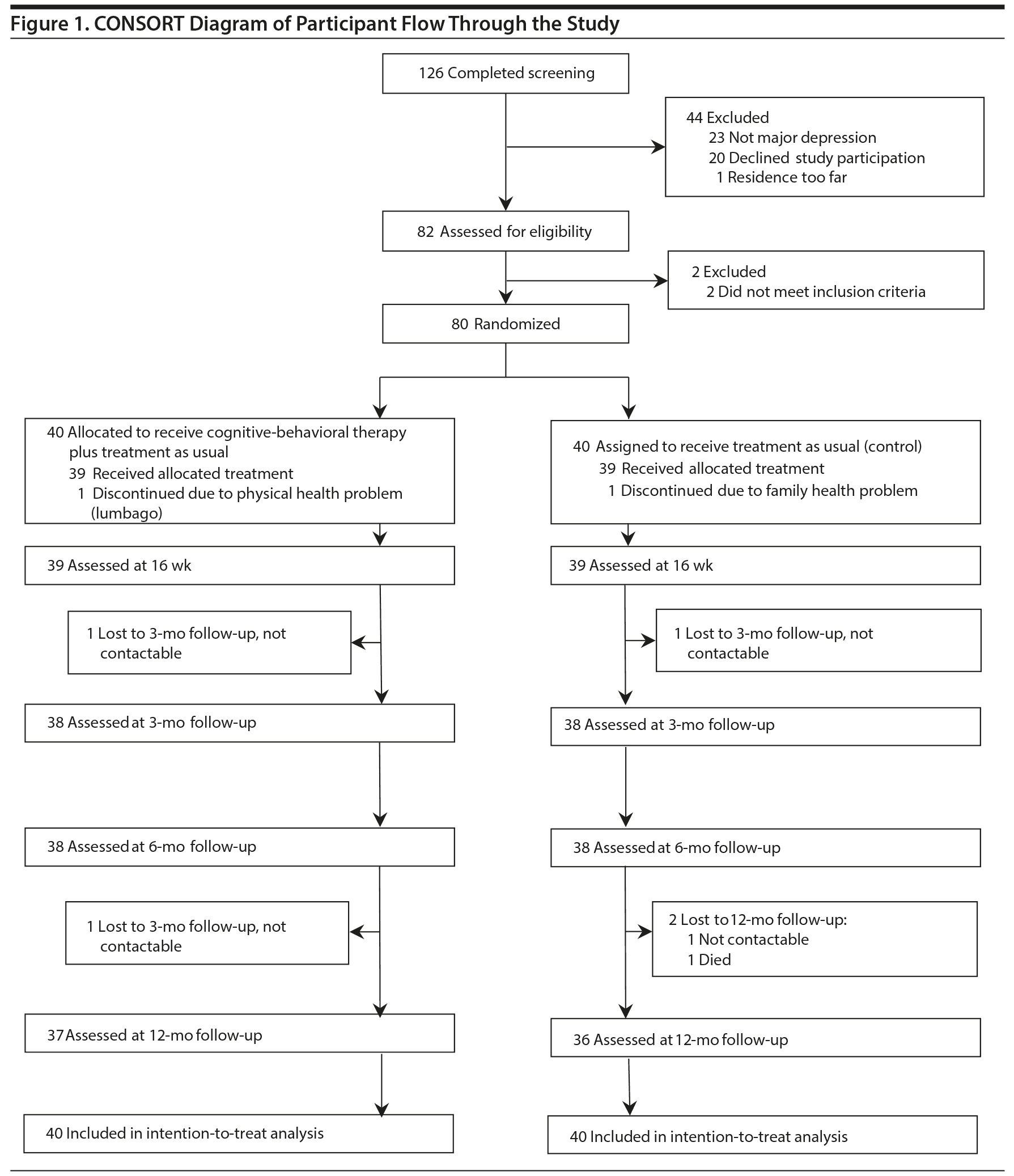
Results: A total of 80 patients were randomized; 78 (97.5%) were assessed for the primary outcome, and 73 (91.3%) were followed up for 12 months. Supplementary CBT significantly alleviated depressive symptoms at 16 weeks, as shown by greater least squares mean changes in GRID-HDRS17 scores in the intervention group than in the control group (−12.7 vs −7.4; difference = −5.4; 95% CI, −8.1 to −2.6; P < .001), and the treatment effect was maintained for at least 12 months (−15.4 vs −11.0; difference = −4.4; 95% CI, −7.2 to −1.6; P = .002).
Conclusions: Patients with pharmacotherapy-resistant depression treated in psychiatric specialty care settings may benefit from supplementing usual medication management with CBT.
Trial Registration: UMIN Clinical Trials Registry identifier: UMIN000001218‘ ‹’ ‹
This work may not be copied, distributed, displayed, published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the publisher’s Terms & Conditions.
Effectiveness of Supplementary Cognitive-Behavioral Therapy for Pharmacotherapy-Resistant Depression:
A Randomized Controlled Trial

ABSTRACT
Objective: Antidepressant medication is efficacious in the treatment of depression, but not all patients improve with antidepressant medication alone. Despite this treatment gap, limited evidence regarding the effectiveness of supplementing psychotherapy for pharmacotherapy-resistant depression is available. Therefore, we investigated the effectiveness of supplementing usual medication management (treatment as usual [TAU]) with cognitive-behavioral therapy (CBT) in patients with pharmacotherapy-resistant depression seeking psychiatric specialty care.
Methods: A 16-week assessor-masked randomized controlled trial with a 12-month follow-up was conducted in 1 university hospital and 1 psychiatric hospital from September 2008 to December 2014. Outpatients aged 20-65 years with pharmacotherapy-resistant depression (taking antidepressant medications for ≥ 8 weeks, 17-item GRID-Hamilton Depression Rating Scale [GRID-HDRS17] score ≥ 16, Maudsley Staging Method for treatment-resistant depression score ≥ 3, and DSM-IV criteria for major depressive disorder) were randomly assigned (1:1) to CBT combined with TAU or to TAU alone. The primary outcome was the alleviation of depressive symptoms, as measured by change in the total GRID-HDRS17 score from baseline to 16 weeks; primary analysis was done on an intention-to-treat basis.
Results: A total of 80 patients were randomized; 78 (97.5%) were assessed for the primary outcome, and 73 (91.3%) were followed up for 12 months. Supplementary CBT significantly alleviated depressive symptoms at 16 weeks, as shown by greater least squares mean changes in GRID-HDRS17 scores in the intervention group than in the control group (−12.7 vs −7.4; difference = −5.4; 95% CI, −8.1 to −2.6; P < .001), and the treatment effect was maintained for at least 12 months (−15.4 vs −11.0; difference = −4.4; 95% CI, −7.2 to −1.6; P = .002).
Conclusions: Patients with pharmacotherapy-resistant depression treated in psychiatric specialty care settings may benefit from supplementing usual medication management with CBT.
Trial Registration: UMIN Clinical Trials Registry identifier: UMIN000001218
J Clin Psychiatry 2017;78(8):1126-1135
https://doi.org/10.4088/JCP.15m10511
© Copyright 2017 Physicians Postgraduate Press, Inc.
aCenter for Clinical Research and bDepartments of Neuropsychiatry and cPreventive Medicine and Public Health, Keio University School of Medicine, Tokyo, Japan
dDepartment of Psychiatry, Sakuragaoka Memorial Hospital, Tokyo, Japan
eDepartment of Neuropsychiatry, Kyorin University School of Medicine, Tokyo, Japan
fCenter for the Development of Cognitive Behavior Therapy Training, Tokyo, Japan
*Corresponding author: Atsuo Nakagawa, MD, PhD, Center for Clinical Research, Keio University School of Medicine, Shinanomachi 35, Shinjuku-ku, Tokyo 160-8582, Japan ([email protected]).
