ABSTRACT
Objective: Patients with early-phase schizophrenia or bipolar I disorder (BD-I) are at greater risk for antipsychotic-associated weight gain. This 12-week, randomized, double-blind study conducted between June 2017 and December 2021 evaluated weight effects of combination olanzapine and samidorphan (OLZ/SAM) versus olanzapine in early-phase illness.
Methods: Young adults (16–39 years) with DSM-5 schizophrenia, schizophreniform disorder, or BD-I, < 4 years since symptom onset, body mass index < 30 kg/m2, and < 24 weeks’ cumulative antipsychotic exposure were randomized to OLZ/SAM (5–20/10 mg/d) or olanzapine (5–20 mg/d). Primary endpoint was percent change from baseline body weight at week 12. Secondary endpoints, tested hierarchically, were proportions of patients with ≥ 10% or ≥ 7% weight gain, waist circumference change, and Clinical Global Impressions-Severity (CGI-S) change.
Results: Of 428 patients (OLZ/SAM, n = 213; olanzapine, n = 215), 408 had ≥ 1 postbaseline weight assessment and were analyzed. Percent weight change was significantly lower with OLZ/SAM versus olanzapine (4.91% vs 6.77%; least-squares mean [LSM] [SE] difference, −1.87% [0.75]; P = .012). Although fewer patients treated with OLZ/SAM had ≥ 10% weight gain, the difference was not statistically significant versus olanzapine (21.9% vs 30.4%, respectively; OR = 0.64; 95% CI = 0.39 to 1.05); hierarchical testing precluded further statistical evaluation of secondary endpoints. Proportions of patients with ≥ 7% weight gain (33.1% vs 44.8%; OR = 0.61, 95% CI = 0.39 to 0.94) and waist circumference change (2.99 vs 3.90 cm; LSM [SE] difference, −0.92 cm [0.58]; 95% CI = −2.06 to 0.22) favored OLZ/SAM. LSM (SE) CGI-S change with OLZ/SAM was −0.82 (0.06). OLZ/SAM and olanzapine had similar safety profiles, including small, similar metabolic parameter changes.
Conclusions: In patients with early-phase schizophrenia, schizophreniform disorder, or BD-I, OLZ/SAM treatment resulted in less weight gain versus olanzapine.
Trial Registration: ClinicalTrials.gov identifier: NCT03187769
J Clin Psychiatry 2023;84(3):22m14674
To cite: Kahn RS, Kane JM, Correll CU, et al. Olanzapine/samidorphan in young adults with schizophrenia, schizophreniform disorder, or bipolar I disorder who are early in their illness: results of the randomized, controlled ENLIGHTEN-Early study. J Clin Psychiatry. 2023;84(3):22m14674.
To share: https://doi.org/10.4088/JCP.22m14674
© 2023 Physicians Postgraduate Press, Inc.
aIcahn School of Medicine at Mount Sinai, New York, New York
bZucker Hillside Hospital, Glen Oaks, New York
cDonald and Barbara Zucker School of Medicine at Hofstra/Northwell, Hempstead, New York
dCharité Universitätsmedizin, Berlin, Germany
eAlkermes, Inc., Waltham, Massachusetts
fAlkermes Pharma Ireland Ltd., Dublin, Ireland
*Corresponding author: David McDonnell, MD, Executive Medical Director, Neuroscience, Alkermes Pharma Ireland Limited, Connaught House, 1 Burlington Rd, Dublin, D04 C5Y6, Ireland ([email protected]).
Early intervention in the course of serious mental illness is considered to be the best opportunity to beneficially alter disease trajectory and potentially improve long-term outcomes in patients with schizophrenia or bipolar I disorder (BD-I).1–5 Clinical practice guidelines recommend antipsychotic monotherapy for patients with first-episode schizophrenia.6 Selection of antipsychotic medications for individual patients is based on efficacy and their safety and tolerability profiles, which commonly include extrapyramidal symptoms and/or metabolic effects,6 including the risk of significant weight gain.7,8 Olanzapine is a highly effective treatment with established antipsychotic efficacy and a low incidence of extrapyramidal symptoms.9–13 However, the antipsychotic efficacy of olanzapine is accompanied by a propensity to cause significant weight gain and increased cardiometabolic risk compared with other atypical antipsychotic drugs.9,10,14–16
Olanzapine is approved for the treatment of schizophrenia and BD-I in adolescents and adults,17 but the Schizophrenia Patient Outcomes Research Team (PORT) treatment guidelines do not recommend olanzapine first-line for patients experiencing their first episode of schizophrenia.18 Olanzapine-associated weight gain and metabolic risk are substantial in patients with schizophrenia or BD-I who are early in their illness.19 Patients in this population who fail to respond to first-line antipsychotics may well be treated with olanzapine because of its known efficacy,20 even though switching to an antipsychotic other than olanzapine may be an option for some patients.21–24 Although some augmentation strategies have been shown to reduce antipsychotic-related weight gain,25,26 few have been studied for that purpose, and none are approved by regulatory agencies for this specific indication. Therefore, a version of olanzapine with a reduced risk for clinically significant weight gain would be a valuable clinical option.
Olanzapine combined with samidorphan (OLZ/SAM) is approved by the US Food and Drug Administration for the treatment of adults with schizophrenia and for adults with BD-I, in whom it may be used for the acute treatment of manic or mixed episodes as a monotherapy or adjunctively to lithium or valproate and as a maintenance monotherapy.27 In healthy volunteers and in phase 2 and 3 clinical trials in adult patients with schizophrenia, treatment with OLZ/SAM mitigated olanzapine-associated weight gain28–30 while providing antipsychotic efficacy29–31 and an adverse event28–31 profile similar to those of olanzapine. The objectives of the ENLIGHTEN-Early study were to evaluate the effect of OLZ/SAM versus olanzapine on body weight in young adults (ages 16–39 years) with schizophrenia, schizophreniform disorder, or BD-I who are early in the course of their illness, and thus at high risk for weight gain and metabolic effects,19 and to assess OLZ/SAM safety and tolerability in this population.
METHODS
The ENLIGHTEN-Early study (ClinicalTrials.gov identifier NCT03187769) was conducted from June 8, 2017, to December 1, 2021, in accordance with Good Clinical Practice principles (International Conference on Harmonization, 1997) and ethical principles derived from the Declaration of Helsinki. The protocol and all amendments were approved by an institutional review board or ethics committee at each study site. All patients provided written informed consent before participating in any study-specific procedures.
