Abstract
Objective: To evaluate the effects of adjunctive cariprazine on anxiety symptoms in adults with a DSM-5 diagnosis of major depressive disorder (MDD) and inadequate response to antidepressant therapy (ADT).
Methods: In this post hoc analysis of a phase 3 study (NCT03738215), we assessed the effects of adjunctive cariprazine 1.5 and 3.0 mg/d on depressive and anxiety symptoms. The modified intent-to-treat (mITT) population was evaluated, as well as subgroups with varying degrees of baseline anxiety, defined by Hamilton Rating Scale for Depression (HAM-D) Anxiety/Somatization factor scores and Hamilton Anxiety Rating Scale (HAM-A) total scores. Least squares mean differences (LSMD) in change from baseline to week 6 in Montgomery Asberg Depression Rating Scale and HAM-A total scores and in HAM-D Anxiety/Somatization factor scores were reported.
Results: The mITT population included 751 patients. At week 6, cariprazine 1.5 mg/d +ADT resulted in significantly greater changes in depressive symptoms versus placebo in patients with elevated baseline anxiety (HAM-D Anxiety/Somatization factor subgroup: LSMD [95% CI], −2.4 [−4.2 to −0.7]); HAM A total score subgroup: −2.8 [−4.6 to −1.0]). Adjunctive cariprazine also significantly reduced anxiety symptoms in the overall mITT population, as measured by mean reductions in HAM-D Anxiety/Somatization factor (1.5 mg/d: −0.8 [−1.2 to −0.3]; 3.0 mg/d: −0.5 [−1.0 to −0.1]) and HAM-A total scores (1.5 mg/d: −1.3 [−2.5 to −0.1]). Similar trends were observed for adjunctive cariprazine 1.5 mg/d in subgroups of patients with elevated anxiety symptoms.
Conclusions: In addition to reducing depressive symptoms, adjunctive cariprazine may also reduce anxiety symptoms in patients with MDD, regardless of the level of baseline anxiety.
J Clin Psychiatry 2025;86(2):24m15506
Author affiliations are listed at the end of this article.
Major depressive disorder (MDD) is a complex mood disorder that often requires adjunctive therapy to achieve effective symptom control, as a significant proportion of patients do not achieve remission following standard antidepressant therapies.1 Many patients with MDD also suffer from clinically meaningful levels of anxiety.2–4 In the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial, 46% of patients with MDD were considered to have significant levels of anxiety or “anxious depression,” defined as a Hamilton Depression Rating Scale (HAM-D) anxiety/somatization factor score ≥7.2 A subsequent analysis of STAR*D patients showed that those who had anxious depression were significantly less likely to respond or remit with antidepressant monotherapy vs patients with nonanxious depression.5 In addition, patients with anxious depression took significantly longer to respond and achieve remission than patients with nonanxious depression,5 suggesting that anxious depression may be more difficult to treat than depression without comorbid anxiety. Anxious depression has also been associated with increased risk of relapse, increased suicide risk, depression severity, severe role impairment, and functional impairment relative to depression without anxiety.2–6 Even among patients with MDD who respond to treatment, residual anxiety symptoms predict more rapid recurrence,7 underscoring the negative effects anxiety may have on MDD outcomes.
Cariprazine is a dopamine D3-preferring D3/D2 and serotonin 5-HT1A receptor partial agonist that is approved by the US Food and Drug Administration (FDA) as an adjunct to antidepressant therapy (ADT) to treat adults with MDD and inadequate response to antidepressants. The efficacy of adjunctive cariprazine in reducing depressive symptoms in patients with MDD and inadequate response to ADT alone was demonstrated in an 8-week, multicenter, double-blind, placebo controlled, phase 2, flexible-dose study8 designed to investigate the effects of adjunctive cariprazine 1–2 mg/d and 2–4.5 mg/d in patients with MDD and an inadequate response to ongoing ADT. Results indicated that the difference in Montgomery-Asberg Depression Rating Scale (MADRS) total score change from baseline to week 8 was significant in favor of the higher dose treatment group (cariprazine 2–4.5 mg/d) vs placebo.8 This was followed by a 6-week, phase 3, fixed-dose study9 in which patients with MDD and an inadequate response to ADT alone were randomized to receive cariprazine 1.5 mg/d+ADT, cariprazine 3.0 mg/d+ADT, or placebo +ADT for 6 weeks of double-blind treatment. At week 6, differences in mean MADRS total score change from baseline were significant in favor of cariprazine 1.5 mg/d, and MADRS response rates (≥50% reduction in MADRS total score from baseline) were significantly greater with cariprazine 1.5 mg/d +ADT vs placebo +ADT.9 In addition to exhibiting depressive symptom improvement, patients treated with cariprazine had greater reductions from baseline in Hamilton Anxiety Rating Scale (HAM-A) total score than placebo treated patients,9 suggesting a potential anxiolytic benefit with adjunctive cariprazine treatment.
Given the high prevalence of anxiety among patients with MDD and the challenges associated with anxious depression, treatment regimens that can help manage both depressive and anxiety symptoms would address a critical need. We conducted post hoc analyses of data from the fixed-dose, randomized, double-blind, placebo controlled, phase 3 study described above to evaluate the effects of adjunctive cariprazine on depressive and anxiety symptoms across various levels of baseline anxiety among patients with MDD and an inadequate response to ADT alone.
METHODS
Study Design
Details of the fixed-dose phase 3 study (NCT03738215) have been provided previously.9 Briefly, adult patients (age 18–65 years) were randomized 1:1: 1 to receive cariprazine 1.5 mg/d +ADT, cariprazine 3.0 mg/d +ADT, or placebo +ADT for 6 weeks of double blind treatment following a 2-week screening period. After the double-blind treatment period, patients entered a 4-week safety follow-up, during which no medication was administered. Key inclusion criteria were a Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), diagnosis of MDD, inadequate response (<50% improvement) to 1–3 ADTs of adequate dose and duration, and baseline 17-item HAM-D total score ≥22, with a score ≥2 on item 1. The primary efficacy parameter was change from baseline to week 6 in MADRS total score.