Inadequate response to antidepressant medication is a common clinical scenario among patients with depression. It is estimated that only a third of patients fully respond to the initial course of antidepressants and only a quarter to the second course.1-3 This sizable proportion of patients who fail to respond to pharmacotherapy is considered to have treatment-resistant depression. Although researchers have investigated the best treatment strategies for treatment-resistant depression, specifically pharmacotherapy-resistant depression, for years,2,3 no standard treatment has yet been established.4
Ample evidence shows that cognitive-behavioral therapy (CBT) is as efficacious as treatment with antidepressant medication alone5 and that combining the two enhances treatment efficacy.6,7 Several studies have investigated the value of adding CBT to antidepressant medication in patients with chronic depression. While one study8 found the cognitive behavioral analysis system of psychotherapy (CBASP), a variant of CBT, to be effective in combination with medication, others found no advantages of adding CBASP9,10 and mindfulness-based cognitive therapy11 over medication alone; these studies, however, did not focus on pharmacotherapy-resistant depression. Despite the evidence, so far only 2 randomized controlled trials (RCTs)12,13 (excluding 1 pilot study14 for the CoBalT Study12) undertaken in primary care settings have tested the benefits of supplementary CBT for patients who show treatment resistance to antidepressant medication. To our knowledge, no previous RCTs have directly examined the benefits of supplementing usual medication visits (treatment as usual [TAU]) with CBT in patients with pharmacotherapy-resistant depression seeking psychiatric specialty care—a subgroup of patients who are likely to have more complicated psychiatric comorbidities.15-17 Therefore, we carried out an assessor-masked RCT to compare the effectiveness of such treatment in psychiatric specialty care settings. We hypothesized that CBT plus TAU would be more clinically effective than TAU alone. The economic evaluation will be reported separately.
METHODS
The present study was a 16-week, assessor-masked, RCT of 2 parallel groups with a 12-month follow-up. The study design and procedures are presented in full in the published study protocol.18 The study was registered in the UMIN Clinical Trials Registry (identifier: UMIN000001218) and was conducted and reported in accordance with the CONSORT guidelines.19,20
Participants
The participants were individuals who sought treatment for major depression at 2 study sites: a university teaching hospital and a psychiatric hospital located in Tokyo. Those who agreed to participate were asked to provide written informed consent and undergo a baseline assessment.
Participants were eligible for inclusion in the study if they were aged 20-65 years and had DSM-IV major depressive disorder (MDD),21 either single or recurrent episodes, without psychotic features as confirmed by the Structured Clinical Interview for DSM-IV-TR Axis I Disorders-Patient Edition (SCID-I/P).22 All participants also met the operationalized criteria of having at least a minimal degree of treatment-resistant depression (Maudsley Staging Method for treatment-resistant depression score23 ≥ 3) and a 17-item GRID-Hamilton Depression Rating Scale (GRID-HDRS17)24,25 score ≥ 16, despite having received adequate therapeutic levels of antidepressant medication for at least 8 weeks as part of their routine care. The Maudsley Staging Method23 is a point-based staging method that measures level of treatment-resistant severity of the index episode by incorporating 3 factors: treatment, severity of illness, and duration of episode. The treatment factors include (1) number of failed antidepressant trials (ie, at least 6 weeks at an adequate dose: 1-2 trial failures [equals score of 1] to > 10 trial failures [equals score of 5]), (2) usage of augmentation strategies (ie, lithium, anticonvulsants, thyroid hormone, or second-generation antipsychotics; score of 1), and (3) usage of electroconvulsive therapy (score of 1). Severity of illness is rated on a 5-point subscale from subsyndromal depression (score of 1) to severe syndromal depression with psychosis (score of 5). Duration of episode is rated on a 3-point subscale from acute (< 12 months = score of 1) to chronic (> 24 months = score of 3). The overall level of treatment resistance varies from minimal (score of 3) to severe (score of 15) and is categorized into 3 levels: mild resistance (scores = 3-6), moderate resistance (scores = 7-10), and severe resistance (scores = 11-15).

- Researchers have found ample evidence that cognitive-behavioral therapy (CBT) is as efficacious as treatment with antidepressants alone and that combining the two enhances treatment efficacy; however, no randomized control trials have tested the benefits of supplementing routine medication management with CBT in patients with pharmacotherapy-resistant depression who receive psychiatric specialty care.
- Supplementary CBT significantly alleviated depressive symptoms at 16 weeks, and the treatment effect was maintained for at least 12 months.
- For patients with pharmacotherapy-resistant depression who receive psychiatric specialty care, supplementary CBT is a viable option.
Exclusion criteria were a primary DSM-IV Axis I diagnosis other than MDD, manic or psychotic episodes, alcohol or substance use disorder or antisocial personality disorder, serious and imminent suicidal ideation, organic brain lesions or major cognitive deficits, and serious or unstable medical illnesses. Those who had received CBT in the past (ie, attended 8 or more CBT sessions) or who were unlikely to attend more than 8 sessions of study treatment (for reasons such as planned relocation) were also excluded.
Randomization and Masking
All eligible participants were randomly allocated (in a ratio of 1:1) to receive either CBT plus TAU or TAU alone. Allocation was concealed with the use of a web-based random allocation system set up and managed independently of the study by the Keio Center for Clinical Research Project Management Office, Tokyo, Japan. Randomization was stratified by study site with the minimization method to balance the age of the participants at study entry (< 40 years, ≥ 40 years) and baseline GRID-HDRS17 score (16-18, ≥ 19).
Psychiatrists and clinical psychologists assessed the assessor-rated outcome measures. Due to the nature of the interventions, neither the participants nor the treating psychiatrists or therapists could be masked to randomization status, but the outcome assessors were masked as much as possible. The assessors were not involved with the treatment delivery or study coordination and were prohibited from accessing any information that could confer participant allocation. Participants were instructed not to disclose their allocated treatment in the assessment interviews. The success of the assessor masking was investigated by randomly evaluating one-third of the primary outcome interviews by asking the assessors to guess the treatment allocations after the assessment interviews, and the percentage of agreement and κ coefficients between the actual and guessed allocations were calculated. The percentage of agreement and κ coefficients were 52.0% and 0.00 (95% CI, −0.39 to 0.39), respectively, based on the available primary outcome interviews (32.1%, n = 25), indicating that masking was successful.
Treatment Procedures
Cognitive-behavioral therapy. Participants allocated to the CBT arm were offered 16 individual 50-minute sessions, scheduled weekly, with up to 4 additional sessions if deemed clinically appropriate by the therapist. Therapists followed the procedures outlined in the CBT Manual for Depression26 developed by the authors (Y.O., D.F., A.N., M.S., and T.K.). This manual is based on Beck’s original treatment manual,27 with some adaptations designed to address the cultural characteristics of Japanese patients, such as their emphasis on interpersonal relationships and consideration of the family as an essential part of treatment.28
Four psychiatrists, 1 clinical psychologist with a master’s degree, and 1 psychiatric nurse provided CBT. Together, the therapists had practiced CBT for a mean (SD) of 4.0 (2.1) years and had used CBT to treat 12.5 (7.3) patients before the study. All received CBT training, which included a 2-day intensive workshop, and the therapists received 2-hour on-site group supervision sessions every 2 weeks from a skilled CBT supervisor (Y.O.), with thorough reviews of the cases and detailed feedback to maintain adherence to CBT protocols and competence during the study. A senior clinician independent of treatment delivery rated one-fourth of the audio tapes (10 tapes) recorded during mid to late sessions, mostly in the tenth session, using the Cognitive Therapy Rating Scale (CTRS)29 to check therapist competence and adherence to CBT protocols. All the therapists were competent, as reflected in their scores of ≥ 40 on the CTRS.