Study Design and Treatments
ENLIGHTEN-Early was a phase 3 multicenter, randomized, double-blind study conducted at 62 sites, including research organizations and academic, state, and private medical centers and practices, in 11 countries (United States, United Kingdom, Ireland, Germany, Poland, Austria, Italy, Israel, Russia, South Korea, and Ukraine). Eligible patients were randomly assigned 1:1 (via a central interactive web response system using codes prepared by an independent biostatistician) to receive either OLZ/SAM or olanzapine for 12 weeks (Supplementary Figure 1), stratified by diagnosis (schizophrenia/schizophreniform disorder vs BD-I), region (US vs non-US region), and baseline body mass index (BMI; <25 vs ≥ 25 kg/m2). Patients who completed the study could enroll in an ongoing 48-month, open-label safety study (ClinicalTrials.gov identifier NCT03201757) or entered a 4-week safety follow-up period.
Treatment assignment was blinded to the sponsor, clinical and study site staff, patients, and caregivers. For patients assigned to OLZ/SAM, the dosage of samidorphan was fixed at 10 mg/d. The olanzapine dose in the OLZ/SAM and olanzapine arms was flexible; dosing schedules are listed in Supplementary Table 1. OLZ/SAM and olanzapine were provided as matched tablets for each dose strength.
Patients were tapered off any previous antipsychotic medication or mood stabilizers within 14 days after randomization; no concomitant antipsychotic medications or mood stabilizers were allowed for the remainder of the study. Varenicline, prescription or over-the-counter weight reduction agents, systemic steroids, topiramate, calcitonin, diabetes treatments, and hypoglycemic agents were prohibited. Selective serotonin reuptake inhibitors (SSRIs) were permitted for patients with schizophrenia or schizophreniform disorder who were stable on that medication for ≥ 30 days before study entry; all patients were permitted to start an SSRI during the treatment period, if clinically indicated.
Patients
Patients aged 18 to < 40 years (US sites: ≥16 to < 40 years) with a BMI < 30 kg/m2 at screening who met Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, criteria for a primary diagnosis of schizophrenia, schizophreniform disorder, or BD-I were enrolled. Diagnoses were confirmed with the Mini International Neuropsychiatric Interview 7.0 for Schizophrenia and Psychotic Disorder Studies32 (patients ≥ 18 years) or the Mini International Neuropsychiatric Interview for Children and Adolescents 7.0 for Schizophrenia and Psychotic Disorder Studies32 (patients < 18 years). To capture an adequate sample of patients early in illness, ENLIGHTEN-Early included patients aged < 18 years and/or without a fully established diagnosis of schizophrenia at screening (ie, with a transitional diagnosis of schizophreniform disorder). Patients with bipolar I disorder were enrolled if they experienced an acute manic episode as defined by DSM-5 within 14 days leading to enrollment. All eligible patients were required to be within 4 years from the initial onset of active symptoms and to have < 24 weeks of cumulative lifetime antipsychotic exposure. Further, history of olanzapine use was restricted to 14 days (cumulatively) within the 6 months before enrollment or 3 weeks’ cumulative lifetime use. Participants could be inpatient or outpatient at screening but were required to be outpatient within 2 weeks after randomization.
Assessments
Patient visits were scheduled at screening and baseline (randomization visit) and at weeks 1, 2, 4, 6, 8, 10, and 12 (or early termination) during the double-blind treatment period; follow-up visits were scheduled at weeks 14 and 16 for patients who chose not to enroll in the 48-month follow-up safety study. Body weight, waist circumference (both measured in triplicate), and body composition were assessed at each visit. Height was measured at screening only. Blood samples for fasting triglycerides, total, low-density lipoprotein, and high-density lipoprotein (HDL) cholesterol, fasting glucose, glycosylated hemoglobin (HbA1c), and fasting insulin were collected at screening, baseline, and weeks 2 and 12 (or early termination). Fasting was based on patient report; patients were instructed not to eat or drink (except water) for 8 hours before scheduled laboratory assessments. Safety assessments included vital sign and 12-lead electrocardiogram measurements, clinical laboratory tests, adverse event (AE) monitoring, and the Columbia-Suicide Severity Rating Scale (C-SSRS).33 The Clinical Global Impressions-Severity scale34 (CGI-S) was administered at baseline and at each visit during the double-blind treatment period to evaluate severity of illness over time.
The primary endpoint was percent change from baseline in body weight at week 12. Key secondary endpoints (all at week 12) were proportion of patients with ≥ 10% weight gain from baseline; proportion of patients with ≥ 7% weight gain from baseline; change from baseline in waist circumference; and change from baseline in the CGI-S score within the OLZ/SAM treatment group. In the psychiatric literature, ≥ 7% weight gain from baseline is considered “clinically significant”; ≥ 10% weight gain, an endpoint used in weight management studies, represents an outcome of greater severity that is also relevant for treatment comparisons. Both the ≥ 7% and ≥ 10% weight gain thresholds were used in previously conducted studies of the effects of samidorphan on olanzapine-associated weight gain.29,30 Additional endpoints assessed changes from baseline in fasting lipids, fasting glucose, HbA1c, and fasting insulin at week 12.
Statistical Analysis
The sample size determination is provided in Supplementary Appendix 1. Safety was assessed in all randomized patients who received ≥ 1 dose of study medication; the overall analysis included all patients who received ≥ 1 dose of study medication and who underwent ≥ 1 postbaseline weight assessment. Demographic and baseline characteristics were summarized using descriptive statistics. The study protocol specified that potentially clinically important differences between treatment arms in baseline characteristics were to be investigated to determine if adjustments in the efficacy and safety analyses were necessary. However, no meaningful imbalances in baseline characteristics were observed.
Percent change in body weight from baseline (primary endpoint) was analyzed using analysis of covariance with multiple imputation for missing data using SAS v9.4 (SAS Institute, Cary, NC). The model included treatment group and randomization strata (diagnosis [schizophrenia/schizophreniform disorder vs bipolar I disorder], region [US vs non-US], and baseline BMI [< 25 vs ≥ 25 kg/m2]) as factors and baseline weight as a covariate. Subgroup analyses of the primary endpoint based on age, sex, race, BMI, region, and diagnosis and an analysis of percent changes in body weight from baseline in study completers versus patients who discontinued early are described in Supplementary Appendix 1. Secondary endpoints were not included in subgroup analyses.
Categorical secondary endpoints were analyzed using a logistic regression model with multiple imputation. Change from baseline in waist circumference at week 12 was analyzed using the analysis-of-covariance method with multiple imputation, with the same factors in the model as in the primary analysis and baseline weight as a covariate. Change from baseline in CGI-S score within the OLZ/SAM group at week 12 and change from baseline in laboratory parameters were analyzed based on a mixed model with repeated measurements; the model included randomization strata, treatment, visit, and treatment-by-visit interaction term as factors and the corresponding baseline value as a covariate.