Post Hoc Analysis
In post hoc analyses, the effects of cariprazine on depressive and anxiety symptoms were evaluated in the overall modified intent-to-treat population (mITT; defined as all randomized participants who had ≥1 postbaseline assessment of the MADRS total score) and in subgroups of patients with varying levels of baseline anxiety, which were based on HAM-D Anxiety/Somatization factor total scores and HAM-A total scores at baseline. The HAM-D Anxiety/Somatization factor score is the sum of the following HAM-D items: psychic anxiety, somatic anxiety, gastrointestinal somatic symptoms, general somatic symptoms, hypochondriasis, and insight. The HAM-D was administered at baseline and at week 6 in the primary study. The HAM-A is a 14-item, clinician-rated evaluation used to assess the severity of anxiety symptoms. The HAM-A was administered at baseline and at weeks 2 and 6 in the primary study.
For the analysis of depressive symptoms, the following subgroups were evaluated:
- HAM-D Anxiety/Somatization factor subgroups were defined as patients with or without anxiety symptoms (score ≥7 or <7, respectively)
- HAM-A subgroups of patients with mild anxiety (score ≤14) or with moderate to severe anxiety (score >14) were also evaluated
For the analysis of anxiety symptoms, the following subgroups were evaluated:
- Patients with at least mild (HAM-A total score of >7), at least moderate (HAM-A total score of >14), or severe anxiety (HAM-A total score >23)
- Patients with and without baseline anxiety symptoms, defined as a HAM-D Anxiety/Somatization factor score ≥7 or <7, respectively
Outcomes included least squares (LS) mean change from baseline to week 6 in depressive symptoms, as measured by MADRS total score, and in anxiety symptoms, as measured by HAM-D Anxiety/Somatization factor score and HAM-A total score. Change from baseline in MADRS total scores or HAM-A scores was analyzed using a mixed-effects model for repeated measures. HAM-D Anxiety/Somatization factor was analyzed using analysis of covariance since the HAM-D was administered only at baseline and week 6. Differences were considered statistically significant in favor of cariprazine when the 95% CI for the LS mean difference (LSMD) vs placebo did not cross zero.
RESULTS
Baseline Characteristics
The mITT population included 751 patients (cariprazine 1.5 mg/d + ADT, n = 250; cariprazine 3.0 mg/d + ADT, n = 252; placebo + ADT, n = 249). Baseline demographic and clinical characteristics were generally similar across adjunctive cariprazine treatment and placebo groups. Most patients (>70%) were female, and the average age was approximately 45 years. Baseline MADRS and HAM-A total scores were approximately 32 and 22, respectively, in the overall mITT population.
Based on HAM-D Anxiety/Somatization Factor scores, 627 patients (83.5%) had anxiety symptoms at baseline. HAM-A total scores indicated that 742 patients (98.8%) had at least mild baseline anxiety, and 131 patients (17.4%) had mild anxiety at baseline. 620 (82.6%) had at least moderate baseline anxiety, and 262 (34.9%) had severe baseline anxiety.
Effects of Adjunctive Cariprazine on Depressive Symptoms
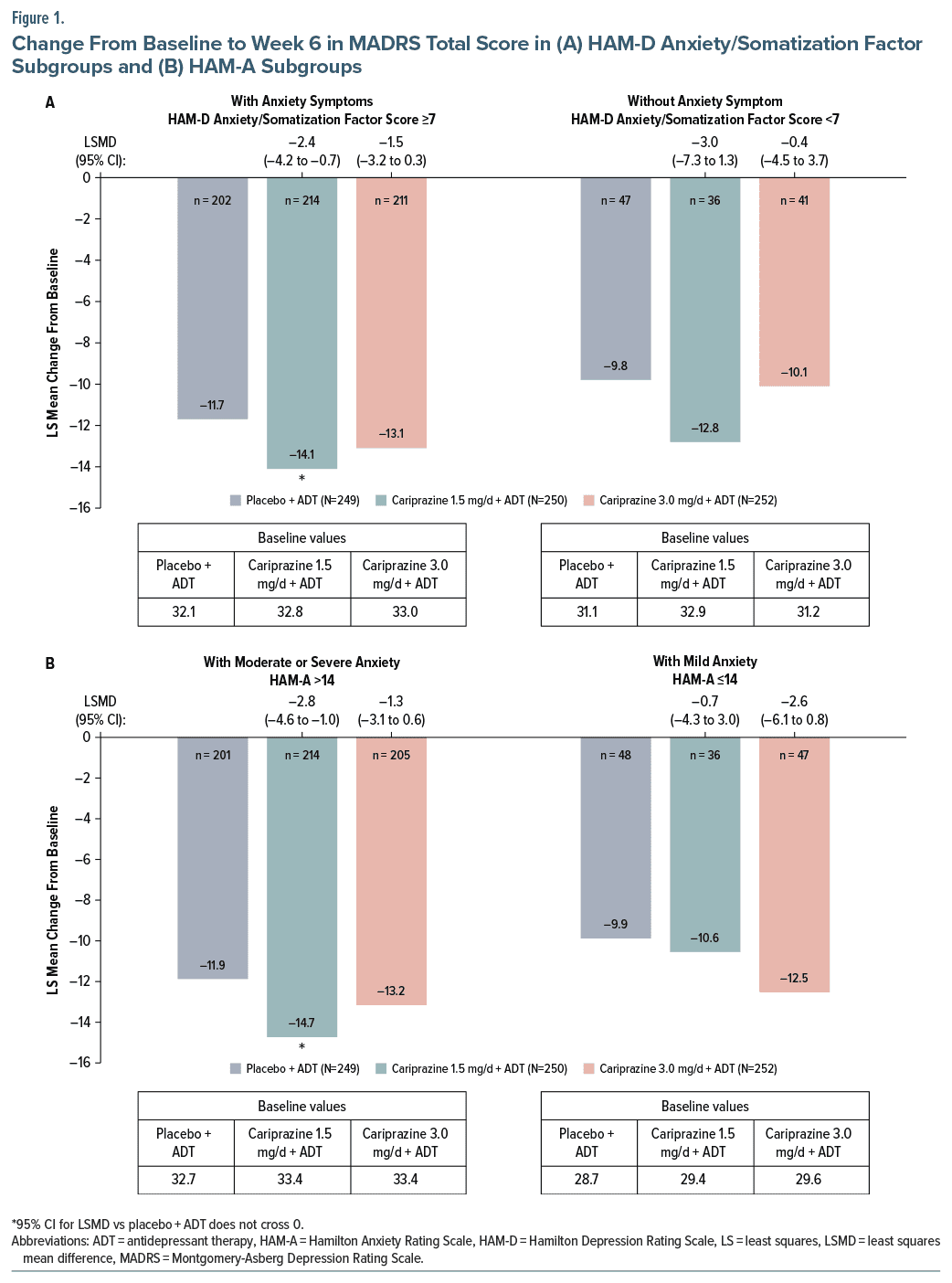
In patients with elevated anxiety symptoms as measured by HAM-D Anxiety/Somatization factor score (anxious depression), the change from baseline in MADRS total score was significantly greater for cariprazine 1.5 mg/d +ADT vs placebo +ADT (Figure 1A). In patients without anxiety symptoms (nonanxious depression), the LSMD for cariprazine 1.5 mg/d +ADT vs placebo was larger than the LSMDs observed in patients with anxiety, but the difference was not significant, potentially due to smaller sample sizes in this subgroup (adjunctive placebo n = 47, adjunctive cariprazine 1.5 mg/d n = 36, adjunctive cariprazine 3.0 mg/d n = 41) (Figure 1A).