Treatment as usual. Treatment as usual consisted of medication management along with education regarding medication and dosage schedules, reviews of adverse effects, and supportive guidance from the treating psychiatrists. Monitoring of depressive symptoms with the 16-item Quick Inventory of Depressive Symptomatology Self-Report (QIDS-SR)30,31 was also conducted at each visit. Although there were no particular restrictions on the pharmacotherapy provided, treatments were in line with practice guidelines for depression care.32 The only constraint was that no CBT or interpersonal therapy was to be offered during the intervention phase. Seven psychiatrists who had practiced psychiatric specialty care for a mean (SD) of 7.3 (4.4) years provided the medication visits, which were offered roughly every 2 weeks, and each lasted 10-15 minutes.
After 16 weeks of CBT, the participants resumed standard care alongside other patients not enrolled in the study. No booster sessions were provided. Participants were assessed at 6 time points: baseline (at randomization); 8 and 16 weeks postrandomization; and 3, 6, and 12 months after the end of the 16-week intervention.
Outcomes
The primary outcome was the alleviation of depressive symptoms as measured by change in the total GRID-HDRS17 score from baseline to 16 weeks. Changes were also assessed at other time points. The GRID-HDRS17 is an amended version of the original Hamilton Depression Rating Scale,24,25 which provides standardized explicit scoring conventions with a structured interview guide for administration and scoring. All assessors received extensive GRID-HDRS17 training, and the interrater reliability (intraclass correlation coefficient) was 0.94-0.98, showing excellent agreement.
The secondary outcomes were also evaluated at the same 6 time points. These included treatment response (≥ 50% reduction in baseline GRID-HDRS17 score), remission (GRID-HDRS17 score ≤ 7), participant-rated measures of depressive symptoms (Beck Depression Inventory-II [BDI-II],33,34 and QIDS-SR score), and quality-of-life status as measured by the mental and physical component summary score of the 36-Item Short-Form Health Survey (SF-36).35,36 In addition, the participants were asked to complete the European Quality of Life Questionnaire-5 Dimensions37,38 and the World Health Organization Health and Work Performance Questionnaire (HPQ)39,40 so that we could conduct an economic evaluation (results from this analysis will be reported separately).
Information on the total daily dose of each antidepressant was expressed as a fraction of the World Health Organization’s defined daily dose,41 which is defined as the assumed average maintenance dose per day for adults calculated from the dosage recommendations for each drug. Adverse events were also monitored. Serious adverse events were defined as death, life-threatening events, events leading to severe impairment or dysfunction, and hospitalization.
Statistical Analysis
On the basis of our 16-week single-arm study of CBT plus TAU for major depression,28 we assumed that a mean difference of 4 points on the GRID-HDRS17 score with an SD of 5.0 between the CBT plus TAU group and the TAU-alone group would be clinically meaningful. With a 2-sided significance level of 5% and statistical power at 90%, and allowing for 15% attrition, a sample size of 40 was required for each arm.
Primary analysis was done on an intention-to-treat basis, and all of the randomized participants were included. For the continuous outcomes, the least squares mean values and their 95% CIs were estimated using a mixed-effects model for repeated measures (MMRM) that contained treatment group, week, and group-by-week interaction as fixed-effects with a compound symmetry covariance matrix among time points; Kenward-Roger degrees of freedom adjustment was performed. Mean values for each group at each time point and between-group mean differences were estimated using appropriate contrasts in the MMRM. Missing values were not imputed. We used the MMRM as the primary analysis model in this study, although an analysis of covariance (ANCOVA) with the last observation carried forward (LOCF) was originally planned in the study protocol.18 The reason for this change was that MMRM gives more accurate estimates than ANCOVA + LOCF under general settings, and it became a more accepted primary analysis model after our study was initiated.42 We also performed sensitivity analyses on GRID-HDRS17 scores at 12 months using pattern-mixture models of 5 imputation strategies based on multiple imputation43 to determine the sensitivity of the study results to departures from a missing at random mechanism.44 For the categorical outcomes, relative risks (RRs) and their 95% CIs were calculated. The number needed to treat (NNT) was calculated when a 95% CI of an RR did not include 1.0. Participants with missing data were considered nonresponders or nonremitters. The significance level was set at .05 (2-tailed) for all analyses. No multiple-testing correction was applied because this study was an RCT with 1 primary null hypothesis. Statistical analyses were performed with SAS version 9.3 (SAS Institute, Cary, North Carolina).
RESULTS
Figure 1 shows the flow of the participants from screening to follow-up. Between September 20, 2008, and August 19, 2013, we screened 126 participants, and the final 12-month follow-up was done on December 26, 2014. Of these participants, 80 (63.5%) met the inclusion criteria and were randomized either to receive CBT plus TAU (CBT group, n = 40) or to continue with TAU alone (TAU group, n = 40).
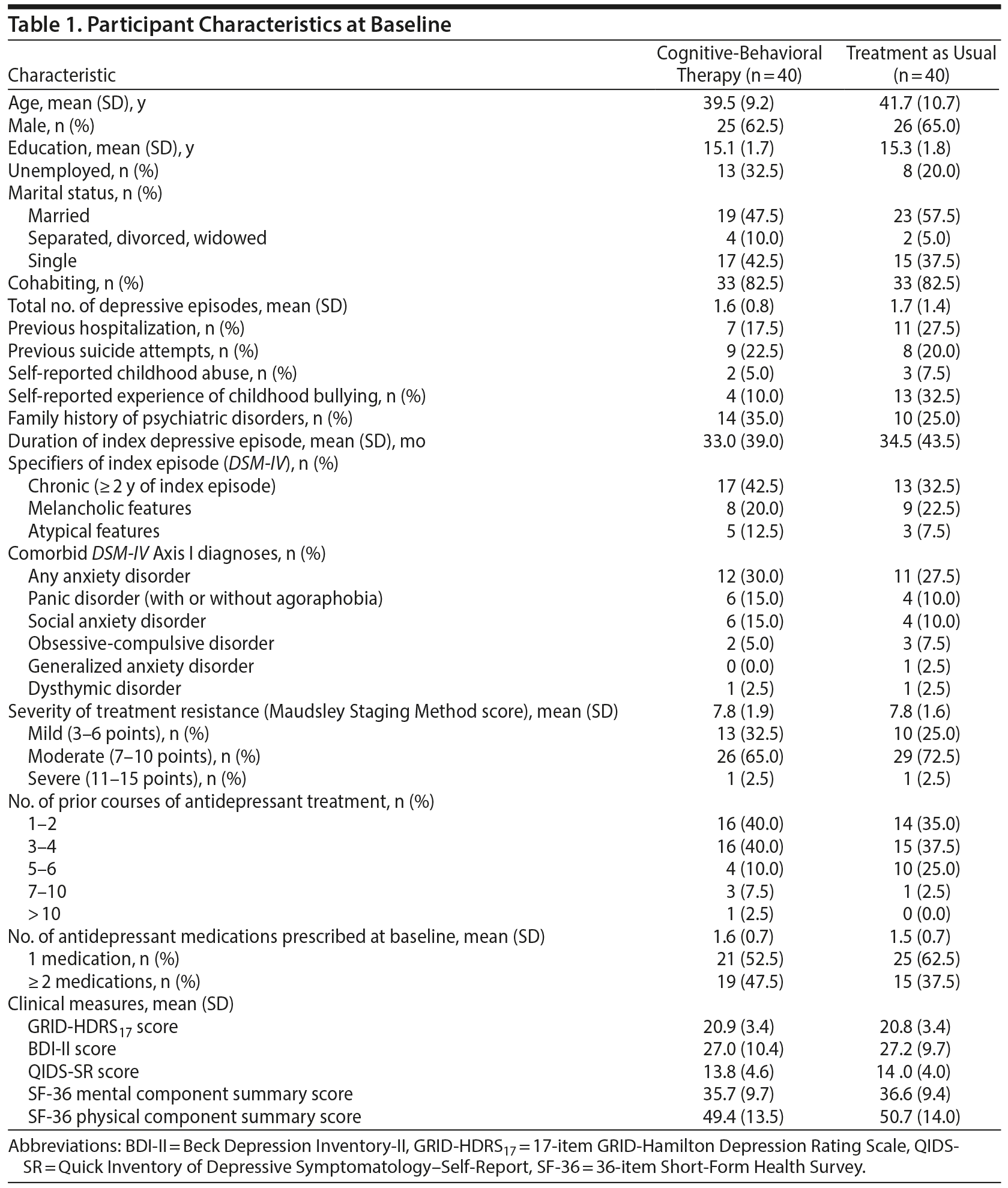
Table 1 summarizes the sociodemographic and clinical characteristics of the participants at baseline. Of the total sample, around one-fifth had a past history of psychiatric hospitalization or previous suicide attempts (18 [22.5%] and 17 [21.3%], respectively). Further, 31 participants (38.6%) had received 3 or 4 courses of antidepressants, and 19 (23.8%) had received 5 or more courses before study entry. None of the participants had received psychotherapy either for the current episode or in the past.