To control the overall type I error rate, hierarchical testing was performed for the primary and key secondary endpoints in the following order: percent change from baseline in body weight; proportion of patients with ≥ 10% weight gain from baseline; proportion of patients with ≥ 7% weight gain from baseline; change from baseline in waist circumference; and change from baseline in CGI-S score within the OLZ/SAM group.
The numbers and percentages of patients with AEs, serious AEs (SAEs), AEs leading to discontinuation, and suicidal ideation (C-SSRS) were summarized using descriptive statistics.
RESULTS
Patient Disposition and Baseline Characteristics
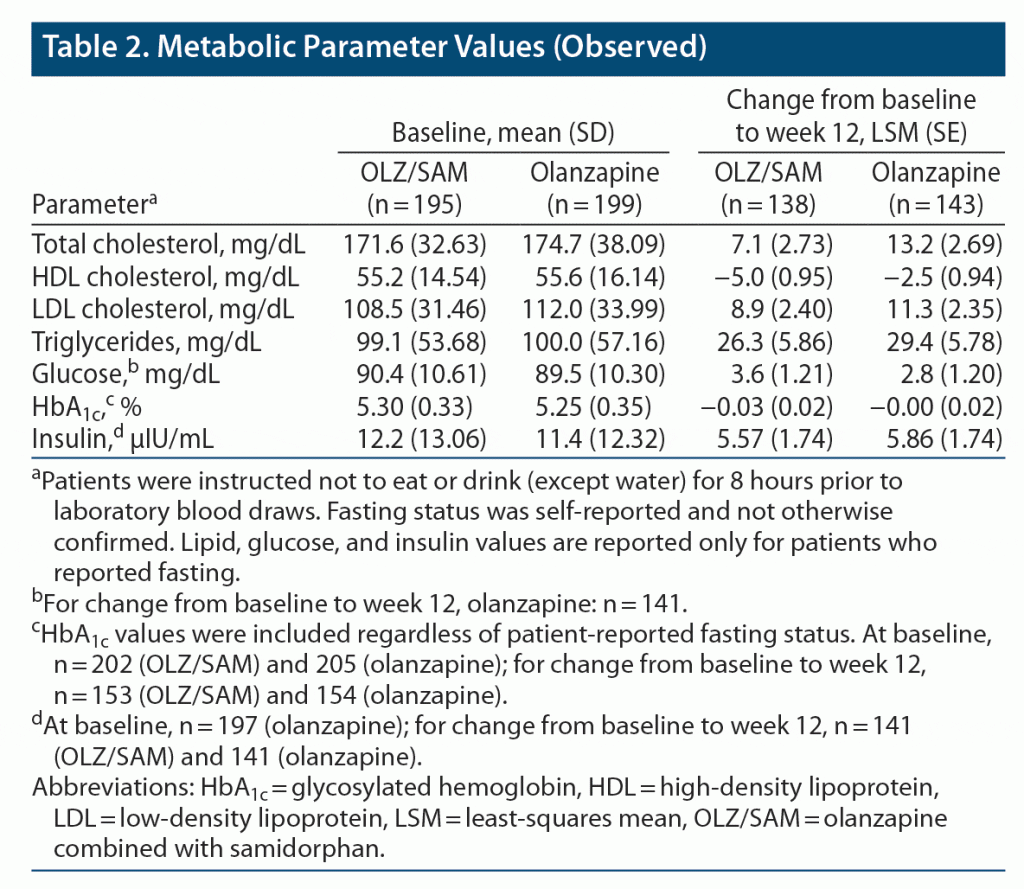
Of the 428 patients randomized to treatment, 426 received ≥ 1 dose of OLZ/SAM (n = 211) or olanzapine (n = 215) and were included in the safety analysis (Supplementary Figure 2). A total of 408 patients (OLZ/SAM, n = 202; olanzapine, n = 206) underwent ≥ 1 postbaseline body weight assessment and were included in the efficacy analysis; 326 (76.5% of the total randomized; 78.2% on OLZ/SAM; 74.9% on olanzapine) completed the 12-week double-blind treatment period. The most common reasons for discontinuation were withdrawal by subject (9.5% and 10.7% for OLZ/SAM and olanzapine, respectively), loss to follow-up (5.2% and 6.0%, respectively), and AEs (4.7% and 6.0%, respectively). Demographic and baseline clinical characteristics were similar between groups (Table 1). Also, the rates of prior use for the most commonly taken antipsychotics were similar between the OLZ/SAM and olanzapine treatment groups.
Olanzapine Exposure and Concomitant Medications
Both groups received a similar mean (SD) olanzapine dosage, calculated as a time-weighted average dosage of olanzapine during the entire study (OLZ/SAM, 11.6 [4.14] mg/d; olanzapine, 11.6 [4.15] mg/d). The final olanzapine dosages were 10 mg/d for 43.5% and 43.9% and 20 mg/d for 17.2% and 18.2% of patients assigned to OLZ/SAM and olanzapine, respectively.
Proportions of patients who used concomitant medication during the treatment period were similar between the OLZ/SAM (53.6% [113/211]) and olanzapine groups (50.2% [108/215]). The most frequently used concomitant medications are listed in Supplementary Table 2. Concomitant non-olanzapine antipsychotics or mood stabilizers may have included prior medications used during the taper period (first 2 weeks).
