Objective: Misdiagnosis of early behavioral variant frontotemporal dementia (bvFTD) with major depressive disorder (MDD) is not uncommon due to overlapping symptoms. The aim of this study was to improve the discrimination between these disorders using a novel facial emotion perception task.
Method: In this prospective cohort study (July 2013-March 2016), we compared 25 patients meeting Rascovsky diagnostic criteria for bvFTD, 20 patients meeting DSM-IV criteria for MDD, 21 patients meeting McKhann diagnostic criteria for Alzheimer’s disease dementia, and 31 healthy participants on a novel emotion intensity rating task comprising morphed low-intensity facial stimuli. Participants were asked to rate the intensity of morphed faces on the congruent basic emotion (eg, rating on sadness when sad face is shown) and on the 5 incongruent basic emotions (eg, rating on each of the other basic emotions when sad face is shown).
Results: While bvFTD patients underrated congruent emotions (P < .01), they also overrated incongruent emotions (P < .001), resulting in confusion of facial emotions. In contrast, MDD patients overrated congruent negative facial emotions (P < .001), but not incongruent facial emotions. Accordingly, ratings of congruent and incongruent emotions highly discriminated between bvFTD and MDD patients, ranging from area under the curve (AUC) = 93% to AUC = 98%. Further, an almost complete discrimination (AUC = 99%) was achieved by contrasting the 2 rating types. In contrast, Alzheimer’s disease dementia patients perceived emotions similarly to healthy participants, indicating no impact of cognitive impairment on rating scores.
Conclusions: Our congruent and incongruent facial emotion intensity rating task allows a detailed assessment of facial emotion perception in patient populations. By using this simple task, we achieved an almost complete discrimination between bvFTD and MDD, potentially helping improve the diagnostic certainty in early bvFTD.
This work may not be copied, distributed, displayed, published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the publisher’s Terms & Conditions.


ABSTRACT
Objective: Misdiagnosis of early behavioral variant frontotemporal dementia (bvFTD) with major depressive disorder (MDD) is not uncommon due to overlapping symptoms. The aim of this study was to improve the discrimination between these disorders using a novel facial emotion perception task.
Method: In this prospective cohort study (July 2013-March 2016), we compared 25 patients meeting Rascovsky diagnostic criteria for bvFTD, 20 patients meeting DSM-IV criteria for MDD, 21 patients meeting McKhann diagnostic criteria for Alzheimer’s disease dementia, and 31 healthy participants on a novel emotion intensity rating task comprising morphed low-intensity facial stimuli. Participants were asked to rate the intensity of morphed faces on the congruent basic emotion (eg, rating on sadness when sad face is shown) and on the 5 incongruent basic emotions (eg, rating on each of the other basic emotions when sad face is shown).
Results: While bvFTD patients underrated congruent emotions (P < .01), they also overrated incongruent emotions (P < .001), resulting in confusion of facial emotions. In contrast, MDD patients overrated congruent negative facial emotions (P < .001), but not incongruent facial emotions. Accordingly, ratings of congruent and incongruent emotions highly discriminated between bvFTD and MDD patients, ranging from area under the curve (AUC) = 93% to AUC = 98%. Further, an almost complete discrimination (AUC = 99%) was achieved by contrasting the 2 rating types. In contrast, Alzheimer’s disease dementia patients perceived emotions similarly to healthy participants, indicating no impact of cognitive impairment on rating scores.
Conclusions: Our congruent and incongruent facial emotion intensity rating task allows a detailed assessment of facial emotion perception in patient populations. By using this simple task, we achieved an almost complete discrimination between bvFTD and MDD, potentially helping improve the diagnostic certainty in early bvFTD.
J Clin Psychiatry 2018;79(1):16m11342
To cite: Chiu I, Piguet O, Diehl-Schmid J, et al. Facial emotion recognition performance differentiates between behavioral variant frontotemporal dementia and major depressive disorder. J Clin Psychiatry. 2018;79(1):16m11342.
To share: https://doi.org/10.4088/JCP.16m11342
© Copyright 2018 Physicians Postgraduate Press, Inc.
aMemory Clinic, University of Basel & University Center for Medicine of Aging, Felix Platter Hospital, Basel, Switzerland
bDepartment of Neurology, University Hospital Basel, Basel, Switzerland
cNeuroscience Research Australia and the University of New South Wales, Sydney, Australia
dARC Centre of Excellence in Cognition and Its Disorders, Sydney, Australia
eDepartment of Psychiatry, Technische Universitפt München, Munich, Germany
fCenter for Affective, Stress and Sleep Disorders, Psychiatric Clinics (UPK), University of Basel, Basel, Switzerland
gCenter of Old Age Psychiatry, Psychiatric Clinics (UPK), University of Basel, Basel, Switzerland
hDepartment of Mathematics and Technology, University of Applied Sciences Koblenz, Koblenz, Germany
*Corresponding author: Marc Sollberger, MD, Memory Clinic, University Center for Medicine of Aging Basel, Felix Platter-Hospital, Burgfelderstrasse 101, 4012 Basel, Switzerland ([email protected]).
Behavioral variant frontotemporal dementia (bvFTD) is a clinical syndrome, characterized by early and prominent behavioral changes,1 resulting from predominantly anterior frontal and temporal lobe dysfunctions due to heterogeneous underlying neurodegenerative processes.2 After Alzheimer’s disease (AD) dementia, it is the most common diagnosis in patients with early-onset dementia with an estimated prevalence of 15.4 (95% CI, 9.1-24.3) per 100,000 individuals 45 to 64 years of age.3,4 Biomarkers are often absent or unreliable in early bvFTD, and diagnosis largely depends on clinical criteria.1 In its early stage, bvFTD is frequently misdiagnosed as psychiatric disorders, most of all major depressive disorder (MDD),5,6 or, vice versa, psychiatric disorders are falsely diagnosed as bvFTD.7 Accordingly, new clinical tools are needed to improve the differentiation between bvFTD and psychiatric disorders that mimic bvFTD such as MDD.