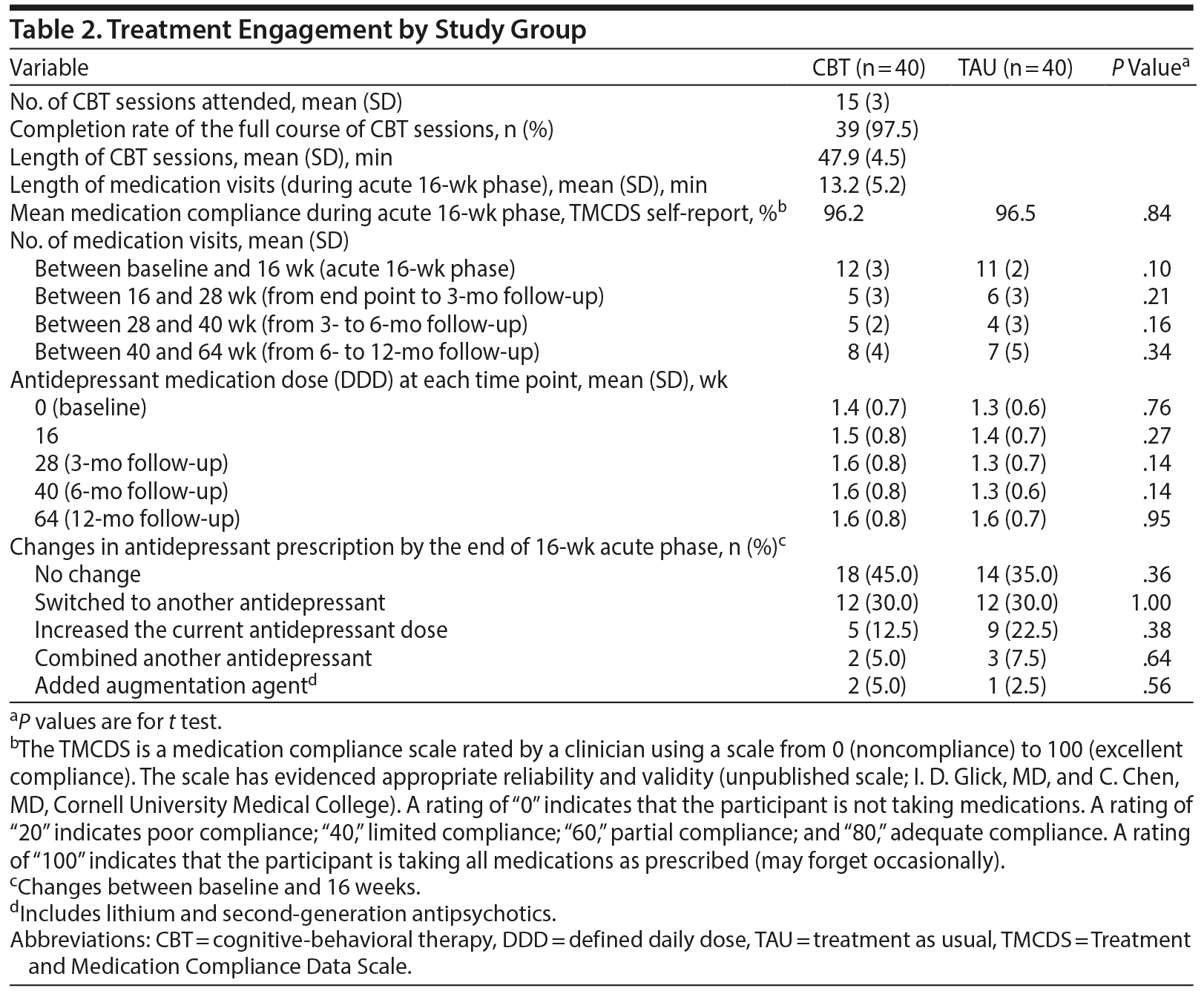
Table 2 shows treatment engagement by treatment group. Nearly all of the members of the CBT group completed the full course of CBT sessions. As for medication management, the mean daily dose of antidepressants was comparable at each time point between the treatment groups, and no significant dose changes were observed during follow-up. Selective serotonin reuptake inhibitors (SSRIs) were the most common antidepressant medication prescribed at baseline (40 participants [50.0%]; see Supplementary eTable 1 at Psychiatrist.com). After completion of the study intervention, all participants resumed standard follow-up care with their treating psychiatrists. There were no differences in the number of medical visits between the groups during the 12-month study period.