Weight and Metabolic Effects
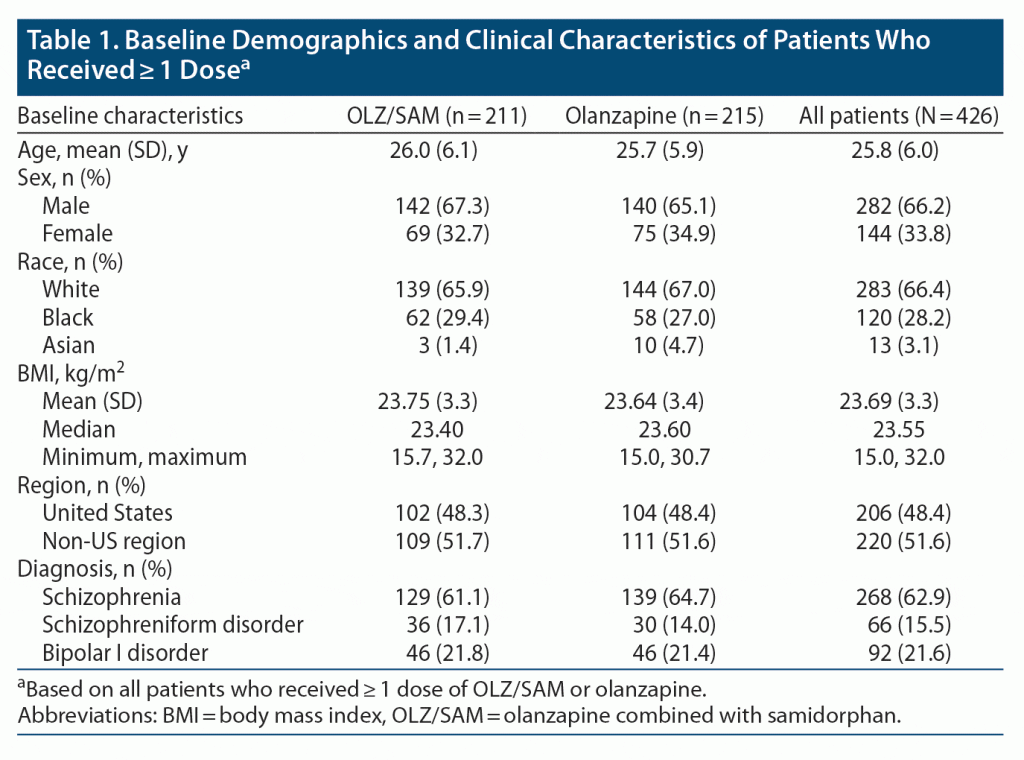
Weight. On the primary endpoint, the least-squares mean (LSM) (SE) percent changes from baseline in body weight at week 12 were 4.91% (0.60%) and 6.77% (0.60%) for the OLZ/SAM and olanzapine groups, respectively (LSM [SE] difference, –1.87% [0.745]; P = .012) (Figure 1A and Supplementary Table 3). The mean absolute change in body weight was 3.37 kg for OLZ/SAM and 4.70 kg for olanzapine. OLZ/SAM and olanzapine weight gain curves separated after week 6 and remained separated through the end of the treatment period.
At week 12, 44/202 (21.9%) OLZ/SAM-treated patients had ≥ 10% weight gain from baseline compared with 63/206 (30.4%) olanzapine-treated patients (number needed to treat [NNT] = 12; Figure 1B). The odds ratio (OR) for ≥ 10% weight gain with OLZ/SAM versus olanzapine was 0.64 (95% CI = 0.39 to 1.05; P = .075). Based on the hierarchical testing procedure and the lack of statistical significance between treatment groups for ≥ 10% weight gain, P values are not reported for the remaining key secondary endpoints. The proportions of patients with ≥ 7% weight gain from baseline were 67/202 (33.1%) and 92/206 (44.8%) for the OLZ/SAM and olanzapine groups, respectively (NNT = 9; Figure 1B), with an OR of 0.61 (95% CI = 0.39 to 0.94). LSM (SE) changes from baseline in waist circumference at week 12 were 2.99 (0.464) cm for OLZ/SAM and 3.90 (0.477) cm for olanzapine (LSM difference, –0.92; 95% CI = –2.06 to 0.22; Figure 1C).
Less weight gain was observed with OLZ/SAM vs olanzapine in all subgroups assessed (Supplementary Figure 3); however, some subgroups had small sample sizes and wide 95% CIs. Within the OLZ/SAM group, patients who discontinued early generally had weight gain profiles similar to those of completers (Supplementary Figure 4). Many patients discontinuing olanzapine treatment experienced substantial weight gain compared with those who completed the study, although weight gain was infrequently reported as a reason for discontinuation. However, the study was not powered to provide statistical comparisons among subgroups.
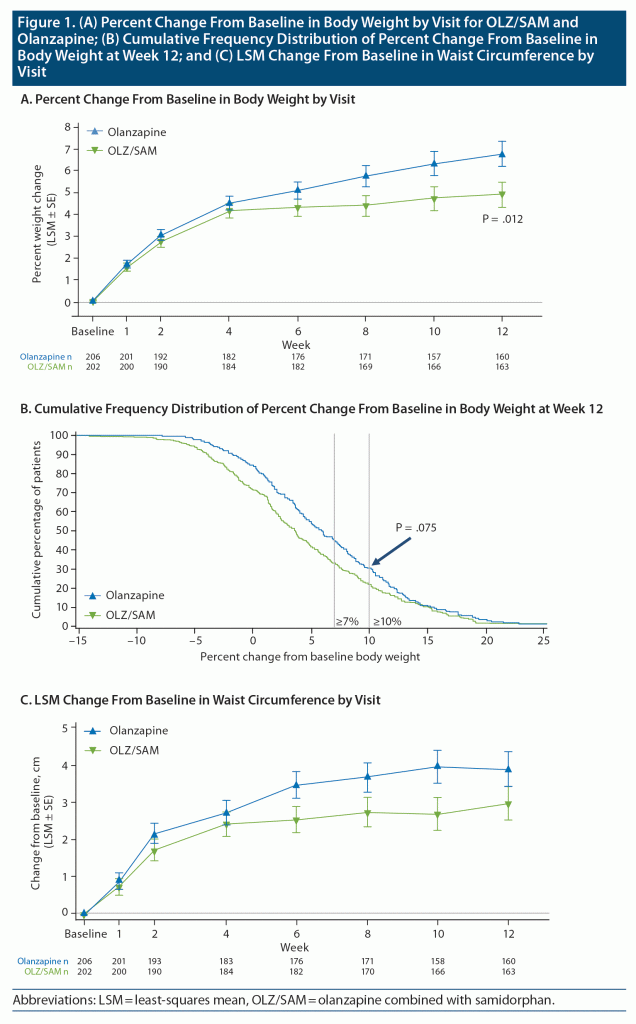
Metabolic laboratory parameters. LSM changes in fasting lipid and glycemic parameters from baseline to week 12 were generally small and similar for OLZ/SAM and olanzapine (Table 2). For both treatment groups, triglycerides, total cholesterol, and low-density lipoprotein (LDL) cholesterol increased and HDL cholesterol decreased. Blood glucose and insulin increased; however, HbA1c remained stable throughout the study. Increases in triglycerides, total cholesterol, LDL cholesterol, glucose, and insulin occurred within the first 2 weeks, with little additional change through week 12.