As shown in recent studies, emotion processing paradigms have emerged as a promising approach to tackle this challenge.8,9 In a previous study,9 we showed that bvFTD and MDD patients dissociate in rating negative facial emotions; indeed, while bvFTD patients underrated negative emotions, MDD patients overrated negative emotions compared to healthy participants. Importantly, bvFTD and MDD patients might differ not only in perceiving the intensity of the primary emotion (ie, the type of emotion we recognize as such), but also in perceiving the intensities of facial emotions that are present but are not the primary emotion. Such detailed assessment of emotion perception may not only further improve the differentiation between bvFTD and MDD but also could be used for a more comprehensive testing of facial emotion perception in patient populations.
The general consensus is that bvFTD patients are impaired in recognizing emotions, particularly negative emotions.10 To the best of our knowledge, no study to date has systematically examined whether bvFTD patients are impaired in discriminating a primary facial emotion from nonprimary facial emotions. Our ability to discriminate primary from nonprimary emotions is critical for successfully navigating the social world as facial expressions are often reflecting not just single (“pure”) emotional states, but a combination of emotional states at varying intensities.11-13 For example, one might not just perceive anger in another person’s facial expression, but anger as the primary emotion in combination with disgust, fear, and surprise at lower intensities. Confusing an emotion such as anger with fear or disgust likely results in interpersonal misunderstandings and conflicts. Small-sample studies in patients with brain lesions that involve regions that are commonly affected in bvFTD suggest that bvFTD patients would be prone to such confusion.14-18
In contrast, MDD patients are generally not impaired in recognizing emotions (for review, see Bourke et al,19 but see also Dalili et al20) and even seem to show a mood-congruent (negative) response bias, especially toward sadness.19,21 In other words, they tend not only to perceive negative emotions but also to perceive positive, neutral, and ambiguous emotions more negatively.19,21
On the basis of these previous studies, we hypothesized that bvFTD and MDD patients would show divergent emotion perception deficits: whereas bvFTD patients would confuse emotions, MDD patients would show a negative response bias for both primary and nonprimary emotions. Further, we hypothesized that these divergent emotion perception deficits would highly discriminate between bvFTD and MDD patients.
To test our hypotheses, we extended our previously established congruent emotion intensity rating task9 by adding incongruent facial emotion stimuli. In this novel task, participants were asked not only to rate the intensity of the primary facial stimulus (congruent rating),9 but also to rate the same facial stimulus on the intensity of each of the other 5 basic emotions (incongruent ratings).
METHODS
Participants
Ninety-seven participants were recruited for the study: 25 patients with probable bvFTD,1 20 inpatients with MDD according to DSM-IV criteria,22 21 patients with probable Alzheimer’s disease (AD) dementia,23 and 31 age-matched healthy participants (HP). Percentages in bvFTD patients fulfilling each of the core diagnostic symptoms were as follows: behavioral disinhibition (75%), apathy (92%), loss of empathy (83%), perseverative or compulsive/ritualistic behavior (71%), hyperorality and dietary changes (63%), and neuropsychological profile suggestive of bvFTD (21%). Two bvFTD patients had a coexisting motor neuron disease. Two MDD patients (10%) had a bipolar disorder, and another 2 MDD patients (10%) were diagnosed with their first episode of depression. Magnetic resonance imaging of the brain was performed in 85% of the MDD patients, showing no signs of neurodegeneration, but signs of moderate to severe microangiopathy in 2 older patients. AD dementia patients were included as a clinical comparison group to test whether impairments in perceiving the intensity of facial emotions are influenced by cognitive deficits.

- Misdiagnosis of early behavioral variant frontotemporal dementia with major depressive disorder is not uncommon and results in potential inappropriate treatment of patients.
- Our congruent and incongruent facial emotion intensity rating task appears to help differentiate between these 2 clinical syndromes.
- Given its simple administration, this task is well suited for a fine-grained assessment of emotion perception in patient populations.
Clinical diagnosis was confirmed at a follow-up assessment, in general 1 year after the baseline assessment (mean time period of 12.73 ± 1.54 months in dementia patients and 13.08 ± 2.08 months in MDD patients) either in the clinic or, if they were unable to attend, by a standardized phone interview. Clinical diagnosis was confirmed in all patients, with the exception of 1 AD dementia patient and 1 MDD patient who could not be reached.
For details on recruitment, medication, and neuropsychological assessment of the participants, see Methods in the Supplementary Material.
The study was approved by the local ethics committee, and all study procedures complied with the Declaration of Helsinki.
Procedure
Congruent and incongruent emotion intensity rating task. Participants were tested on a congruent and incongruent emotion intensity rating task comprising the 6 basic emotions from the morphed facial stimuli of the Facial Expressions of Emotion—Stimuli and Tests.24 The 6 basic emotions (anger, disgust, fear, sadness, surprise, and happiness) were presented for both sexes at threshold intensity levels, as previously established.25 Each facial stimulus was shown 6 times consecutively and was rated on its congruent emotion (eg, rating on sadness when sad face is shown) and its 5 incongruent basic emotions (eg, rating on anger, disgust, fear, surprise, and happiness when sad face is shown) (Supplementary eFigure 1). Stimuli were presented in 2 pseudorandomized versions, whereby no type of emotion was shown more than twice in a row. A total of 72 stimuli (6 emotions ×— 2 sexes ×— 6 ratings) were administered.
Stimuli were displayed on a 15-inch laptop computer using E-Prime 1.2 software (Psychology Software Tools, Pittsburgh, Pennsylvania). Above each facial stimulus, the emotion label and a 7-point intensity rating scale ranging from “no emotion” (1) to “maximum emotion” (7) appeared on the screen. Participants were instructed to rate the intensity of each emotion by pointing at the appropriate circle on the screen. Stimuli remained on the screen until participants provided an answer. The task was untimed, and no feedback was given. Before the experiment started, 4 practice trials were conducted to familiarize the participant with the procedure and clarify any questions. For more details on procedure and stimuli, see the Supplementary Material.