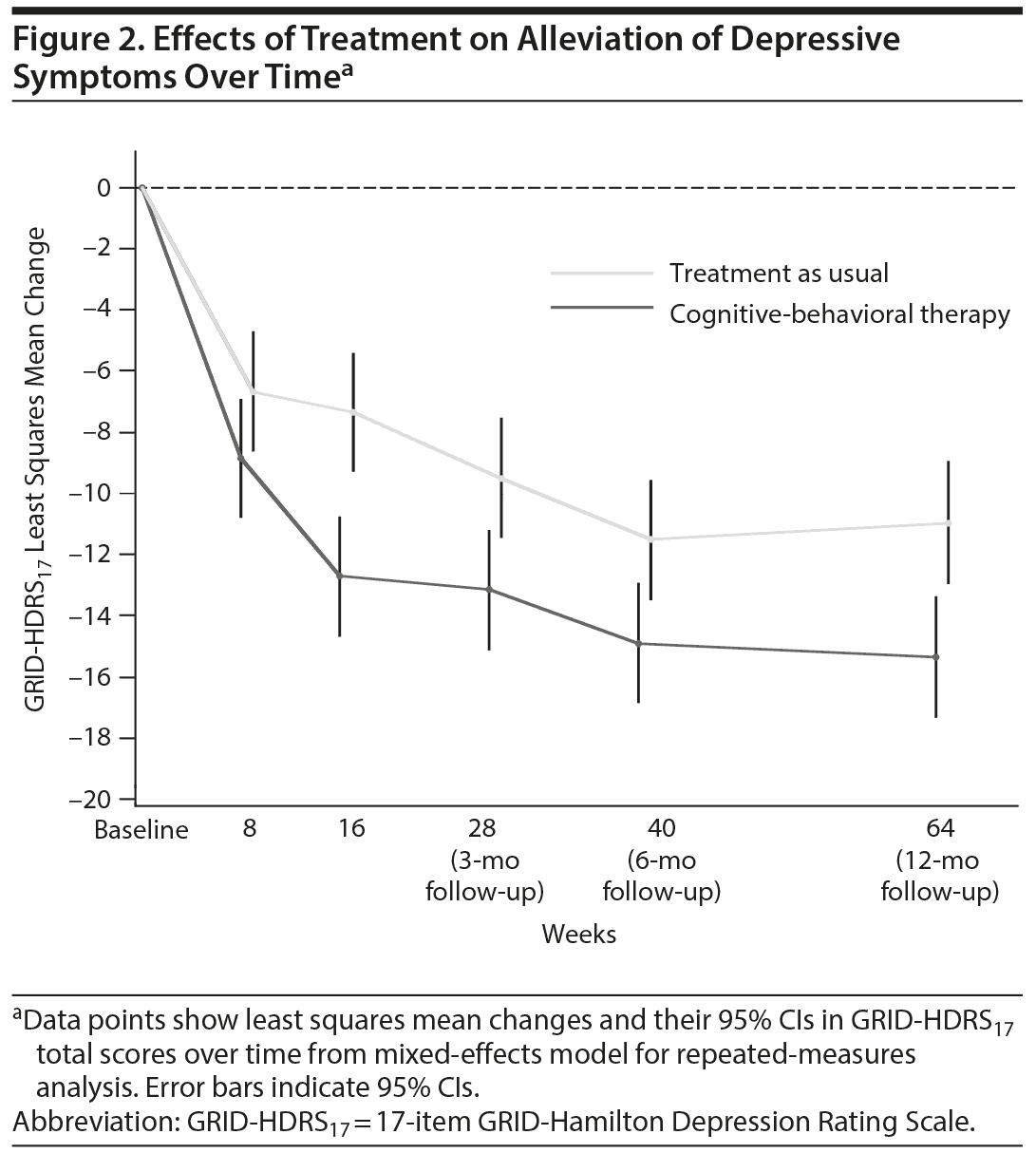
The least squares mean changes in GRID-HDRS17 total scores showed that alleviation of depressive symptoms at 16 weeks was greater in the CBT group than in the TAU group (−12.7 vs −7.4, respectively), and the between-group mean difference was significant (−5.4; 95% CI, −8.1 to −2.6; P < .001) (Figure 2). The beneficial effects of CBT were maintained over the 12-month follow-up and were confirmed at 3 months (−13.2 vs −9.5; difference = −3.7; 95% CI, −6.4 to −0.9; P = .01), at 6 months (−14.9 vs −11.5; difference = −3.4; 95% CI, −6.2 to −0.6; P = .02), and at 12 months (−15.4 vs −11.0; difference = −4.4; 95% CI, −7.2 to −1.6; P = .002). However, we did not note any difference in treatment effect at 8 weeks (P = .11). Similar results were obtained by conducting LOCF analyses (Supplementary eTable 2).
Sensitivity analyses of the GRID-HDRS17 score at 12 months to departures from a missing at random mechanism were performed using pattern-mixture models. The range of the between-group difference in changes in the pattern-mixture models (5 imputation strategies) was −4.1 to −4.3 (P ≤ .02 for all). These results were consistent with those of the MMRM (−4.4, P = .002), due to the high follow-up rate in this study (Supplementary eTable 3).
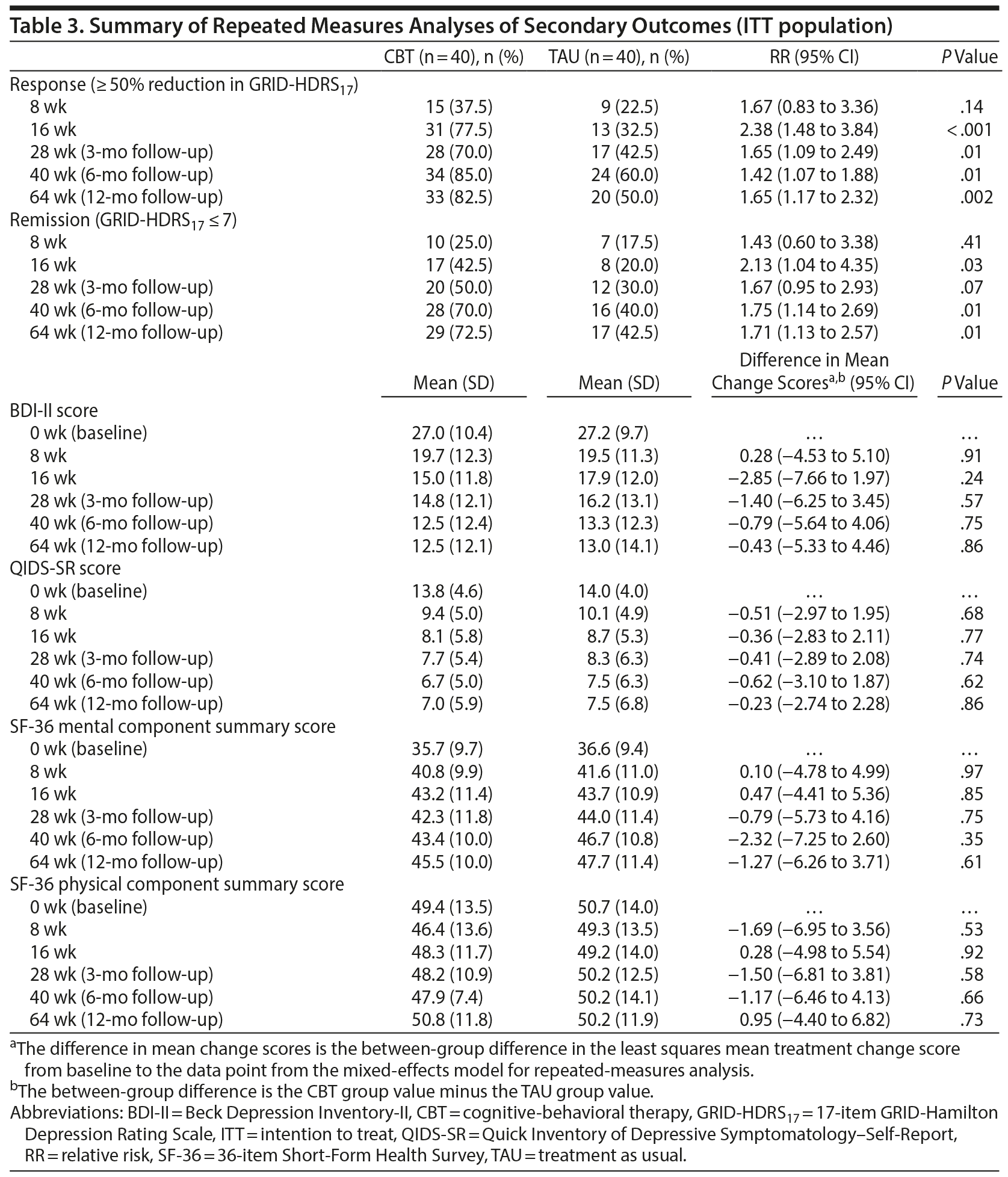
Table 3 summarizes the secondary outcome measures. Participants allocated to the CBT group were 2.4 times more likely to have a treatment response at 16 weeks than members of the TAU group (RR = 2.38; 95% CI, 1.48 to 3.84), resulting in an NNT of 5 (95% CI, 3 to 83). The beneficial effect of the intervention on treatment response was confirmed at 3, 6, and 12 months. Supplementary CBT had a similarly significant effect on the achievement of remission at 16 weeks (RR = 2.13; 95% CI, 1.04 to 4.35; NNT = 5; 95% CI, 3 to 36), and this was maintained over 12 months (except at 3 months: RR = 1.67; 95% CI, 0.95 to 2.93). Although the CBT group participants had milder depressive symptoms as measured by both BDI-II and QIDS-SR than the TAU group members over the study period, the differences were not statistically significant at each time point, nor were there any statistically significant differences between the groups in quality-of-life status as assessed by the SF-36 mental and physical subscales over the study period.