Antipsychotic Efficacy
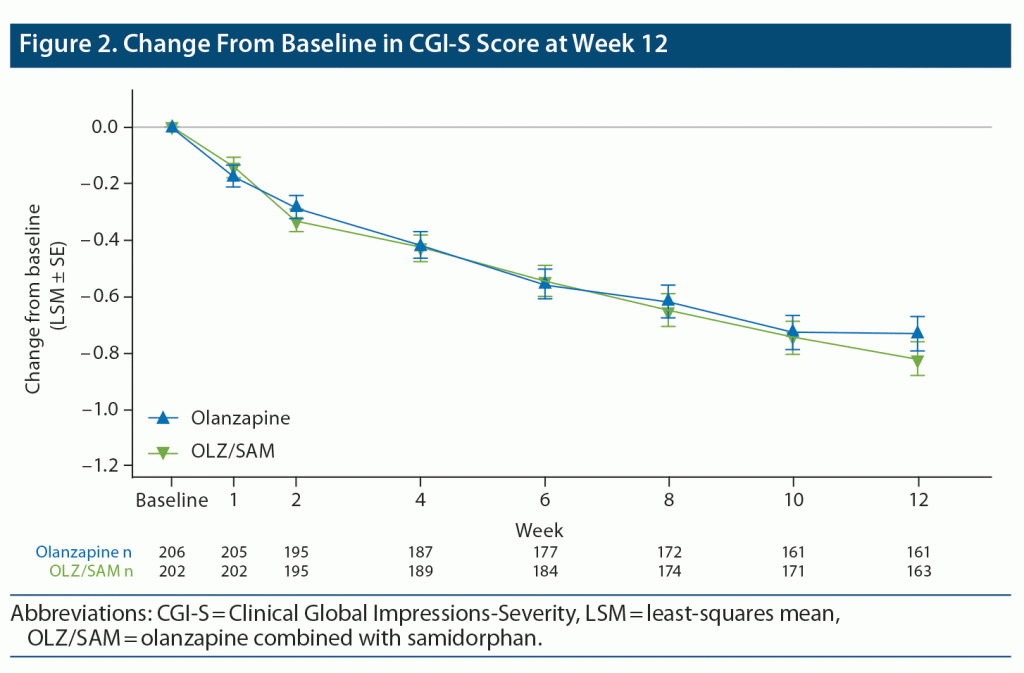
Within the OLZ/SAM group, mean (SD) CGI-S scores were 3.87 (0.809) at baseline and 3.12 (0.939) at week 12 (LSM change from baseline at week 12, –0.82; 95% CI = –0.94 to –0.70); similar changes were observed with olanzapine (Figure 2).
Safety
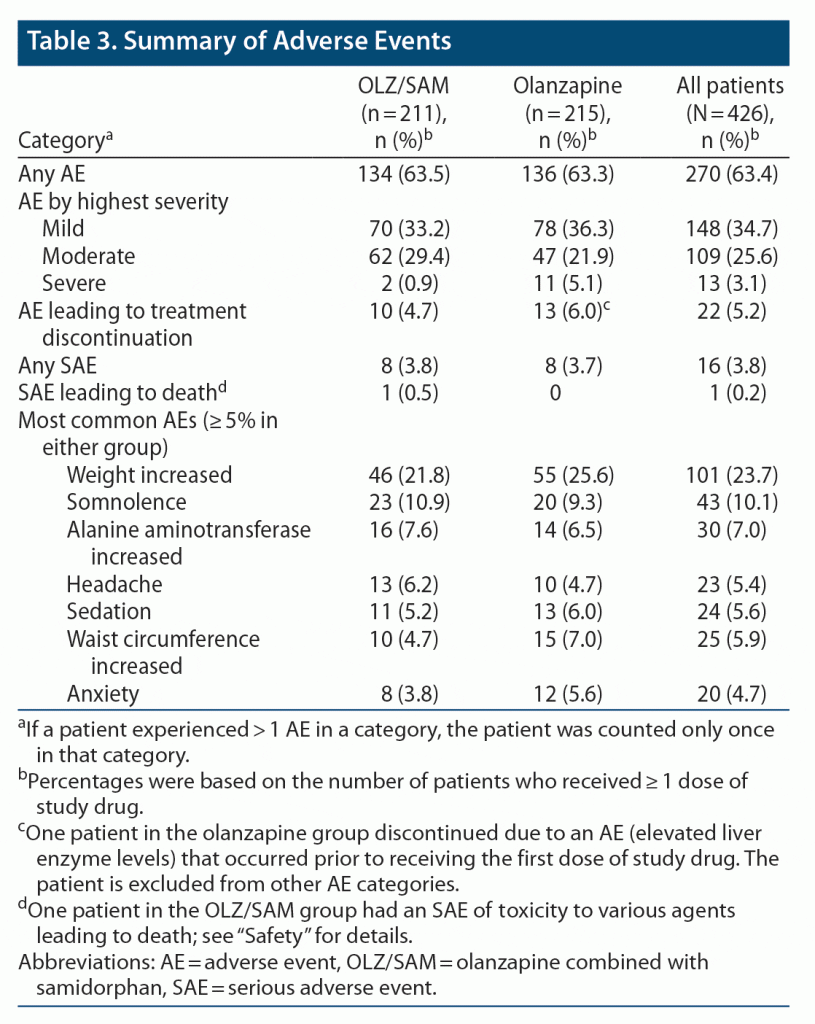
AEs were reported by 134/211 (63.5%) patients who received OLZ/SAM and 136/215 (63.3%) patients who received olanzapine. The most reported AEs (≥ 5%, either group) are listed in Table 3; AEs reported by ≥ 10% of the OLZ/SAM and olanzapine groups were weight increase (21.8% and 25.6%, respectively) and somnolence (10.9% and 9.3%, respectively). A total of 10/211 (4.7%) patients in the OLZ/SAM group and 13/215 (6.0%) patients in the olanzapine group discontinued the study because of an AE (Table 3; Supplementary Figure 2). AEs leading to discontinuation that occurred in more than 1 patient in either group were increased weight (OLZ/SAM, 2 [0.9%]; olanzapine, 3 [1.4%]) and intentional overdose (OLZ/SAM, 0; olanzapine, 2 [0.9%]).
Most AEs were mild to moderate in severity. Severe AEs were experienced by 2 (0.9%) patients receiving OLZ/SAM and 11 (5.1%) patients receiving olanzapine. Sixteen SAEs were reported (OLZ/SAM, 8/211 [3.8%]; olanzapine, 8/215 [3.7%]; Supplementary Table 4); 1 SAE reported in the OLZ/SAM group (seizure, moderate in severity) was considered related to study treatment. One death was reported on study day 39 during safety follow-up in a patient assigned to OLZ/SAM (10/10 mg/d) in whom an SAE of toxicity to various agents (reported as quetiapine and methamphetamine toxicity) resulted in death. This event occurred 21 days after the patient discontinued study medication due to AEs of increased alanine aminotransferase, aspartate aminotransferase, and prolactin levels and switched to quetiapine 300 mg/d for schizophrenia. The fatal event was considered unrelated to study drug treatment by the investigator.
Proportions of patients with potentially clinically significant laboratory values were similar in the OLZ/SAM and olanzapine groups (Supplementary Table 5). There were no clinically meaningful changes in vital sign or electrocardiogram findings in either treatment group.