In patients with moderate or severe anxiety symptoms as measured by HAM-A, the difference in LS mean change from baseline to week 6 in MADRS total score was statistically significant for cariprazine 1.5 mg/d + ADT vs placebo + ADT (Figure 1B), with significantly greater LS mean reductions observed as early as week 2 (Supplementary Figure 1). In the mild anxiety subgroup, the greatest treatment effect was observed in the cariprazine 3.0 mg/d + ADT group (LSMD: −2.6); however, differences vs placebo + ADT were not significant for either adjunctive cariprazine treatment group, potentially due to the small sample size in this subgroup (adjunctive placebo n = 48, adjunctive cariprazine 1.5 mg/d n = 36, adjunctive cariprazine 3.0 mg/d n = 47) (Figure 1B).
Effects of Adjunctive Cariprazine on Anxiety Symptoms
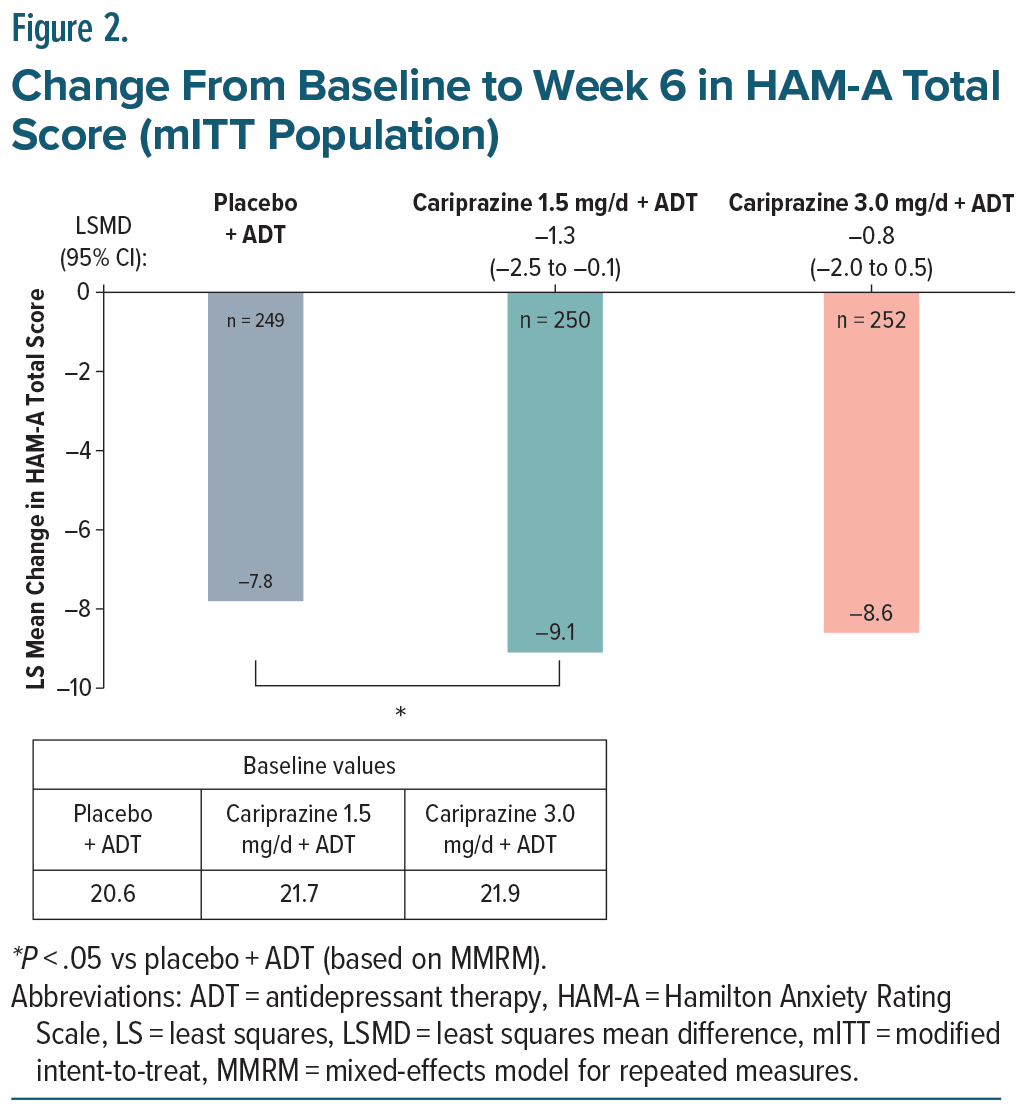
In the overall mITT population, the LSMD from baseline to week 6 in HAM-A total score was statistically significant for cariprazine 1.5 mg/d +ADT vs placebo +ADT (Figure 2). The reduction in HAM-A total score for cariprazine 3.0 mg/d +ADT was greater than that of placebo + ADT, though the difference did not achieve statistical significance (Figure 2).