HP were retested 12 months after baseline to evaluate test-retest reliability.
Data Analyses
Within-group discrimination of the primary emotion from other emotions. To examine the perception of primary versus other emotions within each group, linear mixed-effects models were calculated using package nlme in R.26
Comparison of congruent rating scores between groups. We created mean congruent rating scores for each of the 6 emotions (eg, Sadness_Con = mean of congruent ratings of female and male facial stimuli portraying sadness [ie, 2 ratings]) and 2 congruent composite scores (ie, Congruent Emotion Total score [ET_Con = mean of congruent ratings over all 6 emotions, ie, 2 ×— 6 = 12 ratings] and Congruent Negative Emotion Total score [NET_Con = mean of congruent ratings over the 4 negative emotions, ie, 2 ×— 4 = 8 ratings]).
To achieve normal distribution and homogeneity of variance, all congruent scores were transformed by square transformation (y = x2), with the exception of Surprise_Con, which did not require transformation.
Comparison of incongruent rating scores between groups. Similarly, we calculated 2 types of incongruent rating scores for each of the 6 emotions (eg, Sadness_5Incon = mean of the 5 incongruent ratings of female and male facial stimuli portraying sadness [ie, 2 ×— 5 = 10 ratings] and Sadness_3Incon = mean of the 3 incongruent negative ratings of female and male facial stimuli portraying sadness [ie, 2 ×— 3 = 6 ratings]) and 2 incongruent composite scores (ie, Incongruent Emotion Total score [ET_5Incon = mean of the 5 incongruent ratings over all 6 emotions, ie, 2 ×— 5 ×— 6 = 60 ratings] and Incongruent Negative Emotion total score [NET_3Incon = mean of the 3 incongruent negative ratings over the 4 negative emotions, ie, 2 ×— 3 ×— 4 = 24 ratings]).
To achieve normal distribution and homogeneity of variance, all incongruent scores were transformed by square root transformation (y = −šx), except for the 4 incongruent negative emotion scores (Anger_3Incon, etc), which did not require transformation.
Comparison of contrast rating scores (mean of congruent scores minus mean of incongruent scores) between groups. To examine the ability to discriminate the primary facial emotion from other emotions, we calculated 2 types of contrast scores for each emotion (eg, Sadness_5Contrast = mean of congruent ratings on facial stimuli portraying sadness (ie, 2 ratings) minus mean of the respective incongruent ratings [ie, 2 ×— 5 = 10] and Sadness_3Contrast = mean of congruent ratings on facial stimuli portraying sadness (ie, 2 ratings) minus mean of the respective incongruent negative ratings [ie, 2 ×— 3 = 6]).
Similarly, we calculated 2 contrast composite scores (ie, Contrast Emotion Total score [ET_ Contrast = mean of the 2 ×— 6 congruent ratings minus mean of the 2 ×— 30 incongruent ratings] and Contrast Negative Emotion Total score [NET_ Contrast = mean of the 2 ×— 4 congruent ratings of the negative emotions minus mean of the 2 ×— 12 respective incongruent negative ratings]). All contrast scores showed normal distribution and homogeneity of variance, except for Happiness_5Contrast, which was transformed by square transformation (y = x2).
Analyses of covariance (ANCOVAs) with each demographic variable—ie, age, sex, or education—as a single predictor in the model showed that all 3 variables were significant predictors of the outcome variables. Therefore, they were included as covariates in the models. ANCOVA results of group differences (P < .05) were followed by post hoc comparisons of adjusted means using the Tukey-Kramer option for general linear models in Stata 13.1 software (StataCorp LP, College Station, Texas).
Discriminatory power of congruent, incongruent, and contrast composite scores between bvFTD and MDD (n = 45). To explore which of the 6 composite scores discriminates best between bvFTD and MDD patients, we performed receiver operating characteristic (ROC) curve analyses in the bvFTD and MDD subsample (for more details, see Chiu et al9). Logistic regression analyses with each demographic variable as a single predictor in the model showed that education significantly predicted 2 of the 6 outcome variables, ie, ET_Con and NET_Con. Therefore, all ROC analyses were adjusted for education.
RESULTS
Analyses of covariance followed by Tukey-Kramer post hoc analyses indicated that AD dementia patients had a significantly lower education level than HP (P < .05; Table 1). Based on Mini-Mental State Examination27 and Consortium to Establish a Registry for Alzheimer’s Disease—Neuropsychological Assessment Battery28 scores, bvFTD and AD dementia patients were cognitively more impaired than MDD patients. Importantly, bvFTD and AD dementia patients showed no signs of depression (Table 1).