Suicidal ideation, as assessed by the C-SSRS, was present in 15 (7.1%) OLZ/SAM-treated patients and 9 (4.2%) olanzapine-treated patients. Suicidal behaviors were not reported for patients taking OLZ/SAM and occurred in 1 (0.5%) patient taking olanzapine (aborted suicide attempt with preparatory acts or behavior).
DISCUSSION
In patients with schizophrenia, schizophreniform disorder, or BD-I who had limited prior exposure to antipsychotic medications and were early in the course of their illness, OLZ/SAM treatment resulted in significantly less weight gain than olanzapine. Key secondary endpoints supported trends toward less weight gain with OLZ/SAM, although these endpoints were not statistically significant when evaluated based on a hierarchical testing strategy. Odds of clinically significant weight gain at the ≥ 10% and ≥ 7% thresholds were reduced by 36% and 39%, respectively, with OLZ/SAM in comparison with olanzapine.
The pattern of weight gain mitigation observed in ENLIGHTEN-Early was consistent with that reported in previous studies,29–31 including the 24-week ENLIGHTEN-2 study in patients who were chronically ill and had experienced longer-term antipsychotic treatment (> 1 year since onset of active-phase schizophrenia symptoms and initiation of first antipsychotic treatment) than those enrolled here.30 Patients enrolled in ENLIGHTEN-Early were younger (mean, approximately 26 years) and had a lower BMI (mean, 23.7 kg/m2) than those in ENLIGHTEN-2 (means, 40 years and 25.5 kg/m2, respectively). Despite these differences and the greater vulnerability to olanzapine-associated weight gain in early-in-illness patients19 (~7% weight increase with olanzapine at week 12 in this analysis vs ~5% weight increase at week 12 in ENLIGHTEN-2), mitigation of weight gain through 12 weeks was similar in the 2 studies. In this study, as in ENLIGHTEN 2, body weight increased similarly in OLZ/SAM- and olanzapine-treated patients over the first 4 weeks and began to diverge thereafter.30 Although shorter in duration than the ENLIGHTEN 2 study, the current 12-week study was of sufficient length to observe that weight gain stabilized in patients treated with OLZ/SAM at around week 6, while weight gain continued in patients treated with olanzapine.30 Importantly, OLZ/SAM treatment resulted in clinical symptom improvements that were similar to those observed with olanzapine in both studies, as measured by CGI-S scores in ENLIGHTEN-Early and by the Positive and Negative Syndrome Scale and CGI-S scores in ENLIGHTEN-2.30 Taken together, these results suggest that OLZ/SAM provides antipsychotic efficacy comparable to that of olanzapine but with a reduced risk of weight gain regardless of patients’ stage of illness or prior antipsychotic treatment, even in early-in-illness patients who are at increased risk of olanzapine-associated weight gain.18,19
In this study, changes in most metabolic parameters were generally small for both the OLZ/SAM and olanzapine groups. Changes in lipid and glucose parameters in both treatment groups are consistent with findings from other olanzapine studies in similar (early-in-illness) populations.35–37 The emergence of metabolic changes in both treatment groups within the first weeks of treatment is also in line with reports of early effects of olanzapine on lipid and glucose metabolism independent of weight gain.38,39 The timing of these early metabolic changes suggests that they may be direct effects of olanzapine rather than secondary to antipsychotic-associated weight gain30 and thus not blocked by weight gain mitigation. Weight gain is also a well-established driver of adverse metabolic effects40; however, metabolic benefits of mitigating weight gain would likely be observed over follow-up periods much longer than were feasible in this study.41
Limitations of this study should be noted. Although the study enrolled patients with schizophrenia, schizophreniform disorder, or BD-I, no specific conclusions could be made regarding the effects of OLZ/SAM on mitigation of weight gain among subgroups, including patients with schizophreniform disorder or BD-I, as relatively few patients with those diagnoses were enrolled. Patients < 18 years of age were enrolled in the United States only. The flexible-dose design of this study did not allow assessment of a potential dose effect of olanzapine, alone or in combination with samidorphan, on weight gain. However, prior studies suggest that the dose-related contribution to antipsychotic-associated weight gain is small, both within the regular therapeutic dose range23,42 and even below the therapeutic dose.43 Also, the 12-week duration of the study only allowed assessment of short-term effects of OLZ/SAM on weight gain mitigation and differences in metabolic parameters. Longer-term safety outcomes for this population are being evaluated in an open-label extension study (ClinicalTrials.gov identifier: NCT03201757) of up to 48 months in duration; however, that study will not include an olanzapine comparison arm. Finally, the study design included few laboratory collections, and fasting status was based on self-report and not otherwise confirmed.
CONCLUSIONS
In patients who are early in the course of schizophrenia, schizophreniform disorder, or BD-I illness, and thus at high risk for weight gain, OLZ/SAM resulted in less weight gain at week 12 compared with olanzapine while providing similar antipsychotic efficacy. Small metabolic changes observed in both the OLZ/SAM and olanzapine groups were consistent with previously reported early effects of olanzapine on lipid and glucose metabolism.37,38 Taken together with previously published findings,29,30 these data suggest that OLZ/SAM mitigates olanzapine-associated weight gain across both early and later stages of illness.
Submitted: September 20, 2022; accepted January 25, 2023.
Published online: March 22, 2023.
Author contributions: Study design: David McDonnell, Adam Simmons, René S. Kahn, John M. Kane, Christoph U. Correll, Christine Graham. Study investigator: René S. Kahn, John M. Kane. Enrolled patients: None. Collection and assembly of data: Christina Arevalo, Christine Graham, Sergey Yagoda, Beibei Hu. Data analysis: Christina Arevalo, Christine Graham, Sergey Yagoda, Beibei Hu, David McDonnell, Adam Simmons. Data interpretation: all authors. Manuscript preparation: all authors. Manuscript review and revisions: all authors. Final approval of manuscript: all authors.
