Abstract
Iron is an essential trace element that is important for the development, structure, and functioning of the brain. Iron has been both favorably and unfavorably implicated in neuropsychiatric disorders. For example, iron adequacy in pregnancy and early childhood has been suggested to reduce the risk of neurodevelopmental disorders and schizophrenia, but iron mechanisms have been implicated in neurodegenerative disorders, multiple sclerosis, and stroke. Supplemental iron may be indicated to treat restless legs syndrome, akathisia, and pica, but more commonly to treat iron deficiency associated with poor nutrition in major mental illness. Supplemental iron is commonly orally administered but is poorly absorbed by this route. It is therefore necessary to know what improves and what impairs iron absorption. This article explains that, for best absorption, oral iron supplements are ideally dosed as ferrous salts. The dose should be administered in the morning, on a fasting stomach, along with about 100 mg of vitamin C in the form of a tablet, or with a glass of orange or other citrus juice. If neither vitamin C nor citrus juice is available, as a poorer option, iron should be dosed with plain water. Absorption is markedly reduced if iron is administered in the afternoon, or with food such as cereals and other grains, or with beverages such as milk, tea, and coffee. Calcium supplements, antacids, H2 inhibitors, and proton pump inhibitors also reduce the absorption of orally administered iron. Some data suggest that alternate day dosing improves fractional iron absorption as well as reduces adverse effects of treatment. Finally, to reduce the risk of pill esophagitis, iron should be dosed with a full glass of liquid, and the patient should not recline or lie down for at least the next 30–60 min.
J Clin Psychiatry 2025;86(4):25f16139
Author affiliations are listed at the end of this article.
Iron is an essential trace element1 that plays important roles in the brain. It is involved in fundamental physiological processes, ranging from oxygen transport to energy metabolism. It plays a role in critical cellular processes, such as neural cell differentiation,2 myelination by oligodendrocytes,3 neurotransmitter synthesis and breakdown,2 and programmed nerve cell death through ferroptosis.4 In brief, it is implicated in both cell structure and cell functioning in the brain. This article briefly examines the relevance of iron in neuropsychiatry and focuses on a very practical matter: how to dose oral iron supplements so as to maximize its absorption.
Iron in Neuropsychiatry
Iron adequacy in neonates, infants, and children supports normal brain development and functioning,5,6 and iron deficiency has been suggested as a risk factor for intellectual disability and developmental delays7 as well as autism spectrum disorder and attention-deficit/ hyperactivity disorders.6,8 Maternal iron deficiency during pregnancy may be a risk factor for schizophrenia.9,10
On the flip side, iron has been implicated in the pathogenesis of neurodegenerative disorders,11 as in Alzheimer’s disease ,12 Parkinson’s disease,4 Huntington’s disease,13 amyotrophic lateral sclerosis,14 and Friedreich’s ataxia.15 Iron has been implicated in the pathogenesis of other neurological disorders, as well, ranging from multiple sclerosis16 to stroke.17
Iron may be therapeutic in many neuropsychiatric conditions, such as pica,18 pagophagia,19 antipsychotic-induced akathisia,20 and restless legs syndrome (RLS),21 including RLS during pregnancy22 and pediatric RLS.23 Ferroptosis may be leveraged in glioma therapy.24,25
Iron deficiency is often part of the nutritional deficiency that may be observed in patients with psychiatric disorders, such as schizophrenia and depression.26–28 Correcting iron deficiency requires clinicians to know how to dose supplemental iron. Specific issues, listed in Box 1, were addressed in a recent clinical trial.
The Absorption of Supplemental Iron: Clinical Trial
Much literature has been published on factors that influence the absorption of iron from food, but questions remain regarding the absorption of oral iron supplements. In this context, von Siebenthal et al29 described an open-label randomized controlled crossover trial of the effects of food, beverages, and time of day on the absorption of supplemental iron.