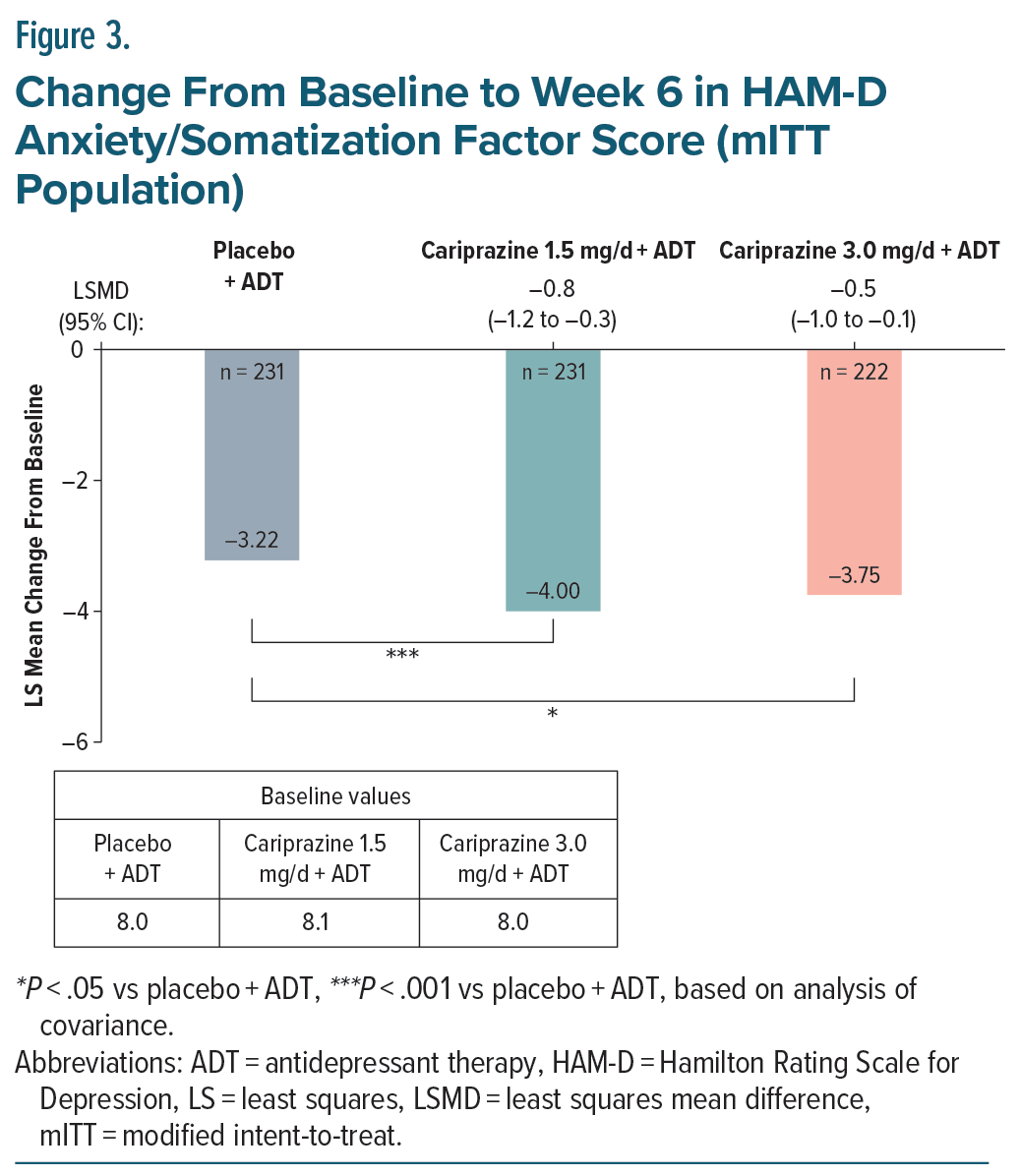
When anxiety was further evaluated by change from baseline to week 6 in HAM-D Anxiety/Somatization factor score in the overall mITT population, differences vs placebo +ADT were statistically significant for both cariprazine 1.5 and 3.0 mg/d +ADT (Figure 3).
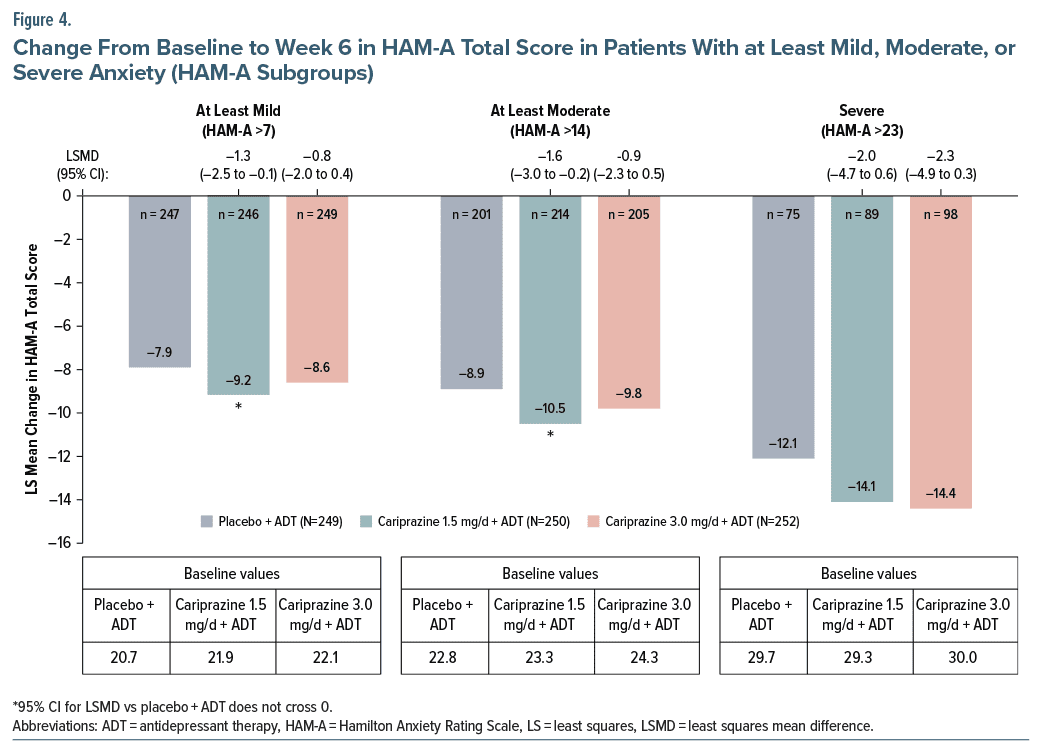
In subgroups of patients with anxiety as measured by HAM-A total score, the difference in change from baseline to week 6 in HAM-A total score was statistically significant for cariprazine 1.5 mg/d +ADT vs placebo +ADT in patients with at least mild or at least moderate anxiety.