Within-Group Discrimination of the Primary Emotion From Other Emotions
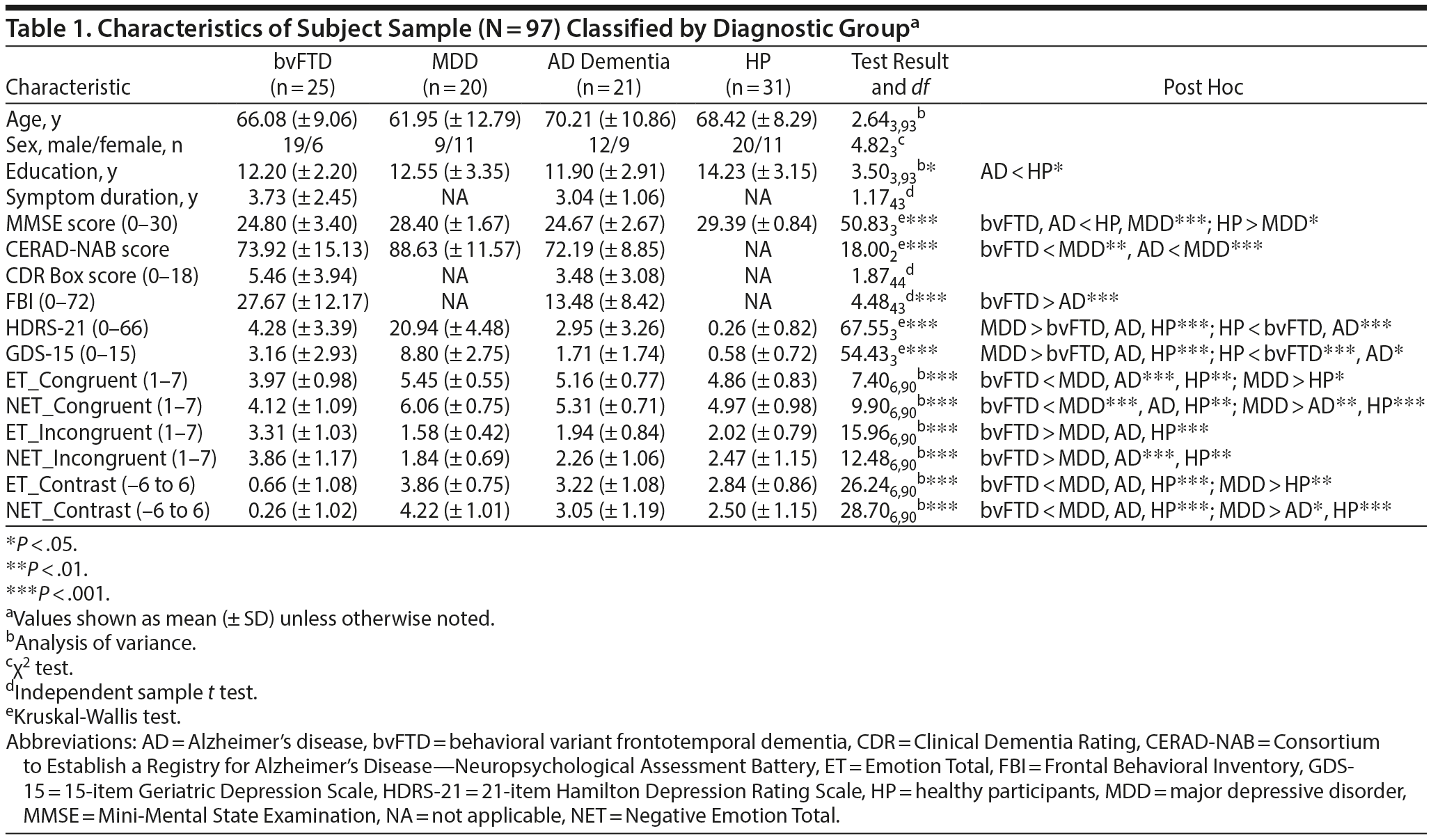
As demonstrated by the means of estimates and 95% confidence intervals (ie, lower and upper estimates) of the emotion scores from the linear mixed-effects models, bvFTD patients confused negative emotions with each other, ie, they were unable to discriminate the primary negative emotion from other negative emotions (Figure 1). However, they were able to discriminate between the valences of emotions, ie, they discriminated any of the 4 primary negative emotions from the positive emotion happiness and, vice versa, the primary positive emotion happiness from the 4 negative emotions. In contrast, they were unable to discriminate the ambiguous emotion surprise from any other emotion.
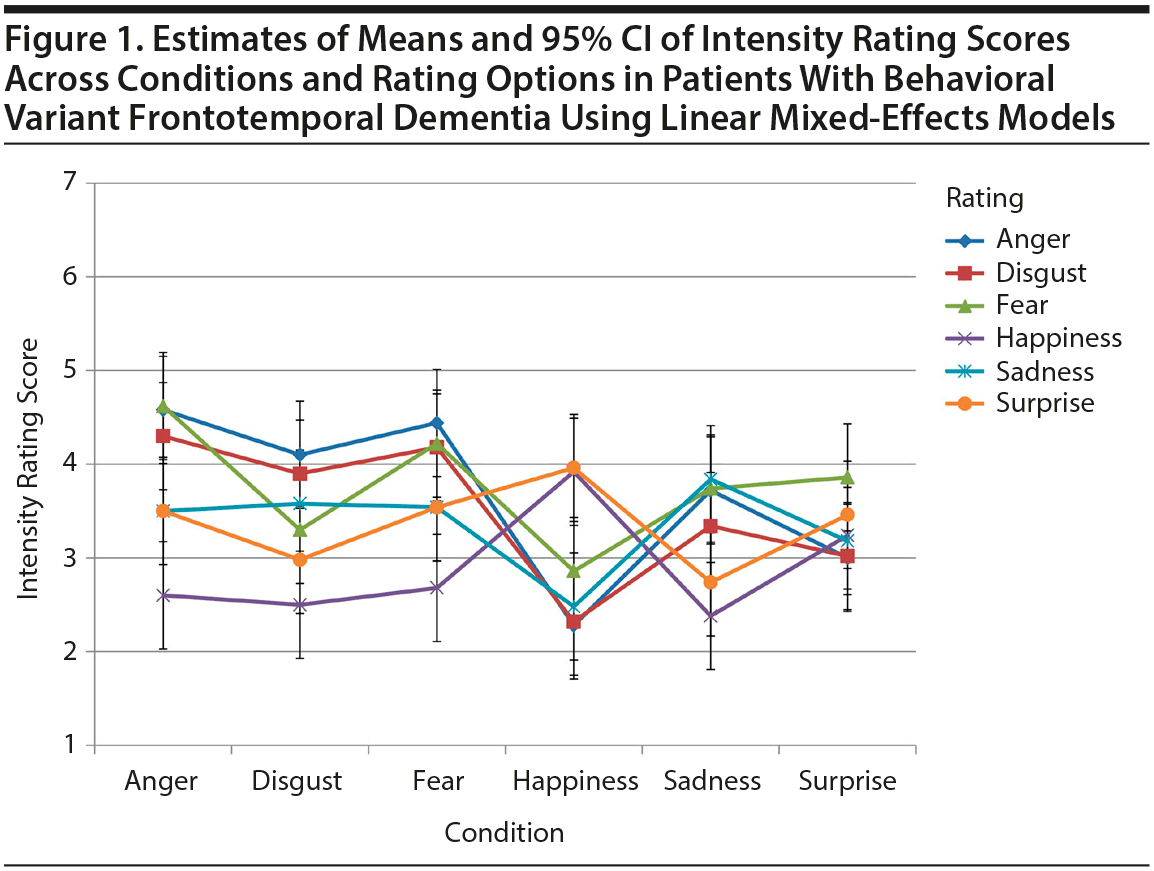
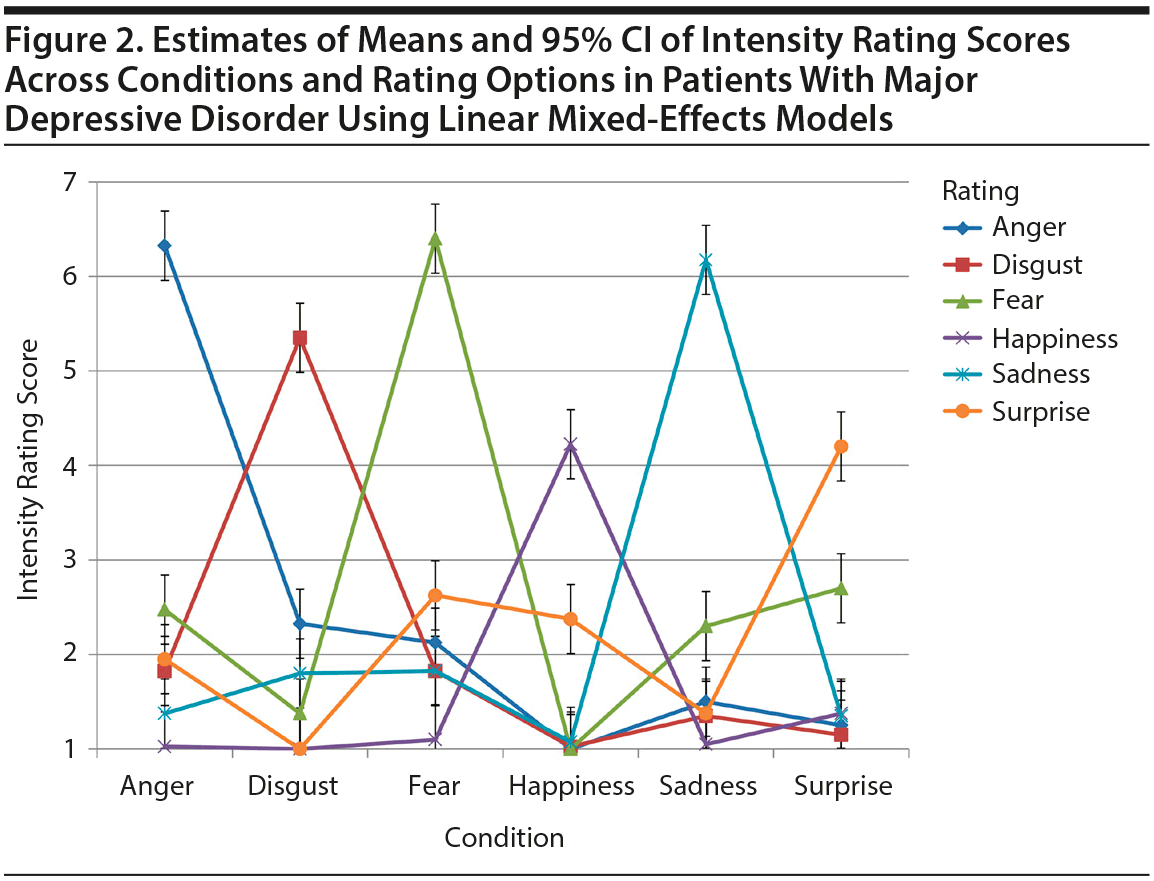
In contrast to bvFTD patients, HP as well as MDD and AD dementia patients were able to discriminate each primary emotion from the other emotions (Figures 2 and 3, Supplementary eFigure 2). Notably, MDD patients’ congruent rating scores (ie, ratings of the primary emotion) of negative emotions were higher than congruent rating scores of the 2 non-negative emotions happiness and surprise (Figure 2).
Comparison of Congruent Rating Scores Between Groups
ANCOVAs revealed significant main effects of group for all 6 congruent emotion scores ranging from F3,90 = 4.04 (Happiness_Con) to F3,90 = 15.88 (Fear_Con). Post hoc analyses showed that anger (t90 = −2.82), happiness (t90 = −3.05), and surprise (t90 = −3.26) congruent scores were significantly lower in bvFTD patients than in HP, whereas fear (t90 = 4.61) and sadness (t90 = 4.80) congruent scores were significantly higher in MDD patients than in HP.
All 4 negative congruent emotion scores were significantly higher in MDD patients than in bvFTD patients, ranging from t90 = 4.22 (Disgust_Con) to t90 = 6.78 (Fear_Con). In contrast, the 2 non-negative congruent emotion scores did not differ between the 2 groups. Unlike bvFTD and MDD patients, AD dementia patients showed similar congruent emotion ratings as HP (Supplementary eFigure 2, Figure 3).