The study was conducted in Zurich, Switzerland. The sample comprised 34 women aged 18–45 years, all of whom were iron deficient (plasma ferritin <30 μg/L) but none of whom were anemic (hemoglobin >12 g/dL). The median age of the women was 28 years. The mean body weight was 57 kg, and the mean body mass index (BMI) was 20 kg/m2. All women were nonsmokers and were generally healthy.
On each of different days and in random order, and with (mostly) 1-day intervals between dosing conditions, these women received 100 mg of radiolabeled ferrous fumarate as follows:
- In the morning, with water (reference condition)
- In the morning, with 80 mg of ascorbic acid (vitamin C)
- In the morning, with 500 mg of ascorbic acid
- In the morning, with coffee
- In the morning, with breakfast that included coffee and orange juice
- In the afternoon, with water
In these 6 treatment conditions, morning dosing was at 7:00–9:00 am after an overnight fast, and afternoon dosing, at 4:00–6:00 pm after a 4 hour fast. Breakfast comprised a bread roll, butter, honey, yogurt, orange juice (containing about 90 mg of ascorbic acid), and coffee. After dosing, food and beverages were not permitted for 3 h.
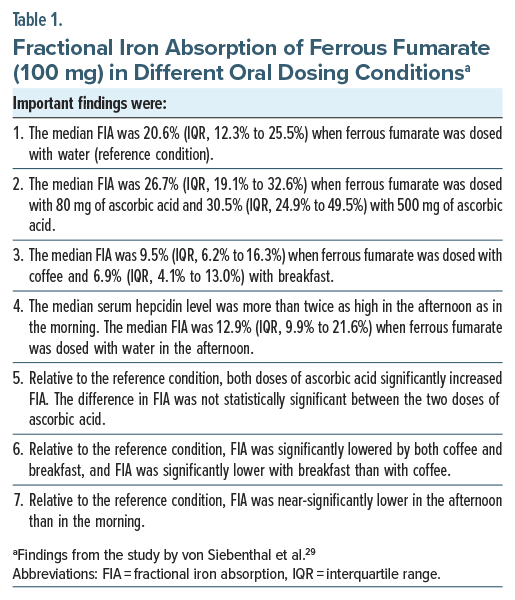
Important findings from the study are presented in Table 1. In summary, the median fractional iron absorption was 21% when iron was dosed in the morning with water (reference condition); 27% with ascorbic acid, 80 mg; 31% with ascorbic acid, 500 mg; 10% with coffee; and 7% with breakfast. Fractional iron absorption was 13% when dosed with water in the afternoon. Notably, administration of iron, along with ascorbic acid, in a fasting condition and in the morning, was associated with a 4-fold greater absorption relative to administration of iron with breakfast (despite the breakfast containing ascorbic acid in orange juice).
A limitation of this study is that it was conducted in a purposive sample: iron-deficient, nonanemic, generally healthy young women. Furthermore, the BMI of the sample was in the low normal range. Generalization of the findings to the population at large should therefore be made with caution. Generalization to clinical populations, including special populations, such as patients with iron-deficiency anemia, pregnant or postmenopausal women, and persons with nutritional deficiencies, should also be made with caution.