Although larger differences vs placebo +ADT were observed for the cariprazine 3.0 mg/d +ADT group, differences were not statistically significant (Figure 4). Changes from baseline to week 6 in HAM-A total score were largest in the severe anxiety subgroup, but the differences between adjunctive cariprazine and placebo were not significant, possibly due to the small sample sizes in this group (adjunctive placebo n = 75, adjunctive cariprazine 1.5 mg/d n = 89, adjunctive cariprazine 3.0 mg/d n = 98) (Figure 4).
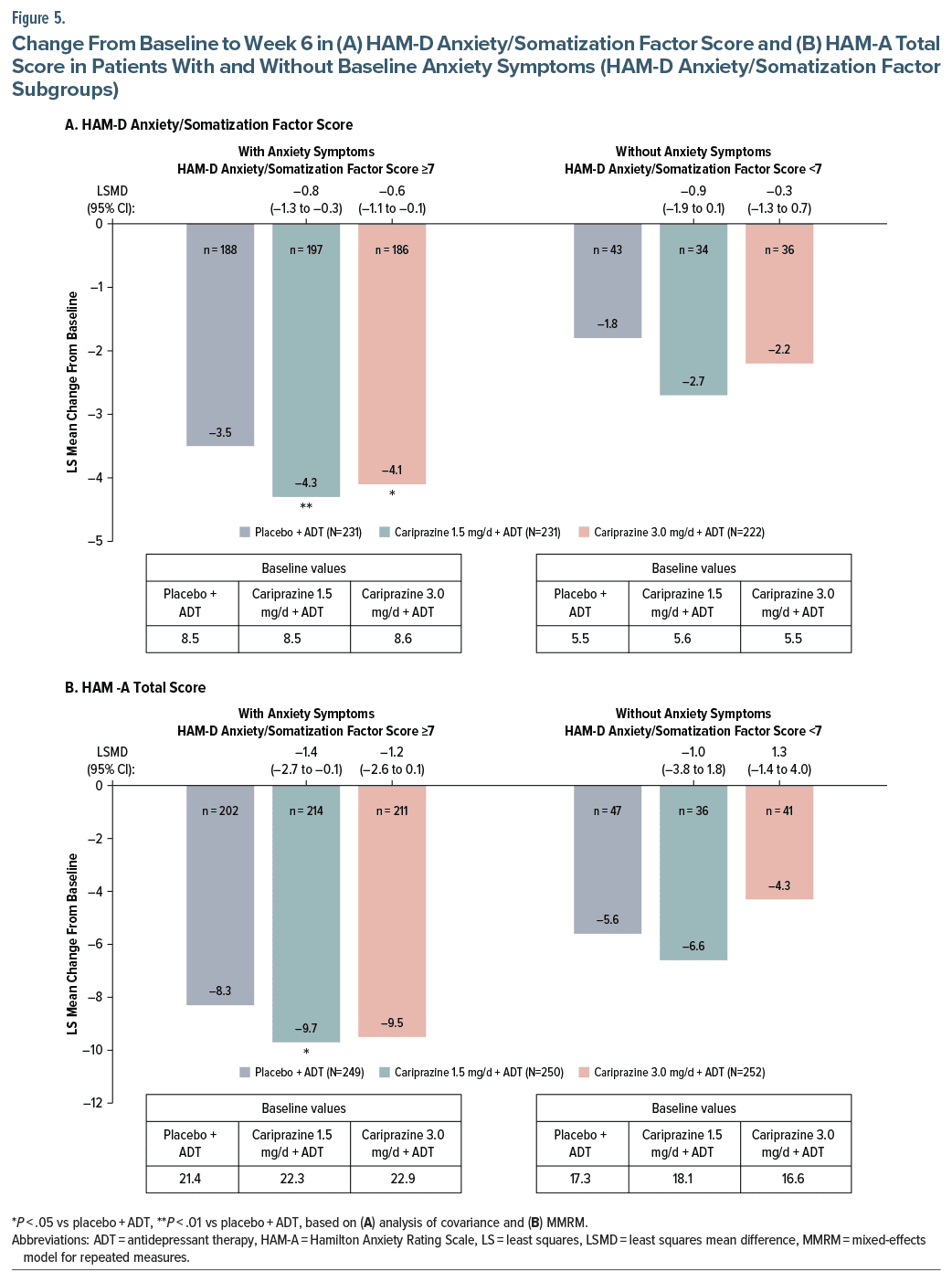
In subgroups of patients with baseline anxiety as measured by HAM-D Anxiety/Somatization factor score, LS mean changes from baseline to week 6 in HAM-D Anxiety/Somatization factor scores were significantly greater for both cariprazine 1.5 mg/d + ADT and 3.0 mg/d + ADT vs placebo + ADT (Figure 5A). Numerically greater changes were observed in favor of adjunctive cariprazine in patients without anxiety symptoms, yet the differences were not statistically significant (Figure 5A). Cariprazine 1.5 mg/d + ADT was associated with significantly greater reductions in HAM-A total score from baseline to week 6 in patients with HAM-D Anxiety/Somatization factor scores ≥7; no significant differences were observed for patients with HAM-D Anxiety/Somatization factor scores <7 (Figure 5B). Among patients without baseline anxiety, there was no worsening of anxiety symptoms as measured by HAM-D Anxiety/Somatization factor scores and HAM-A total scores (Figure 5A and 5B).