We also found significant main effects of group for the 2 congruent composite scores, ie, ET_Con (F3,90 = 14.35) and NET_Con (F3,90 = 18.84) (Table 1). Post hoc analyses showed that both scores dissociated between bvFTD and MDD patients (ET_Con [t90 = 6.17], NET_Con [t90 = 7.44]), ie, scores were lower (ET_Con [t90 = −3.90], NET_Con [t90 = −3.36]) in bvFTD patients than in HP but higher (ET_Con [t90 = 2.69], NET_Con [t90 = 4.50]) in MDD patients than in HP (Table 1, Supplementary eFigure 3).
Comparison of Incongruent Rating Scores Between Groups
ANCOVAs revealed significant main effects of group for all incongruent emotion and composite scores, ranging from F3,90 = 9.63 (Disgust_3Incon) to F3,90 = 17.90 (ET_5Incon). Post hoc comparisons revealed that all incongruent scores were significantly higher in the bvFTD group than in the other groups (Table 1, Supplementary eFigure 4).
Comparison of Contrast Scores Between Groups
ANCOVAs revealed significant main effects of group for each contrast emotion score, ranging from F3,90 = 6.92 (Happiness_5Contrast) to F3,90 = 34.24 (Fear_5Contrast). Apart from Disgust_3Contrast, all contrast negative emotion scores dissociated between bvFTD and MDD patients, ranging between t90 = 7.95 (Sadness_3Contrast) and t90 = 9.62 (Fear_5Contrast).
Significant main effects of group were also found for the 2 contrast composite scores, ranging from F3,90 = 43.61 (ET_Contrast) to F3,90 = 45.41 (NET_Contrast) (Table 1). Both scores dissociated between bvFTD and MDD patients (ET_Contrast [t90 = 10.32], NET_Contrast [t90 = 10.97]; Table 1, Supplementary eFigure 5).
Apart from Fear_3Contrast (t90 = 2.72), all contrast scores were comparable between AD dementia patients and HP.
Twenty-eight HP (90%) were retested after a mean of 11.40 ± 0.65 months, revealing a test-retest reliability of r = 0.78 (95% CI, 0.57-0.89) for the 72 emotional stimuli. Test-retest reliabilities of each composite score ranged from r = 0.75 (95% CI, 0.53-0.88; ET_Con) to r = 0.88 (95% CI, 0.75-0.94; NET_Incon) (Supplementary Material).
Discriminatory Power of the Congruent, Incongruent, and Contrast Composite Scores Between bvFTD and MDD
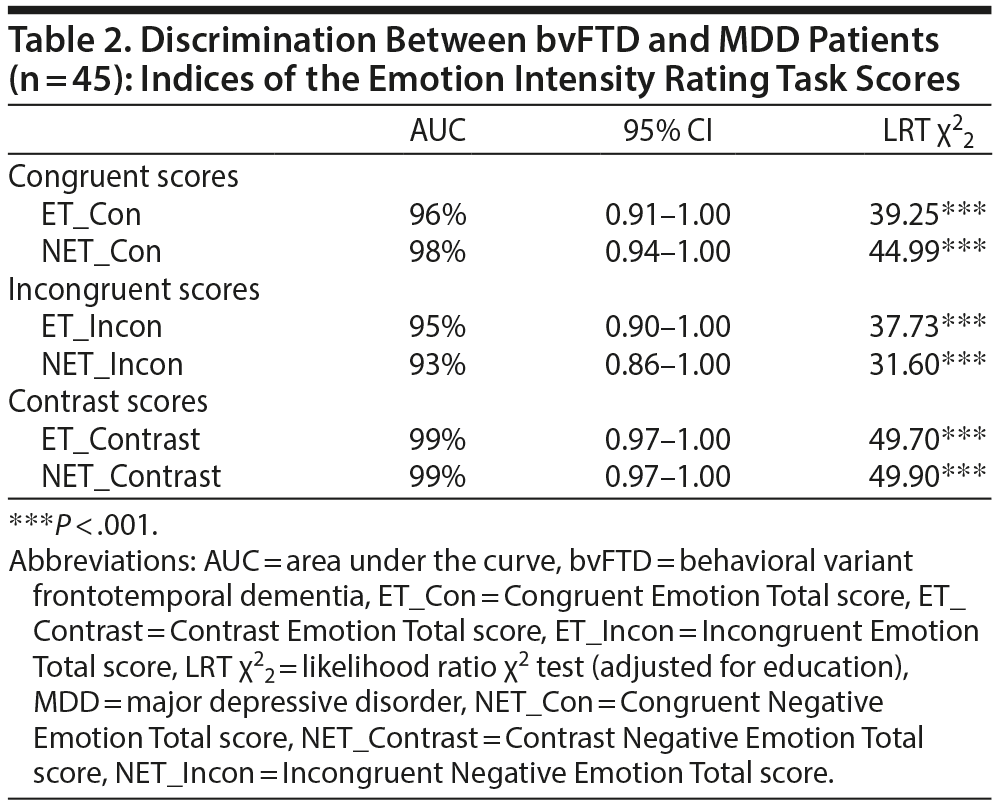
All 6 composite scores showed a high discriminatory power ranging from AUC = 93% (NET_Incon) to AUC = 99% (contrast composite scores) (Table 2). The scores did not significantly differ in their discriminatory power.
DISCUSSION
Using a novel congruent and incongruent facial emotion intensity rating task for a fine-grained assessment of facial emotion perception, we found varying emotion perception patterns in bvFTD and MDD patients, allowing their almost complete discrimination: bvFTD patients were impaired not only in perceiving the intensity of primary emotions, but also in discriminating the primary emotion from other emotions of the same valence. Contrary to bvFTD patients, MDD patients perceived primary negative emotions more intensively than healthy participants, while they discriminated adequately among the different emotions. Notably, AD dementia patients, who showed a similar degree of cognitive dysfunction to that of bvFTD patients overall (albeit with differences in their cognitive profiles), perceived emotions similarly to HP. These latter findings suggest that impaired emotion perception in bvFTD patients was unlikely to be influenced by cognitive dysfunction.