Dietary Influences on the Absorption of Iron
Iron in meat-based foods is in the ferrous form in heme molecules. Iron in vegetables is in the ferric form. Ferrous iron is better absorbed than ferric iron.30
The acid environment in the stomach, and vitamin C in diet, help reduce ferric to ferrous iron, thereby facilitating the absorption of iron.31 This explains why drugs that impair iron absorption include antacids, H2 receptor blockers, and proton pump inhibitors.30 Vitamin C improves the absorption of ferrous iron through other mechanisms, as well.29
Polyphenols in fruits such as berries and in beverages such as coffee and tea, phytates in plant-based foods, and casein in milk form complexes with iron, reducing its absorption.31 Calcium supplements also impair iron absorption.32
Hepcidin
Hepcidin is a peptide hormone that regulates many aspects of iron homeostasis, including the absorption of iron; higher hepcidin levels are associated with lower fractional iron absorption. Hepcidin levels show diurnal variation, being higher in the afternoon than in the morning. Hepcidin levels are also increased, as a feedback mechanism, by iron administration and by rising levels of iron in blood and in the liver.33 These observations explain why iron absorption is poorer with afternoon dosing relative to morning dosing,29 why taking higher doses of iron are associated with lower fractional iron absorption,34 and why at least some35–37 but not all38 studies find that administration of twice the daily dose on alternate days results in better fractional iron absorption and higher end point hemoglobin levels relative to daily dosing with oral iron supplements. Alternate day iron supplementation may also be better tolerated, especially in the elderly.39
Summary
For best absorption, oral iron supplements should be administered in the morning, on a fasting stomach, along with about 100 mg of vitamin C in the form of a tablet, or with a glass of orange or other citrus juice (which could be expected to provide about the same quantity of vitamin C). If neither vitamin C nor citrus juice is available, as the next best option, iron should be dosed with plain water. Absorption is markedly reduced if iron is administered in the afternoon, or with food such as cereals and other grains, or with beverages such as tea and coffee. Clinical messages relevant to improved absorption of oral iron supplements are presented in Box 2.
Parting Notes
Oral iron supplements can cause constipation but less often can cause diarrhea; in the latter event, taking iron with a full meal may reduce the risk of diarrhea. The unavoidable consequence is that it will take longer to correct the iron deficiency and to replenish iron stores after the deficiency has been corrected.
Oral iron supplementation should be continued until the reason for supplementation, such as normalizing the hemoglobin level, has been addressed; subsequently, it is usual to continue supplementation for a further 3 months, or to a target ferritin level of >100 μg/L. The continuation serves to replenish iron reserves in the body, as reflected by the ferritin level.39 Iron supplementation should not be continued beyond necessity because it can result in iron deposits in tissues and damage to organs such as the liver, heart, and pancreas. For more information, including discussions on dosing with intravenous iron, readers are referred to publicly available guidelines.39,40 For information about how iron is handled in the body, readers may consult the review by Anderson and Frazer.1
As a final note, iron supplements should be ingested with plenty of fluids and with the body erect. The body should remain erect, that is, not supine, for at least 30–60 min afterwards. These two precautions reduce the risk of pill esophagitis.41
Article Information
Published Online: October 8, 2025. https://doi.org/10.4088/JCP.25f16139
© 2025 Physicians Postgraduate Press, Inc.
To Cite: Andrade C. Dosing patients with oral iron supplements: practical guidance. J Clin Psychiatry. 2025;86(4):25f16139.
Author Affiliations: Department of Clinical Psychopharmacology and Neurotoxicology, National Institute of Mental Health and Neurosciences, Bangalore, India; Department of Psychiatry, Kasturba Medical College, Manipal Academy of Higher Education, Manipal, India.
Corresponding Author: Chittaranjan Andrade, MD, Department of Clinical Psychopharmacology and Neurotoxicology, National Institute of Mental Health and Neurosciences, Bangalore 560029, India ([email protected]).
Relevant Financial Relationships: None.
Funding/Support: None.
 Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Department of Clinical Psychopharmacology and Neurotoxicology, National Institute of Mental Health and Neurosciences, Bangalore, India. Please contact Chittaranjan Andrade, MD, at Psychiatrist.com/contact/andrade.