Safety
In the overall safety population, the most common adverse events occurring in at least 5% of patients in either adjunctive cariprazine treatment group and twice the rate of adjunctive placebo were akathisia (cariprazine 1.5 mg/d +ADT: 5.2%; cariprazine 3.0 mg/d +ADT: 7.9%; placebo +ADT: 0.8%) and nausea (cariprazine 1.5 mg/d +ADT: 7.9%; cariprazine 3.0 mg/d +ADT: 6.3%; placebo +ADT: 2.4%).
DISCUSSION
In patients with MDD who experienced an inadequate response to ADT alone and had elevated anxiety symptoms at baseline, defined using either HAM-D Anxiety/Somatization factor scores or HAM-A scores, cariprazine 1.5 mg/d +ADT resulted in significantly greater changes in depressive symptoms vs placebo +ADT. In patients without anxiety symptoms, cariprazine 1.5 mg/d +ADT was also associated with greater reductions in depressive symptoms, though differences vs placebo were not significantly different, likely due to the relatively small sample size. Further, there was no worsening of anxiety symptoms with adjunctive cariprazine in patients without baseline anxiety (ie, HAM-D Anxiety/Somatization factor score <7). Cariprazine +ADT also significantly reduced anxiety symptoms in the mITT population, as measured by mean reductions in HAM-D Anxiety/Somatization factor scores and HAM-A total scores. Significant differences in HAM-A total scores were observed for cariprazine 1.5 mg/d +ADT in the subgroups with at least mild and at least moderate anxiety and for patients with anxious depression. The largest anxiolytic effects were observed for patients with severe anxiety, though small sample sizes likely prevented detection of significant differences between adjunctive cariprazine and placebo. Both adjunctive cariprazine doses were associated with significantly greater changes in HAM-D Anxiety/Somatization factor scores vs placebo in patients with anxious depression at baseline. Results of our post hoc analyses are consistent with previous findings that anxiety is a common feature of MDD. Of note, the percentages of patients with anxiety symptoms in this study varied based on which criterion was used (HAM-D Anxiety/Somatization factor score: 83.5% with baseline anxiety symptoms; HAM-A total score: 98.8% with at least mild baseline anxiety), suggesting that there may be some differences between these measures in defining anxiety symptoms. Regardless of the scale used to define anxiety symptoms and the level of baseline anxiety in this population of patients with MDD, results suggest that adjunctive cariprazine reduces both depressive symptoms and anxiety symptoms.
These findings support previous preclinical cariprazine results suggesting that cariprazine may have anxiolytic properties. In animal model studies, cariprazine displayed antidepressant and anxiolytic effects, which may have been at least partially mediated by its preferential binding of the D3 receptor.10 Results from previous studies have suggested that the D3 receptor may be involved in the regulation of anxiety symptoms.11 The affinity of cariprazine for the D3 receptor is 10-fold that of the D2 receptor, and cariprazine displays a greater affinity for the D3 receptor than other atypical antipsychotics.12 This unique pharmacodynamic feature may play a role in reducing anxiety symptoms in patients with MDD, though future research is needed to confirm this hypothesis. Further, a recent post hoc analysis of pooled data from 2 cariprazine studies for the treatment of bipolar I depression showed that cariprazine 1.5 mg/d resulted in a significantly greater reduction from baseline to week 6 in HAM-A total score vs placebo (LSMD, −1.5; P = .0027) in the pooled ITT population.13 In a patient subgroup with higher levels of baseline anxiety, changes from baseline to week 6 were statistically significant in favor of cariprazine 1.5 mg/d vs placebo on MADRS total score (LSMD, −2.44; P = .0200) and HAM-A total score vs placebo (LSMD, −1.87; P = .0105). In patients with lower levels of anxiety, cariprazine 3.0 mg/d resulted in significantly greater reductions from baseline to week 6 in MADRS total score (LSMD, −4.43; P < .0001) and HAM-A total score (LSMD, −1.30; P = .0441), while cariprazine 1.5 mg/d resulted in significantly greater reductions in MADRS total score (LSMD, −2.23; P = .0457). Though bipolar and unipolar depression are distinct disorders, the trends of reduced anxiety symptoms with cariprazine observed in patients with bipolar I depression and MDD are comparable. Together, results from the present analysis and previous studies indicate that cariprazine may reduce both depressive and anxiety symptoms, regardless of whether a major depressive episode is associated with bipolar or unipolar depression.
Although some antidepressants can also be used to address anxiety in patients with MDD,14 anxiety symptoms are among the most common residual symptoms following ADT,15 which may complicate treatment, negatively affect psychosocial functioning, and make remission more difficult to achieve.2,5,16 Further, many patients with MDD do not achieve adequate response or remission with ADT alone17 and may require adjunctive therapy to effectively manage their symptoms. Our analysis demonstrates the ability of adjunctive cariprazine to reduce overall depressive and anxiety symptoms in patients with MDD across varying levels of baseline anxiety. Other studies of adjunctive atypical antipsychotics (AAs) for the treatment of MDD have also demonstrated significant effects for reducing anxiety symptoms in patients with higher or lower levels of baseline anxiety18–21 and support the use of an adjunctive AA for managing patients with MDD, regardless of the degree of baseline anxiety.
Our finding of a significant reduction in symptoms of anxiety with adjunctive cariprazine may have implications for managing anxious depression in clinical practice, which can be challenging. Clinicians may have the impression that a sedating agent, such as a sedating adjunctive AA or a benzodiazepine, is required to help manage anxiety symptoms in patients with MDD. However, more sedating AAs may result in persistent daytime somnolence,22,23 and benzodiazepines have been associated with dependence, cognitive impairment, and worsened anhedonia symptoms.22,24,25 Daytime sedation caused by medication can negatively affect physical and cognitive function, leading to subsequent negative effects on medication adherence and quality of life in some cases.22,23,26 Compared with patients in remission who attain normal functioning, those who do not attain normal functioning have a substantially greater risk of MDD relapse,27 underscoring the importance of minimizing functional impairment in this patient population. While sedation is a common adverse effect associated with many AAs,28,29 cariprazine is considered a low somnolence compound compared with other AAs28 and has demonstrated relatively low levels of daytime sedation in MDD clinical trials.9,30 Further, in post hoc analyses of data from patients with bipolar I depression in a phase 2b trial, cariprazine 1.5 mg/d demonstrated significant improvements vs placebo across 5 of 6 Functional Assessment Short Test (FAST) subscale scores,31 suggesting that cariprazine may improve functional outcomes in patients with bipolar depression. While no firm conclusions can be made from post hoc analyses, these findings are important because cariprazine may offer clinicians an option to manage anxiety symptoms in patients with MDD without a significant risk of sedation and potential functional deficits.