Similarly to our previous study with the congruent facial emotion intensity rating task,9 bvFTD and MDD patients differed in perceiving primary negative facial emotions overall in that bvFTD patients underrated primary negative emotions overall, whereas MDD patients overrated these emotions overall compared to HP.
While bvFTD patients underrated the intensity of primary emotions, they overrated nonprimary emotions, suggesting confusion in perceiving facial emotions. Indeed, they failed in discriminating the primary emotion from the other emotions. Yet, they were still able to discriminate between emotional valences, ie, while they failed in discriminating primary negative emotions from other negative emotions, they were able to distinguish primary negative emotions from the positive emotion happiness. Similarly, they were able to discriminate the positive emotion happiness from any negative emotion but failed to discriminate the ambiguous emotion surprise, containing both negative and positive valences, from any other emotion. These findings fit with previous small-sample lesion studies in patients whose brain lesions (ie, amygdala, ventrolateral, and ventromedial prefrontal cortices) are parts of networks that are commonly affected in bvFTD patients.14-16,18 For example, in 5 patients with bilateral amygdala damage, perception of sadness, but not happiness, was confused with other basic emotions.16 Similarly, another lesion study reported misperception of negative emotions in 9 patients with left ventrolateral prefrontal cortex lesions.18 In contrast to our bvFTD patients, all of these patients did not underrate primary emotions, probably because of more focal and minor involvement of relevant brain regions.
In contrast to bvFTD patients, HP and MDD and AD dementia patients adequately discriminated emotions from each other. MDD patients, however, differed from AD dementia patients and HP in perceiving more intensively primary negative emotions. These findings support the mood-congruent processing bias reported in our previous study9 as well as in other clinical and neuroimaging studies.19,29,30 However, contrary to our hypothesis, MDD patients showed a response bias neither toward sadness nor toward negative emotions overall in rating nonprimary emotions (eg, they perceived neither more sadness nor more disgust, fear, and sadness altogether in an angry face than HP). These findings differ from previous studies in MDD patients (for review, see Bourke et al19) and may reflect differences in stimulus characteristics (eg, type of stimuli, degree of emotional intensity, types of emotions), testing paradigms (eg, discrimination task versus intensity rating task, timed vs untimed stimulus presentation), and statistical analyses used to examine response biases.
bvFTD and MDD patients strongly differed not only in perceiving the intensity of primary emotions as measured by the congruent emotion rating scores, but also in perceiving the intensity of nonprimary emotions as measured by the incongruent emotion rating scores. Accordingly, the resulting congruent and incongruent composite rating scores discriminated highly between bvFTD and MDD, reaching AUC scores of 93% and above. Consequently, by contrasting congruent and incongruent composite scores, ie, by looking at participants’ ability in discriminating primary emotions from nonprimary emotions, we reached an almost complete discrimination between bvFTD and MDD, reaching AUC scores of 99%.
In addition to their relevance for the better differentiation between early bvFTD and MDD, our findings may contribute to a better understanding of the breakdown in behavior in bvFTD. Given the importance of adequately perceiving facial emotions in social interactions,31 it is plausible that the misperception of facial emotions by bvFTD patients contributes to the many misunderstandings in their social environment, impacting negatively on interpersonal relationships. It is therefore crucial to raise awareness of bvFTD patients’ deficits in emotion processing through specific psychoeducation to help understand and adjust to the patients’ behavior.
The study is limited by the fact that MDD patients showed few cognitive deficits and typical signs of depression such as rumination, allowing clear separation of them from bvFTD patients, even without data on emotion perception. For proof of concept, our facial emotion rating task should be administered in MDD patients showing more phenotypical overlaps with bvFTD patients. Further, no postmortem pathological confirmation was available in our bvFTD sample. Consequently, we cannot exclude the possibility that in some patients bvFTD was not caused by frontotemporal lobar degeneration pathologies.
In routine clinical care, our task might be limited by its relatively long time of administration (~30 minutes). As negative emotion scores alone discriminated bvFTD and MDD patients equally well as all emotion scores together, one might consider testing only the 4 negative emotions (32 stimuli instead of 72) or the 4 negative emotions plus happiness (50 stimuli). Negative emotions and happiness may be worthwhile to administer together to test whether the individual confuses emotions of the same valence or independently of the valences.
In conclusion, we found that bvFTD patients confuse emotions of the same valence, whereas MDD patients showed preserved facial emotion perception apart from a negative (mood-congruent) bias for primary negative emotions. By using rating scores on different aspects of emotion perception, we achieved an almost complete discrimination between bvFTD and MDD. We suggest that our simple emotion intensity rating task, potentially in a shortened version, is well suited for a fine-grained assessment of emotion perception in clinical routine, both for diagnostic purposes and for follow-up assessments.
Submitted: November 15, 2016; accepted March 16, 2017.
Published online: January 16, 2018.
Potential conflicts of interest: All authors report no conflict of interest.
Funding/support: Drs Chiu and Sollberger are supported by the Research Fund of the University of Basel (grant number DMS2223), the Velux Foundation (grant number 831), and the Swiss Alzheimer’s Association. Dr Piguet is supported by a National Health and Medical Research Council of Australia Senior Research Fellowship (grant number APP1103258).
Role of the sponsor: The sponsors were not involved in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; and the preparation, review, or approval of the manuscript.
Previous presentation: Poster presented at the 10th International Conference on Frontotemporal Dementias; August 31-September 2, 2016; Munich, Germany.
Acknowledgments: The authors thank U. Schreiter-Gasser, MD; Ch. Koch, MD; T. Baumann, MD; H. Pihan, MD; and J.-M. Annoni, MD, of the Swiss Memory Clinic Association (Switzerland) and M. Muehlhauser, MD, and Th. Sauer, MD, of the Psychiatric Clinics of the University of Basel (Switzerland) for their help with patient recruitment. All persons have no conflicts of interest to declare.
Supplementary material: See accompanying pages.