Relevant financial relationships: Dr Kahn has served as a consultant for and/or has received grants or speaking fees from Alkermes, Janssen-Cilag, Lundbeck, Merck, Minerva Neuroscience, Otsuka, Roche, Sunovion, and Teva. Dr Correll has been a consultant and/or advisor to or has received honoraria from AbbVie, Acadia, Alkermes, Allergan, Angelini, Aristo, Boehringer-Ingelheim, Cardio Diagnostics, Cerevel, CNX Therapeutics, Compass Pathways, Darnitsa, Gedeon Richter, Hikma, Holmusk, Intra-Cellular Therapies, Janssen/J&J, Karuna, LB Pharma, Lundbeck, MedAvante-ProPhase, MedinCell, Merck, MindPax, Mitsubishi Tanabe Pharma, Mylan, Neurocrine, Newron, Noven, Otsuka, Pharmabrain, PPD Biotech, Recordati, Relmada, Reviva, Rovi, Seqirus, SK Life Science, Sunovion, Sun Pharma, Supernus, Takeda, Teva, and Viatris; provided expert testimony for Janssen and Otsuka; served on a Data Safety Monitoring Board for Lundbeck, Relmada, Reviva, Rovi, Supernus, and Teva; received grant support from Janssen and Takeda; received royalties from UpToDate; and is a stock option holder of Cardio Diagnostics, MindPax, and LB Pharma. Dr Kane has been a consultant for or received honoraria from Alkermes, Allergan, Boehringer Ingelheim, Cerevel, Click Therapeutics, Dainippon Sumitomo, HLS, Indivior, Intra-Cellular Therapies, Janssen, J&J, Karuna, LB Pharmaceuticals, Lundbeck, Merck, Minerva, Neumora, Neurocrine Biosciences, Newron, Novartis, Otsuka, Reviva, Roche, Saladax Biomedical, Sunovion, Takeda, and Teva; has received grant support from Otsuka, Lundbeck, Janssen, and Sunovion; and is a shareholder of LB Pharmaceuticals and The Vanguard Research Group. Mss Arevalo and Hu, Mr Simmons, and Drs Graham, Yagoda, and McDonnell are or were employees of Alkermes, Inc., and may own stock/options in the company.
Funding/support: This study was sponsored by Alkermes, Inc.
Role of the sponsor: Alkermes, Inc., is a pharmaceutical company that developed and markets OLZ/SAM, a combination of olanzapine and samidorphan for the treatment of adults with schizophrenia and bipolar I disorder, in the United States and funded this study. Alkermes, Inc., was involved in the design, collection, and analysis of the data. Interpretation of the results was performed by the authors, and the decision to submit the manuscript for publication was made by the authors.
Previous presentations: Presented at the following: Schizophrenia International Research Society Annual Congress, April 6–10, 2022, Florence, Italy (oral presentation); American Society of Clinical Psychopharmacology Annual Meeting, May 31–June 3, 2022, Scottsdale, AZ; Psych Congress, September 17–20, 2022, New Orleans, LA (Poster Number: 27); American Psychiatric Nurses Association Annual Conference, October 19–22, 2022, Long Beach, CA (Poster Number: 29922); and Neuroscience Education Institute Congress, November 3–6, 2022; Colorado Springs, CO.
Acknowledgments: The authors thank the ALK3831-A307 Study Group, the Vanguard Research Group, and the Department of Psychiatry, UMC Brain Center, University Medical Center Utrecht, Utrecht University in Utrecht, The Netherlands, as well as Mark S. Todtenkopf, PhD, of Alkermes, Inc., who assisted in the preparation and proofreading of the manuscript. Medical writing and editorial support were provided by Kathleen M. Dorries, PhD, and John H. Simmons, MD, of Peloton Advantage, LLC, an OPEN Health company, and funded by Alkermes, Inc. Dr Todtenkopf is an employee of Alkermes and may own stock. Drs Dorries and Simmons are employees of Peloton Advantage, LLC, an OPEN Health company, which provided medical writing and editorial support funded by Alkermes, Inc.
ORCID ID: David McDonnell: https://orcid.org/0000-0002-6400-1286
Supplementary material: Available at Psychiatrist.com.
Clinical Points
- In previously reported clinical trials, treatment with olanzapine combined with samidorphan (OLZ/SAM) mitigated olanzapine-associated weight gain in patients with schizophrenia while providing antipsychotic efficacy similar to that of olanzapine.
- Before this clinical study, the effects of combination olanzapine and samidorphan (OLZ/SAM) on antipsychotic-associated weight gain had not been assessed in patients with early-phase illness, who are at greater risk for antipsychotic-associated weight gain.
- Treatment with OLZ/SAM resulted in significantly less weight gain at week 12 than treatment with olanzapine, while antipsychotic efficacy in patients early in the course of schizophrenia, schizophreniform disorder, or bipolar I disorder illness was similar between the OLZ/SAM and olanzapine groups.
References (43)

- Berk M, Malhi GS, Hallam K, et al. Early intervention in bipolar disorders: clinical, biochemical and neuroimaging imperatives. J Affect Disord. 2009;114(1-3):1–13. PubMed CrossRef
- Berk M, Brnabic A, Dodd S, et al. Does stage of illness impact treatment response in bipolar disorder? empirical treatment data and their implication for the staging model and early intervention. Bipolar Disord. 2011;13(1):87–98. PubMed CrossRef
- Lieberman JA, Jarskog LF, Malaspina D. Preventing clinical deterioration in the course of schizophrenia: the potential for neuroprotection. J Clin Psychiatry. 2006;67(6):983–990. PubMed CrossRef
- Tohen M, Vieta E, Gonzalez-Pinto A, et al; European Mania in Bipolar Longitudinal Evaluation of Medication (EMBLEM) Advisory Board. Baseline characteristics and outcomes in patients with first episode or multiple episodes of acute mania. J Clin Psychiatry. 2010;71(3):255–261. PubMed CrossRef
- Lieberman JA, Small SA, Girgis RR. Early detection and preventive intervention in schizophrenia: from fantasy to reality. Am J Psychiatry. 2019;176(10):794–810. PubMed CrossRef
- Correll CU, Martin A, Patel C, et al. Systematic literature review of schizophrenia clinical practice guidelines on acute and maintenance management with antipsychotics. Schizophrenia (Heidelb). 2022;8(1):5. PubMed CrossRef
- Huhn M, Nikolakopoulou A, Schneider-Thoma J, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet. 2019;394(10202):939–951. PubMed CrossRef
- Pillinger T, McCutcheon RA, Vano L, et al. Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: a systematic review and network meta-analysis. Lancet Psychiatry. 2020;7(1):64–77. PubMed CrossRef
- Lieberman JA, Stroup TS, McEvoy JP, et al; Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209–1223. PubMed CrossRef
- Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951–962. PubMed CrossRef
- Cipriani A, Barbui C, Salanti G, et al. Comparative efficacy and acceptability of antimanic drugs in acute mania: a multiple-treatments meta-analysis. Lancet. 2011;378(9799):1306–1315. PubMed CrossRef
- Yildiz A, Nikodem M, Vieta E, et al. A network meta-analysis on comparative efficacy and all-cause discontinuation of antimanic treatments in acute bipolar mania. Psychol Med. 2015;45(2):299–317. PubMed CrossRef
- Rummel-Kluge C, Komossa K, Schwarz S, et al. Second-generation antipsychotic drugs and extrapyramidal side effects: a systematic review and meta-analysis of head-to-head comparisons. Schizophr Bull. 2012;38(1):167–177. PubMed CrossRef
- Allison DB, Mentore JL, Heo M, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry. 1999;156(11):1686–1696. PubMed CrossRef
- Wirshing DA, Wirshing WC, Kysar L, et al. Novel antipsychotics: comparison of weight gain liabilities. J Clin Psychiatry. 1999;60(6):358–363. PubMed CrossRef
- Meyer JM, Davis VG, Goff DC, et al. Change in metabolic syndrome parameters with antipsychotic treatment in the CATIE Schizophrenia Trial: prospective data from phase 1. Schizophr Res. 2008;101(1–3):273–286. PubMed CrossRef
- Zyprexa [package insert]. Indianapolis, IN: Eli Lilly and Company; 2021.