References (41)

- Anderson GJ, Frazer DM. Current understanding of iron homeostasis. Am J Clin Nutr. 2017;106(suppl 6):1559S–1566S. PubMed CrossRef
- Gao Q, Zhou Y, Chen Y, et al. Role of iron in brain development, aging, and neurodegenerative diseases. Ann Med. 2025;57(1):2472871. PubMed CrossRef
- Wade QW, Connor JR. What does iron mean to an oligodendrocyte? Glia. 2025;73(9):1784–1804. PubMed CrossRef
- Wang W, Thomas ER, Xiao R, et al. Targeting mitochondria-regulated ferroptosis: a new frontier in Parkinson’s disease therapy. Neuropharmacology. 2025;274:110439. PubMed CrossRef
- Gisslen T, Rao R, Georgieff MK. Anemia, iron supplementation, and the brain. Clin Perinatol. 2023;50(4):853–868. PubMed CrossRef
- Fiani D, Engler S, Fields S, et al. Iron deficiency in attention-deficit hyperactivity disorder, autism spectrum disorder, internalizing and externalizing disorders, and movement disorders. Child Adolesc Psychiatr Clin N Am. 2023;32(2):451–467. PubMed CrossRef
- Chen MH, Su TP, Chen YS, et al. Association between psychiatric disorders and iron deficiency anemia among children and adolescents: a nationwide population-based study. BMC Psychiatry. 2013;13:161. PubMed CrossRef
- McWilliams S, Singh I, Leung W, et al. Iron deficiency and common neurodevelopmental disorders: a scoping review. PLoS One. 2022;17(9):e0273819. PubMed CrossRef
- Insel BJ, Schaefer CA, McKeague IW, et al. Maternal iron deficiency and the risk of schizophrenia in offspring. Arch Gen Psychiatry. 2008;65(10):1136–1144. PubMed CrossRef
- Sørensen HJ, Nielsen PR, Pedersen CB, et al. Association between prepartum maternal iron deficiency and offspring risk of schizophrenia: population-based cohort study with linkage of Danish national registers. Schizophr Bull. 2011;37(5):982–987. PubMed
- Levi S, Ripamonti M, Moro AS, et al. Iron imbalance in neurodegeneration. Mol Psychiatry. 2024;29(4):1139–1152. PubMed CrossRef
- Zhou B, Li J, Wu A, et al. Insights into targeted ferroptosis in mechanisms, biology, and role of Alzheimer’s disease: an update. Front Aging Neurosci. 2025;17:1587986. PubMed CrossRef
- Mi Y, Gao X, Xu H, et al. The emerging roles of ferroptosis in Huntington’s disease. Neuromolecular Med. 2019;21(2):110–119. PubMed CrossRef
- Shibata N, Kataoka I, Okamura Y, et al. Implications for soluble iron accumulation, oxidative stress, and glial glutamate release in motor neuron death associated with sporadic amyotrophic lateral sclerosis. Neuropathology. 2025;45(3):177–201. PubMed CrossRef
- Reetz K, Lischewski SA, Dogan I, et al. Friedreich’s ataxia: a rare multisystem disease. Lancet Neurol. 2025;24(7):614–624. PubMed CrossRef
- Kłodnicka K, Januszewski J, Forma A. ett al. Iron in multiple sclerosis -from pathophysiology to disease progression -a narrative literature review. Acta Neurobiol Exp (Wars). 2025;85(2):75–93. PubMed CrossRef
- Shi Z, Chen K, Wang Y, et al. The crosstalk between ferritinophagy and ferroptosis in ischemic stroke: regulatory mechanisms and therapeutic implications. Cell Mol Neurobiol. 2025;45(1):73. PubMed CrossRef
- Borgna-Pignatti C, Zanella S. Pica as a manifestation of iron deficiency. Expert Rev Hematol. 2016;9(11):1075–1080. PubMed CrossRef
- Kumar S, Jain S, Sinha SK, et al. Pagophagia: a case series. Ind Psychiatry J. 2024;33(1):175–178. PubMed CrossRef
- Schoretsanitis G, Nikolakopoulou A, Guinart D, et al. Iron homeostasis alterations and risk for akathisia in patients treated with antipsychotics: a systematic review and meta-analysis of cross-sectional studies. Eur Neuropsychopharmacol. 2020;35:1–11. PubMed CrossRef
- Short V, Allen R, Earley CJ, et al. A randomized double-blind pilot study to evaluate the efficacy, safety, and tolerability of intravenous iron versus oral iron for the treatment of restless legs syndrome in patients with iron deficiency anemia. Am J Hematol. 2024;99(6):1077–1083. PubMed CrossRef