Results of this post hoc analysis should be interpreted within the context of its limitations, which include the lack of adjustment for multiple comparisons. Also, the primary study was not powered for subgroup analyses and consisted of a relatively short study duration, which could have influenced the results of the present analysis. Patients with primary anxiety disorders were excluded, and while many patients with MDD have anxiety symptoms, there was no requirement for patients to have anxiety at baseline, although very few patients in this study did not. These factors may limit generalizability to some patients with MDD.
Overall, adjunctive cariprazine was more effective than adjunctive placebo for reducing overall depressive and anxiety symptoms in patients with MDD and various levels of baseline anxiety. The outcomes of this post hoc analysis are exploratory and further study is needed to evaluate the potential benefits of adjunctive cariprazine on anxiety symptoms in patients with MDD.
Article Information
Published Online: April 30, 2025. https://doi.org/10.4088/JCP.24m15506
© 2025 Physicians Postgraduate Press, Inc.
Submitted: July 10, 2024; accepted February 20, 2025.
To Cite: Fava M, Masand PS, Maletic V, et al. Efficacy of adjunctive cariprazine on anxiety symptoms in patients with major depressive disorder: post hoc analysis of a randomized placebo-controlled trial. J Clin Psychiatry 2025;86(2):24m15506.
Author Affiliations: Department of Psychiatry, Massachusetts General Hospital, Boston, Massachusetts (Fava); Duke-NUS, Singapore (Masand); University of South Carolina School of Medicine, Columbia, South Carolina (Maletic); AbbVie, North Chicago, Illinois (Chen, Adams, Kerolous).
Corresponding Author: Maurizio Fava, MD, Department of Psychiatry, Massachusetts General Hospital, 55 Fruit St, Bulfinch 351, Boston, MA 02114 ([email protected]).
Relevant Financial Relationships: Dr Fava’s disclosures can be viewed at https://mghcme.org/app/uploads/2022/04/MF-Disclosures-Lifetime-updated-April-2022.pdf. Dr Masand has served as a consultant for AbbVie, Intra-Cellular Therapies, Karuna Therapeutics, Neumora Therapeutics, and Neurocrine Biosciences; has served on speaker’s bureaus for AbbVie and Neurocrine Biosciences; and has stock ownership in Relmada Therapeutics. Dr Maletic has received speaking/consulting fees from AbbVie/Allergan, Acadia Pharmaceuticals, Inc., Alfasigma, USA, Inc., Alkermes, Inc., Biogen, Boehringer Ingelheim, Cerevel Therapeutics, LLC, Corium, Intra-Cellular Therapies, Ironshore, Janssen, LivaNova, Lundbeck A/S, Jazz Pharmaceuticals, Neurelis, Neumora, Noven Pharmaceuticals Inc, Otsuka America Pharmaceutical, Inc., Pax Medica, Relmada Therapeutics, Sage Pharmaceuticals, Sunovion Pharmaceuticals Inc., Supernus Pharmaceuticals, Inc., Takeda Pharmaceutical Company Limited, and Tris Pharma. Drs Chen, Adams, and Kerolous are employees of AbbVie and may hold stock.
Funding/Support: The study was funded by AbbVie.
Role of the Sponsor: AbbVie participated in the study design, research, analysis, data collection, interpretation of data, reviewing, and approval of the publication. All authors had access to relevant data and participated in the drafting, review, and approval of this publication. No honoraria or payments were made for authorship.
Previous Presentation: Presented at the US Psych Congress; September 17–20, 2022; New Orleans, Louisiana, and the American College of Neuropsychopharmacology (ACNP) Annual Meeting; December 4–7, 2022; Phoenix, Arizona.
Data Availability Statement: AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual, and trial-level data (analysis data sets), as well as other information (eg, protocols, clinical study reports, or analysis plans), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent, scientific research, and will be provided following review and approval of a research proposal, Statistical Analysis Plan (SAP), and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time after approval in the US and Europe and after acceptance of this manuscript for publication. The data will be accessible for 12 months, with possible extensions considered. For more information on the process or to submit a request, visit the following link: https://www.abbvieclinicaltrials.com/hcp/data-sharing/.
Acknowledgments: Medical writing support was provided by Caroline Warren, PharmD, of Prescott Medical Communications Group, a Citrus Health Group, Inc, company (Chicago, Illinois), and funded by AbbVie.
Additional Information: Coauthor Vladimir Maletic, MD, died December 11, 2024.
Supplementary Material: Available at Psychiatrist.com.
Clinical Points
- Patients with major depressive disorder (MDD) commonly experience anxiety symptoms that can complicate treatment; therefore, regimens that can address both depressive and anxiety symptoms are clinically relevant.
- Adjunctive cariprazine reduced both depressive symptoms and anxiety symptoms in adults with MDD and various levels of baseline anxiety.
- There was no worsening of anxiety symptoms with adjunctive cariprazine in patients with MDD who had no baseline anxiety symptoms (ie, HAM-D Anxiety/Somatization factor score <7).
References (31)

- Papakostas GI, Jackson WC, Rafeyan R, et al. Overcoming challenges to treat inadequate response in major depressive disorder. J Clin Psychiatry. 2020;81(3):OT19037BR4. PubMed CrossRef
- Fava M, Alpert JE, Carmin CN, et al. Clinical correlates and symptom patterns of anxious depression among patients with major depressive disorder in STAR*D. Psychol Med. 2004;34(7):1299–1308. PubMed CrossRef
- Fava M, Rush AJ, Alpert JE, et al. What clinical and symptom features and comorbid disorders characterize outpatients with anxious major depressive disorder: a replication and extension. Can J Psychiatry. 2006;51(13):823–835. PubMed CrossRef
- Kessler RC, Sampson NA, Berglund P, et al. Anxious and non-anxious major depressive disorder in the World Health Organization World Mental Health Surveys. Epidemiol Psychiatr Sci. 2015;24(3):210–226. PubMed CrossRef
- Fava M, Rush AJ, Alpert JE, et al. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Am J Psychiatry. 2008;165(3):342351.