- Buchanan RW, Kreyenbuhl J, Kelly DL, et al; Schizophrenia Patient Outcomes Research Team (PORT). The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull. 2010;36(1):71–93. PubMed CrossRef
- Correll CU, Robinson DG, Schooler NR, et al. Cardiometabolic risk in patients with first-episode schizophrenia spectrum disorders: baseline results from the RAISE-ETP study. JAMA Psychiatry. 2014;71(12):1350–1363. PubMed CrossRef
- Robinson DG, Schooler NR, Correll CU, et al. Psychopharmacological treatment in the RAISE-ETP study: outcomes of a manual and computer decision support system based intervention. Am J Psychiatry. 2018;175(2):169–179. PubMed CrossRef
- Siskind D, Gallagher E, Winckel K, et al. Does switching antipsychotics ameliorate weight gain in patients with severe mental illness? a systematic review and meta-analysis. Schizophr Bull. 2021;47(4):948–958. PubMed CrossRef
- Ostuzzi G, Vita G, Bertolini F, et al. Continuing, reducing, switching, or stopping antipsychotics in individuals with schizophrenia-spectrum disorders who are clinically stable: a systematic review and network meta-analysis. Lancet Psychiatry. 2022;9(8):614–624. PubMed CrossRef
- Speyer H, Westergaard C, Albert N, et al. Reversibility of antipsychotic-induced weight gain: a systematic review and meta-analysis. Front Endocrinol (Lausanne). 2021;12:577919. PubMed CrossRef
- Correll CU, Vanover KE, Davis RE, et al. Safety and tolerability of lumateperone 42 mg: an open-label antipsychotic switch study in outpatients with stable schizophrenia. Schizophr Res. 2021;228:198–205. PubMed CrossRef
- Lee K, Abraham S, Cleaver R. A systematic review of licensed weight-loss medications in treating antipsychotic-induced weight gain and obesity in schizophrenia and psychosis. Gen Hosp Psychiatry. 2022;78:58–67. PubMed CrossRef
- Vancampfort D, Firth J, Correll CU, et al. The impact of pharmacological and non-pharmacological interventions to improve physical health outcomes in people with schizophrenia: a meta-review of meta-analyses of randomized controlled trials. Focus Am Psychiatr Publ. 2021;19(1):116–128. PubMed CrossRef
- Lybalvi [package insert]. Waltham, MA: Alkermes, Inc.; 2021.
- Silverman BL, Martin W, Memisoglu A, et al. A randomized, double-blind, placebo-controlled proof of concept study to evaluate samidorphan in the prevention of olanzapine-induced weight gain in healthy volunteers. Schizophr Res. 2018;195:245–251. PubMed CrossRef
- Martin WF, Correll CU, Weiden PJ, et al. Mitigation of olanzapine-induced weight gain with samidorphan, an opioid antagonist: a randomized double-blind phase 2 study in patients with schizophrenia. Am J Psychiatry. 2019;176(6):457–467. PubMed CrossRef
- Correll CU, Newcomer JW, Silverman B, et al. Effects of olanzapine combined with samidorphan on weight gain in schizophrenia: a 24-week phase 3 study. Am J Psychiatry. 2020;177(12):1168–1178. PubMed CrossRef
- Potkin SG, Kunovac J, Silverman BL, et al. Efficacy and safety of a combination of olanzapine and samidorphan in adult patients with an acute exacerbation of schizophrenia: outcomes from the randomized, phase 3 ENLIGHTEN-1 study. J Clin Psychiatry. 2020;81(2):19m12769. PubMed CrossRef
- Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33, quiz 34–57. PubMed
- Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168(12):1266–1277. PubMed CrossRef
- Guy W. CGI Clinical Global Impressions. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: US Department of Health, Education, and Welfare, National Institute of Mental Health; 1976:217–222.
- Huang M, Yu L, Pan F, et al. A randomized, 13-week study assessing the efficacy and metabolic effects of paliperidone palmitate injection and olanzapine in first-episode schizophrenia patients. Prog Neuropsychopharmacol Biol Psychiatry. 2018;81:122–130. PubMed CrossRef
- Ou JJ, Xu Y, Chen HH, et al. Comparison of metabolic effects of ziprasidone versus olanzapine treatment in patients with first-episode schizophrenia. Psychopharmacology (Berl). 2013;225(3):627–635. PubMed CrossRef
- Perez-Iglesias R, Crespo-Facorro B, Amado JA, et al. A 12-week randomized clinical trial to evaluate metabolic changes in drug-naive, first-episode psychosis patients treated with haloperidol, olanzapine, or risperidone. J Clin Psychiatry. 2007;68(11):1733–1740. PubMed CrossRef
- Teff KL, Rickels MR, Grudziak J, et al. Antipsychotic-induced insulin resistance and postprandial hormonal dysregulation independent of weight gain or psychiatric disease. Diabetes. 2013;62(9):3232–3240. PubMed CrossRef
- Rickels MR, Perez EM, Peleckis AJ, et al. Contribution of parasympathetic muscarinic augmentation of insulin secretion to olanzapine-induced hyperinsulinemia. Am J Physiol Endocrinol Metab. 2018;315(2):E250–E257. PubMed CrossRef
- Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs. 2005;19(suppl 1):1–93. PubMed CrossRef
- Citrome L, Holt RI, Walker DJ, et al. Weight gain and changes in metabolic variables following olanzapine treatment in schizophrenia and bipolar disorder. Clin Drug Investig. 2011;31(7):455–482. PubMed CrossRef
- Wu H, Siafis S, Hamza T, et al. Antipsychotic-induced weight gain: dose-response meta-analysis of randomized controlled trials. Schizophr Bull. 2022;48(3):643–654. PubMed CrossRef
- Højlund M, Kemp AF, Haddad PM, et al. Standard versus reduced dose of antipsychotics for relapse prevention in multi-episode schizophrenia: a systematic review and meta-analysis of randomised controlled trials. Lancet Psychiatry. 2021;8(6):471–486. PubMed CrossRef
This PDF is free for all visitors!