- Phillips AM, Sward LB, Manning N, et al. Restless leg syndrome and pregnancy. South Med J. 2025;118(5):269–274. PubMed CrossRef
- Dye T, Simakajornboon N. Iron metabolism and the role of iron therapy in pediatric restless leg syndrome. Sleep Med Clin. 2025;20(2):231–238. PubMed CrossRef
- Zhou Y, Fang C, Xu H, et al. Ferroptosis in glioma treatment: current situation, prospects and drug applications. Front Oncol. 2022;12:989896. PubMed CrossRef
- Yeon Kim S, Tang M, Lu T, et al. Ferroptosis in glioma therapy: advancements in sensitizing strategies and the complex tumor-promoting roles. Brain Res. 2024;1840:149045. PubMed CrossRef
- Kuloglu M, Atmaca M, Ustündag B, et al. Serum iron levels in schizophrenic patients with or without akathisia. Eur Neuropsychopharmacol. 2003;13(2):67–71. PubMed CrossRef
- Leung BM, Kaplan BJ. Perinatal depression: prevalence, risks, and the nutrition link: a review of the literature. J Am Diet Assoc. 2009;109(9):1566–1575. PubMed CrossRef
- Stewart R, Hirani V. Relationship between depressive symptoms, anemia, and iron status in older residents from a national survey population. Psychosom Med. 2012;74(2):208–213. PubMed CrossRef
- von Siebenthal HK, Moretti D, Zimmermann MB, et al. Effect of dietary factors and time of day on iron absorption from oral iron supplements in iron deficient women. Am J Hematol. 2023;98(9):1356–1363. PubMed CrossRef
- Bardal SK, Waechter JE, Martin DS. Hematology. In: Bardal SK, Waechter JE, Martin DS, eds. Applied Pharmacology. WB Saunders; 2011:193–214.
- Malik ZI, Ghafoor MU, Shah SHBU, et al. Unlocking iron: nutritional origins, metabolic pathways, and systemic significance. Front Nutr. 2025;12:1637316. PubMed CrossRef
- Abioye AI, Okuneye TA, Odesanya AMO, et al. Calcium intake and iron status in human studies: a systematic review and dose-response meta-analysis of randomized trials and crossover studies. J Nutr. 2021;151(5):1084–1101. PubMed CrossRef
- Girelli D, Nemeth E, Swinkels DW. Hepcidin in the diagnosis of iron disorders. Blood. 2016;127(23):2809–2813. PubMed CrossRef
- Lo JO, Benson AE, Martens KL, et al. The role of oral iron in the treatment of adults with iron deficiency. Eur J Haematol. 2023;110(2):123–130. PubMed CrossRef
- Stoffel NU, Cercamondi CI, Brittenham G, et al. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: two open-label, randomised controlled trials. Lancet Haematol. 2017;4(11):e524–e533. PubMed CrossRef
- Stoffel NU, Zeder C, Brittenham GM, et al. Iron absorption from supplements is greater with alternate day than with consecutive day dosing in iron-deficient anemic women. Haematologica. 2020;105(5):1232–1239. PubMed CrossRef
- Dhanush M, Vinod KV, Manivannan P, et al. Daily versus alternate day oral iron replacement for women with iron deficiency anaemia: a randomized controlled trial. Indian J Hematol Blood Transfus. 2025;41(2):245–251. PubMed CrossRef
- Pasupathy E, Kandasamy R, Thomas K, et al. Alternate day versus daily oral iron for treatment of iron deficiency anemia: a randomized controlled trial. Sci Rep. 2023;13(1):1818. PubMed CrossRef
- Iolascon A, Andolfo I, Russo R, et al. Recommendations for diagnosis, treatment, and prevention of iron deficiency and iron deficiency anemia. Hemasphere. 2024;8(7):e108. PubMed CrossRef
- DeLoughery TG, Jackson CS, Ko CW, et al. AGA clinical practice update on management of iron deficiency anemia: expert review. Clin Gastroenterol Hepatol. 2024;22(8):1575–1583. PubMed CrossRef
- Andrade C. Learning from history: how to swallow a pill. J Clin Psychiatry. 2013;74(10):e949-51. PubMed
This PDF is free for all visitors!