None of the participants experienced serious adverse events during the intervention period. However, during the postintervention follow-up, 2 participants (2.5%), both in the TAU group, were hospitalized for exacerbation of depression. One of these committed suicide shortly after discharge from the psychiatric ward, which was 10 months after the end of the intervention period.
DISCUSSION
We found that adding CBT to usual pharmacotherapy was effective in reducing depressive symptoms and in improving treatment response and remission in patients with pharmacotherapy-resistant depression receiving psychiatric specialty care and that these beneficial effects lasted for at least 12 months. This outcome is consistent with favorable effects observed in a previous large-scale study12 that was also carried out over a period of 12 months. The current findings underscore the benefits of supplementary CBT toward improving depressive symptoms, even in patients with pharmacotherapy-resistant depression treated in psychiatric specialty care settings.
In contrast to our findings, however, participants in the largest depression study, the Sequenced Treatment Alternatives to Relieve Depression (STAR*D),3 which involved patients in both psychiatric and primary care settings, showed no beneficial effects of CBT augmentation among participants who opted for augmentation (n = 65); these participants’ response to treatment was similar to that of those who did not receive CBT augmentation.45 This discrepancy may be explained in part by the unique equipoise-stratified randomization design adopted in the STAR*D, which allowed participants to decline the nonpreferred treatment option. In the STAR*D, participants had to pay most of the charges for the CBT sessions, and some had to go to a different site to receive CBT.45 These factors must have dampened enthusiasm for the CBT option, as demonstrated by the fact that only a quarter of the STAR*D participants agreed to be randomized to CBT. Further, the comparator of CBT augmentation in the STAR*D was medication augmentation and switch strategies as opposed to TAU as in the present study.
Although the present study’s primary outcome measure (the GRID-HDRS17) indicated significant alleviation of depressive symptoms, the self-rated secondary outcome measures of depressive symptoms (the BDI-II and the QIDS-SR) showed negligible differences between the groups. Several explanations can be posited to account for these findings. First, the present study was powered to detect effectiveness based on the primary outcome measure, so it is possible that the secondary outcome measures were underpowered. Second, clinician or patient biases in describing symptomatology may have played a role. Corruble et al46 reported that patients with high anxiety tend to underestimate changes in their depressive symptoms in comparison with clinicians’ assessments; nearly a third of our participants had comorbid anxiety disorders. Third, different depression scales evaluate different components of depressive symptomatology. For example, the GRID-HDRS17 measures more somatic and anxiety items than the BDI-II and QIDS-SR, which focus more on depressive items.47 Waza et al48 reported that depressed Japanese present more somatic symptoms and less psychological symptoms compared to their Western counterparts. Our patients may be less astute at distinguishing between symptoms than trained assessors who carefully evaluate each item on a depression scale independently from others. Fourth, it has not been demonstrated conclusively that patient-rated measurements reliably provide assessments that are consistent with those of clinician-administrated measurements in assessing the level of depressive severity throughout a trial.46,49 Consistent with previous reports,49 we found that the correlation between the GRID-HDRS17 and the BDI-II was poor at baseline and improved over time (baseline, r = 0.44; 16 weeks, r = 0.78; post 3 months, r = 0.81; post 6 months, r = 0.74; post 12 months, r = 0.81).
The SF-36 mental component summary score also did not differ between groups in our sample. The reason for this finding is unclear; however, in addition to the explanations discussed previously, it may be explained in part by studies50,51 raising concerns regarding the validity of orthogonal scoring methods to compute the SF-36 mental component summary score. Simon et al50 reported that improvement in the SF-36 subscales of physical function and role-physical make a negative contribution to the computed mental component summary and therefore might produce no apparent effect on the mental component summary score.
Cognitive-behavioral therapy was clearly an acceptable form of treatment as far as the participants in our study were concerned, as shown by the high rate of therapy completion (97.5%). Although none of the participants allocated to the CBT group experienced serious adverse events, 2 of the participants allocated to the TAU group were hospitalized for exacerbation of depression, 1 of whom committed suicide after discharge. More than 60% of our participants had received 3 or more courses of antidepressants before study entry, and more than a fifth had made previous suicide attempts and had past histories of psychiatric hospitalization. Thus, we were treating considerably disturbed psychiatric patients, mirroring clinically representative patients.
The main limitation of this study was the relatively small sample size, although the number of participants exceeded that required for power analysis. Also, the participants were recruited from 2 sites with a remarkably high treatment adherence (97.5%) and low dropout (8.8%), suggesting a sample of highly motivated treatment-seeking patients, which may limit generalizability. Further, although our small sample size did not allow direct comparison of CTRS scores among therapists, the possible contribution of the variance of non-protocol-based therapeutic effects among different therapists should be acknowledged. Patients with difficult-to-treat depression were reported to show differences in CBT response that were associated with therapists’ experience levels.52 Therefore, comprehensive training that includes supervision and technical assistance is crucial for implementing evidence-based CBT.53,54 Another possible limitation is that although similar antidepressant medication was used for both of our treatment groups, the nature of usual care means that we could not fully control antidepressant medication. Further, the benefits of CBT observed in the present study cannot be specifically derived solely from CBT because there was no treatment control. In other words, nonspecific treatment effects such as patient expectations may also account for the observed efficacy of CBT. Nevertheless, the aim of the present study was to examine the effectiveness of augmenting TAU with CBT rather than to evaluate the effects of CBT itself. Finally, although we used central randomization with the minimization method to ensure good balance of patient characteristics between study groups, the present study took place over 6 years and we cannot totally eliminate the potential effects of time.
In conclusion, the present study suggests that supplementing medication management with CBT is effective in reducing depressive symptoms and in improving treatment response and remission in patients with pharmacotherapy-resistant depression treated in psychiatric specialty care settings; these beneficial effects were maintained for at least 12 months. Additional research is now needed to replicate our results with a larger sample size before definite conclusions can be drawn. Nevertheless, our study provides promising findings in the context of depression therapeutics, and we hope our results will lead to improvements in the options for patients with pharmacotherapy-resistant depression.