- Duffy L, Lewis G, Marston L, et al. Clinical factors associated with relapse in depression in a sample of UK primary care patients who have been on long-term antidepressant treatment. Psychol Med. 2024;54(5):951–961. PubMed CrossRef
- Israel JA. The impact of residual symptoms in major depression. Pharmaceuticals. 2010;3(8):2426–2440. PubMed CrossRef
- Durgam S, Earley W, Guo H, et al. Efficacy and safety of adjunctive cariprazine in inadequate responders to antidepressants: a randomized, double-blind, placebo controlled study in adult patients with major depressive disorder. J Clin Psychiatry. 2016;77(3):371–378. PubMed CrossRef
- Sachs GS, Yeung PP, Rekeda L, et al. Adjunctive cariprazine for the treatment of patients with major depressive disorder: a randomized, double-blind, placebocontrolled phase 3 study. Am J Psychiatry. 2023;180(3):241–251. PubMed CrossRef
- Duric V, Banasr M, Franklin T, et al. Cariprazine exhibits anxiolytic and dopamine D3 receptor-dependent antidepressant effects in the chronic stress model. Int J Neuropsychopharmacol. 2017;20(10):788–796. PubMed CrossRef
- Steiner H, Fuchs S, Accili D. D3 dopamine receptor-deficient mouse: evidence for reduced anxiety. Physiol Behav. 1997;63(1):137–141. PubMed CrossRef
- Kiss B, Horváth A, Némethy Z, et al. Cariprazine (RGH-188), a dopamine D(3) receptorpreferring, D(3)/D(2) dopamine receptor antagonist-partial agonist antipsychotic candidate: in vitro and neurochemical profile. J Pharmacol Exp Ther. 2010;333(1):328–340. PubMed CrossRef
- Jain R, McIntyre RS, Cutler AJ, et al. Efficacy of cariprazine in patients with bipolar depression and higher or lower levels of baseline anxiety: a pooled post hoc analysis. Int Clin Psychopharmacol. 2024;39(2):82–92. PubMed CrossRef
- Bandelow B, Michaelis S, Wedekind D. Treatment of anxiety disorders. Dialogues Clin Neurosci. 2017;19(2):93–107. PubMed CrossRef
- Hiranyatheb T, Nakawiro D, Wongpakaran T, et al. The impact of residual symptoms on relapse and quality of life among Thai depressive patients. Neuropsychiatr Dis Treat. 2016;12:3175–3181. PubMed CrossRef
- Hopwood M. Anxiety symptoms in patients with major depressive disorder: commentary on prevalence and clinical implications. Neurol Ther. 2023;12(suppl 1):5–12. PubMed CrossRef
- Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905–1917. PubMed CrossRef
- Bandelow B, Bauer M, Vieta E, et al. Extended release quetiapine fumarate as adjunct to antidepressant therapy in patients with major depressive disorder: pooled analyses of data in patients with anxious depression versus low levels of anxiety at baseline. World J Biol Psychiatry. 2014;15(2):155–166. PubMed CrossRef
- McIntyre RS, Weiller E, Zhang P, et al. Brexpiprazole as adjunctive treatment of major depressive disorder with anxious distress: results from a post-hoc analysis of two randomised controlled trials. J Affect Disord. 2016;201:116–123. PubMed CrossRef
- Thase ME, Weiller E, Zhang P, et al. Adjunctive brexpiprazole in patients with major depressive disorder and anxiety symptoms: post hoc analyses of three placebo-controlled studies. Neuropsychiatr Dis Treat. 2019;15:37–45. PubMed CrossRef
- Trivedi MH, Thase ME, Fava M, et al. Adjunctive aripiprazole in major depressive disorder: analysis of efficacy and safety in patients with anxious and atypical features. J Clin Psychiatry. 2008;69(12):1928–1936. PubMed CrossRef
- Citrome LL, McIntyre RS, Manning JS, et al. Activating and sedating properties of medications used for the treatment of major depressive disorder and their effect on patient functioning. J Clin Psychiatry. 2019;80(3):lu18052ah1. PubMed CrossRef
- Kane JM, Sharif ZA. Atypical antipsychotics: sedation versus efficacy. J Clin Psychiatry. 2008;69(suppl 1):18–31. PubMed
- Baldwin DS, Aitchison K, Bateson A, et al. Benzodiazepines: risks and benefits. A reconsideration. J Psychopharmacol. 2013;27(11):967–971. PubMed CrossRef
- Rizvi SJ, Sproule BA, Gallaugher L, et al. Correlates of benzodiazepine use in major depressive disorder: the effect of anhedonia. J Affect Disord. 2015;187:101–105. PubMed CrossRef
- Lim R, Kalisch Ellett LM, Widagdo IS, et al. Analysis of anticholinergic and sedative medicine effects on physical function, cognitive function, appetite and frailty: a cross-sectional study in Australia. BMJ Open. 2019;9(9):e029221. PubMed CrossRef
- Ishak WW, Greenberg JM, Cohen RM. Predicting relapse in major depressive disorder using patient-reported outcomes of depressive symptom severity, functioning, and quality of life in the Individual Burden of Illness Index for Depression (IBI-D). J Affect Disord. 2013;151(1):59–65. PubMed CrossRef
- Fang F, Sun H, Wang Z, et al. Antipsychotic drug-induced somnolence: incidence, mechanisms, and management. CNS Drugs. 2016;30(9):845–867. PubMed CrossRef
- Miller DD. Atypical antipsychotics: sleep, sedation, and efficacy. Prim Care Companion J Clin Psychiatry. 2004;6(suppl 2):3–7. PubMed
- Riesenberg R, Yeung PP, Rekeda L, et al. Cariprazine for the adjunctive treatment of major depressive disorder in patients with inadequate response to antidepressant therapy: results of a randomized, double-blind, placebo controlled study. J Clin Psychiatry. 2023;84(5):22m14643. PubMed CrossRef
- Vieta E, Calabrese JR, Whelan J, et al. The efficacy of cariprazine on function in patients with bipolar depression: a post hoc analysis of a randomized controlled trial. Curr Med Res Opin. 2021;37(9):1635–1643. PubMed CrossRef
This PDF is free for all visitors!