Submitted: November 3, 2015; accepted April 15, 2016.
Online first: February 28, 2017.
Author contributions: Drs Nakagawa and Sado conceived and designed the study. Dr Nakagawa drafted the study protocol, organized and supervised study implementation, and drafted the manuscript. Drs Fujisawa and Kikuchi refined the study protocol and study implementation. Dr Abe provided methodological and statistical expertise. Drs Abe and Nakagawa conducted the statistical analyses. Drs Nakagawa, Sado, Fujisawa, Kikuchi, and Ono interpreted the results. Dr Ono provided cognitive-behavioral therapy expertise, supervised the therapists, drafted the grant proposal, and was responsible for study implementation. Dr Nakagawa was responsible for study management, staff training, and supervision. Dr Mitsuda managed day-to-day study responsibilities, including monitoring the recruitment of participants, collecting data, and liaising between the recruitment sites. Drs Iwashita and Mimura are the directors of the 2 study sites and provided clinical expertise and on-site management of the study. Drs Nakagawa and Abe had full access to all of the data throughout the study and take responsibility for the integrity of the data and the accuracy of the analyses. All authors critically reviewed the manuscript for content and approved the final version.
Potential conflicts of interest: Dr Ono has received research support from the Japanese Ministry of Health, Labour, and Welfare and royalties from Igaku-Shoin, Seiwa-Shoten, Sogensha, and Kongo-Shuppan. Dr Fujisawa has received royalties from Seiwa-Shoten. The other authors have no conflicts of interest to declare.
Funding/support: This study was funded by Health Labour Sciences Research grants from the Japanese Ministry of Health, Labour, and Welfare.
Role of the sponsor: The funding source had no role in the study design, data collection, management, analysis, interpretation of the data, or in the writing of the paper.
Acknowledgments: The authors thank Kimio Yoshimura, MD, PhD, of Keio University School of Medicine, for his comments as a member of the Data Safety Monitoring Committee. The authors also appreciate the support provided by Yoko Ito, MS, and Kayoko Kikuchi, PhD, of the Project Management Office at the Keio Center for Clinical Research for the construction of the electronic system and data management. These individuals report no relevant financial disclosures.
Supplementary material: Available at Psychiatrist.com.
REFERENCES
1. Souery D, Amsterdam J, de Montigny C, et al. Treatment resistant depression: methodological overview and operational criteria. Eur Neuropsychopharmacol. 1999;9(1-2):83-91. PubMed doi:10.1016/S0924-977X(98)00004-2
2. Rush AJ, Trivedi MH, Wisniewski SR, et al; STAR*D Study Team. Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. N Engl J Med. 2006;354(12):1231-1242. PubMed doi:10.1056/NEJMoa052963
3. Trivedi MH, Rush AJ, Wisniewski SR, et al; STAR*D Study Team. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28-40. PubMed doi:10.1176/appi.ajp.163.1.28
4. Connolly KR, Thase ME. If at first you don’ t succeed: a review of the evidence for antidepressant augmentation, combination and switching strategies. Drugs. 2011;71(1):43-64. PubMed doi:10.2165/11587620-000000000-00000
5. Cuijpers P, Berking M, Andersson G, et al. A meta-analysis of cognitive-behavioural therapy for adult depression, alone and in comparison with other treatments. Can J Psychiatry. 2013;58(7):376-385. PubMed
6. Cuijpers P, Dekker J, Hollon SD, et al. Adding psychotherapy to pharmacotherapy in the treatment of depressive disorders in adults: a meta-analysis. J Clin Psychiatry. 2009;70(9):1219-1229. PubMed doi:10.4088/JCP.09r05021
7. Cuijpers P, Sijbrandij M, Koole SL, et al. Adding psychotherapy to antidepressant medication in depression and anxiety disorders: a meta-analysis. World Psychiatry. 2014;13(1):56-67. PubMed doi:10.1002/wps.20089
8. Keller MB, McCullough JP, Klein DN, et al. A comparison of nefazodone, the cognitive behavioral-analysis system of psychotherapy, and their combination for the treatment of chronic depression. N Engl J Med. 2000;342(20):1462-1470. PubMed doi:10.1056/NEJM200005183422001
9. Kocsis JH, Gelenberg AJ, Rothbaum BO, et al; REVAMP Investigators. Cognitive behavioral analysis system of psychotherapy and brief supportive psychotherapy for augmentation of antidepressant nonresponse in chronic depression: the REVAMP Trial. Arch Gen Psychiatry. 2009;66(11):1178-1188. PubMed doi:10.1001/archgenpsychiatry.2009.144
10. Wiersma JE, Van Schaik DJF, Hoogendorn AW, et al. The effectiveness of the cognitive behavioral analysis system of psychotherapy for chronic depression: a randomized controlled trial. Psychother Psychosom. 2014;83(5):263-269. PubMed doi:10.1159/000360795
11. Michalak J, Schultze M, Heidenreich T, et al. A randomized controlled trial on the efficacy of mindfulness-based cognitive therapy and a group version of cognitive behavioral analysis system of psychotherapy for chronically depressed patients. J Consult Clin Psychol. 2015;83(5):951-963. PubMed doi:10.1037/ccp0000042
12. Wiles N, Thomas L, Abel A, et al. Cognitive behavioural therapy as an adjunct to pharmacotherapy for primary care based patients with treatment resistant depression: results of the CoBalT randomised controlled trial. Lancet. 2013;381(9864):375-384. PubMed doi:10.1016/S0140-6736(12)61552-9
13. Watkins ER, Mullan E, Wingrove J, et al. Rumination-focused cognitive-behavioural therapy for residual depression: phase II randomised controlled trial. Br J Psychiatry. 2011;199(4):317-322. PubMed doi:10.1192/bjp.bp.110.090282
14. Wiles NJ, Hollinghurst S, Mason V, et al. A randomized controlled trial of cognitive behavioural therapy as an adjunct to pharmacotherapy in primary care based patients with treatment resistant depression: a pilot study. Behav Cogn Psychother. 2008;36(01):21-33. doi:10.1017/S135246580700389X
15. Gaynes BN, Rush AJ, Trivedi M, et al. A direct comparison of presenting characteristics of depressed outpatients from primary vs specialty care settings: preliminary findings from the STAR*D clinical trial. Gen Hosp Psychiatry. 2005;27(2):87-96. PubMed doi:10.1016/j.genhosppsych.2004.10.003
16. Gaynes BN, Rush AJ, Trivedi MH, et al. Major depression symptoms in primary care and psychiatric care settings: a cross-sectional analysis. Ann Fam Med. 2007;5(2):126-134. PubMed doi:10.1370/afm.641
17. Souery D, Oswald P, Massat I, et al; Group for the Study of Resistant Depression. Clinical factors associated with treatment resistance in major depressive disorder: results from a European multicenter study. J Clin Psychiatry. 2007;68(7):1062-1070. PubMed doi:10.4088/JCP.v68n0713
18. Nakagawa A, Sado M, Mitsuda D, et al. Effectiveness of cognitive behavioural therapy augmentation in major depression treatment (ECAM study): study protocol for a randomised clinical trial. BMJ Open. 2014;4(10):e006359. PubMed doi:10.1136/bmjopen-2014-006359
19. Boutron I, Moher D, Altman DG, et al; CONSORT Group. Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Ann Intern Med. 2008;148(4):295-309. PubMed doi:10.7326/0003-4819-148-4-200802190-00008
20. Schulz KF, Altman DG, Moher D; CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. PubMed doi:10.1136/bmj.c332
21. American Psychiatric Association. Diagnostic and Statistical Manual for Mental Disorders. Fourth Edition. Washington, DC: American Psychiatric Association; 1994.
22. First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV-TR Axis I Disorders-Patient Edition (SCID-I/P). New York, NY: Biometrics Research Department; 2007.
23. Fekadu A, Wooderson S, Donaldson C, et al. A multidimensional tool to quantify treatment resistance in depression: the Maudsley Staging Method. J Clin Psychiatry. 2009;70(2):177-184. PubMed doi:10.4088/JCP.08m04309
24. Tabuse H, Kalali A, Azuma H, et al. The new GRID Hamilton Rating Scale for Depression demonstrates excellent inter-rater reliability for inexperienced and experienced raters before and after training. Psychiatry Res. 2007;153(1):61-67. PubMed doi:10.1016/j.psychres.2006.07.004
25. Williams JBW, Kobak KA, Bech P, et al. The GRID-HAMD: standardization of the Hamilton Depression Rating Scale. Int Clin Psychopharmacol. 2008;23(3):120-129. PubMed doi:10.1097/YIC.0b013e3282f948f5
26. CBT Manual for Depression, a Therapist’s Manual. Japanese Ministry of Health, Labour and Welfare Web site. http://www.mhlw.go.jp/bunya/shougaihoken/kokoro/dl/01.pdf. Accessed April 11, 2016.
27. Beck AT, Rush AJ, Shaw BF, et al. Cognitive Therapy of Depression. New York, NY: Guilford Press; 1979.
28. Fujisawa D, Nakagawa A, Tajima M, et al. Cognitive behavioral therapy for depression among adults in Japanese clinical settings: a single-group study. BMC Res Notes. 2010;3:160. PubMed doi:10.1186/1756-0500-3-160
29. Young J, Beck AT. Cognitive Therapy Rating Scale Manual. Philadelphia, PA: University of Pennsylvania, Psychotherapy Research Unit; 1980.
30. Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54(5):573-583. PubMed doi:10.1016/S0006-3223(02)01866-8
31. Fujisawa D, Nakagawa A, Tajima M, et al. Development of the Japanese version of Quick Inventory of Depressive Symptomatology Self-Reported (QIDS-SR16-J) [in Japanese]. Stress Science. 2010;25:43-52.
32. Gelenberg AJ, Freeman MP, Markowitz JC, et al; Work Group on Major Depressive Disorder. Practice Guideline for the Treatment of Patients With Major Depressive Disorder. 3rd ed. Arlington, VA: American Psychiatric Association; 2010.
33. Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II (BDI-II). San Antonio, TX: Psychological Corporation; 1996.
34. Kojima M, Furukawa TA, Takahashi H, et al. Cross-cultural validation of the Beck Depression Inventory-II in Japan. Psychiatry Res. 2002;110(3):291-299. PubMed doi:10.1016/S0165-1781(02)00106-3
35. Brazier JE, Harper R, Jones NM, et al. Validating the SF-36 Health Survey questionnaire: new outcome measure for primary care. BMJ. 1992;305(6846):160-164. PubMed doi:10.1136/bmj.305.6846.160
36. Fukuhara S, Ware JE Jr, Kosinski M, et al. Psychometric and clinical tests of validity of the Japanese SF-36 Health Survey. J Clin Epidemiol. 1998;51(11):1045-1053. PubMed doi:10.1016/S0895-4356(98)00096-1
37. EuroQol Group. EuroQol—a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199-208. PubMed doi:10.1016/0168-8510(90)90421-9
38. Fukuhara S, Ikegami N, Torrance GW, et al. The development and use of quality-of-life measures to evaluate health outcomes in Japan. Pharmacoeconomics. 2002;20(suppl 2):17-23. PubMed doi:10.2165/00019053-200220002-00003
39. Kessler RC, Barber C, Beck A, et al. The World Health Organization Health and Work Performance Questionnaire (HPQ). J Occup Environ Med. 2003;45(2):156-174. PubMed doi:10.1097/01.jom.0000052967.43131.51
40. Kessler RC, Petukhova M, Mcinnes K. World Health Organization Health and Work Performance Questionnaire (HPQ). Japanese version of the HPQ Short Form (Absenteeism and Presenteeism Questions and Scoring Rules). Harvard Medical School Department of Health Care Policy Web site. http://www.hcp.med.harvard.edu/hpq/info.php. Accessed April 11, 2016.
41. World Health Organization. Guidelines for ATC Classification and DDD Assignment. WHO Collaboration Center for Drug Statistics Methodology. Geneva, Switzerland: World Health Organization; 2014.
42. Little RJ, D’ Agostino R, Cohen ML, et al. The prevention and treatment of missing data in clinical trials. N Engl J Med. 2012;367(14):1355-1360. PubMed doi:10.1056/NEJMsr1203730
43. Carpenter J, Kenward M. Multiple Imputation and its Application. Hoboken, NJ: Wiley; 2013. doi:10.1002/9781119942283
44. Rubin DB. Inference and missing data. Biometrika. 1976;63(3):581-592. doi:10.1093/biomet/63.3.581
45. Thase ME, Friedman ES, Biggs MM, et al. Cognitive therapy versus medication in augmentation and switch strategies as second-step treatments: a STAR*D report. Am J Psychiatry. 2007;164(5):739-752. PubMed doi:10.1176/ajp.2007.164.5.739
46. Corruble E, Legrand JM, Zvenigorowski H, et al. Concordance between self-report and clinician’s assessment of depression. J Psychiatr Res. 1999;33(5):457-465. PubMed doi:10.1016/S0022-3956(99)00011-4
47. Uher R, Farmer A, Maier W, et al. Measuring depression: comparison and integration of three scales in the GENDEP study. Psychol Med. 2008;38(2):289-300. PubMed doi:10.1017/S0033291707001730
48. Waza K, Graham AV, Zyzanski SJ, et al. Comparison of symptoms in Japanese and American depressed primary care patients. Fam Pract. 1999;16(5):528-533. PubMed doi:10.1093/fampra/16.5.528
49. Dunlop BW, Li T, Kornstein SG, et al. Correlation between patient and clinician assessments of depression severity in the PREVENT study. Psychiatry Res. 2010;177(1-2):177-183. PubMed doi:10.1016/j.psychres.2010.02.008
50. Simon GE, Revicki DA, Grothaus L, et al. SF-36 summary scores: are physical and mental health truly distinct? Med Care. 1998;36(4):567-572. PubMed doi:10.1097/00005650-199804000-00012
51. Taft C, Karlsson J, Sullivan M. Do SF-36 summary component scores accurately summarize subscale scores? Qual Life Res. 2001;10(5):395-404. PubMed doi:10.1023/A:1012552211996
52. DeRubeis RJ, Hollon SD, Amsterdam JD, et al. Cognitive therapy vs medications in the treatment of moderate to severe depression. Arch Gen Psychiatry. 2005;62(4):409-416. PubMed doi:10.1001/archpsyc.62.4.409
53. Karlin BE, Brown GK, Trockel M, et al. National dissemination of cognitive behavioral therapy for depression in the Department of Veterans Affairs health care system: therapist and patient-level outcomes. J Consult Clin Psychol. 2012;80(5):707-718. PubMed doi:10.1037/a0029328
54. Branson A, Shafran R, Myles P. Investigating the relationship between competence and patient outcome with CBT. Behav Res Ther. 2015;68:19-26. PubMed doi:10.1016/j.brat.2015.03.002
This PDF is free for all visitors!