REFERENCES
1. Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134(Pt 9):2456-2477. PubMed doi:10.1093/brain/awr179
2. Mackenzie IR, Neumann M, Bigio EH, et al. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol. 2010;119(1):1-4. PubMed doi:10.1007/s00401-009-0612-2
3. Harvey RJ, Skelton-Robinson M, Rossor MN. The prevalence and causes of dementia in people under the age of 65 years. J Neurol Neurosurg Psychiatry. 2003;74(9):1206-1209. PubMed doi:10.1136/jnnp.74.9.1206
4. Vieira RT, Caixeta L, Machado S, et al. Epidemiology of early-onset dementia: a review of the literature. Clin Pract Epidemol Ment Health. 2013;9:88-95. PubMed doi:10.2174/1745017901309010088
5. Woolley JD, Khan BK, Murthy NK, et al. The diagnostic challenge of psychiatric symptoms in neurodegenerative disease: rates of and risk factors for prior psychiatric diagnosis in patients with early neurodegenerative disease. J Clin Psychiatry. 2011;72(2):126-133. PubMed doi:10.4088/JCP.10m06382oli
6. Velakoulis D, Walterfang M, Mocellin R, et al. Frontotemporal dementia presenting as schizophrenia-like psychosis in young people: clinicopathological series and review of cases. Br J Psychiatry. 2009;194(4):298-305. PubMed doi:10.1192/bjp.bp.108.057034
7. Shinagawa S, Catindig JA, Block NR, et al. When a little knowledge can be dangerous: false-positive diagnosis of behavioral variant frontotemporal dementia among community clinicians. Dement Geriatr Cogn Disord. 2016;41(1-2):99-108. PubMed doi:10.1159/000438454
8. Bertoux M, Delavest M, de Souza LC, et al. Social cognition and emotional assessment differentiates frontotemporal dementia from depression. J Neurol Neurosurg Psychiatry. 2012;83(4):411-416. PubMed doi:10.1136/jnnp-2011-301849
9. Chiu I, Piguet O, Diehl-Schmid J, et al. Dissociation in rating negative facial emotions between behavioral variant frontotemporal dementia and major depressive disorder. Am J Geriatr Psychiatry. 2016;24(11):1017-1027. PubMed doi:10.1016/j.jagp.2016.06.011
10. Bora E, Velakoulis D, Walterfang M. Meta-analysis of facial emotion recognition in behavioral variant frontotemporal dementia: comparison with Alzheimer disease and healthy controls. J Geriatr Psychiatry Neurol. 2016;29(4):205-211. PubMed doi:10.1177/0891988716640375
11. Matsumoto D, Keltner D, Shiota MN, et al. Facial expressions of emotion. In: Lewis M, Haviland-Jones JM, Feldman Barrett L, eds. Handbook of Emotions. 3rd ed. New York, NY: The Guilford Press; 2008:211-234.
12. Watson D, Stanton K. Emotion blends and mixed emotions in the hierarchical structure of affect [published online ahead of print January 30, 2017]. Emot Rev. doi:10.1177/1754073916639659
13. Larsen JT, McGraw AP. The case for mixed emotions. Soc Personal Psychol Compass. 2014;8(6):263-274. doi:10.1111/spc3.12108
14. Seeley WW, Crawford RK, Zhou J, et al. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62(1):42-52. PubMed doi:10.1016/j.neuron.2009.03.024
15. Bickart KC, Brickhouse M, Negreira A, et al. Atrophy in distinct corticolimbic networks in frontotemporal dementia relates to social impairments measured using the Social Impairment Rating Scale. J Neurol Neurosurg Psychiatry. 2014;85(4):438-448. PubMed doi:10.1136/jnnp-2012-304656
16. Adolphs R, Tranel D. Impaired judgments of sadness but not happiness following bilateral amygdala damage. J Cogn Neurosci. 2004;16(3):453-462. PubMed doi:10.1162/089892904322926782
17. Heberlein AS, Padon AA, Gillihan SJ, et al. Ventromedial frontal lobe plays a critical role in facial emotion recognition. J Cogn Neurosci. 2008;20(4):721-733. PubMed doi:10.1162/jocn.2008.20049
18. Tsuchida A, Fellows LK. Are you upset? distinct roles for orbitofrontal and lateral prefrontal cortex in detecting and distinguishing facial expressions of emotion. Cereb Cortex. 2012;22(12):2904-2912. PubMed doi:10.1093/cercor/bhr370
19. Bourke C, Douglas K, Porter R. Processing of facial emotion expression in major depression: a review. Aust N Z J Psychiatry. 2010;44(8):681-696. PubMed doi:10.3109/00048674.2010.496359
20. Dalili MN, Penton-Voak IS, Harmer CJ, et al. Meta-analysis of emotion recognition deficits in major depressive disorder. Psychol Med. 2015;45(6):1135-1144. PubMed doi:10.1017/S0033291714002591
21. Naranjo C, Kornreich C, Campanella S, et al. Major depression is associated with impaired processing of emotion in music as well as in facial and vocal stimuli. J Affect Disord. 2011;128(3):243-251. PubMed doi:10.1016/j.jad.2010.06.039
22. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition. Washington, DC: American Psychiatric Associaton; 1994.
23. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263-269. PubMed doi:10.1016/j.jalz.2011.03.005
24. Young A, Perrett D, Calder A, et al. Facial Expressions of Emotion—Stimuli and Tests (FEEST). 1.0 ed. Bury St Edmunds, England: Thames Valley Test Company; 2002.
25. Chiu I, Gfrorer RI, Piguet O, Berres M, Monsch AU, Sollberger M. “Now I see it, now I don’ t”: determining threshold levels of facial emotion recognition for use in patient populations. J Int Neuropsychol Soc. 2015:21(7):568-572.
26. R Development Core Team. R: A Language and Environment for Statistical Computing [computer program]. Vienna, Austria: The R Foundation for Statistical Computing. 2016.
27. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. PubMed doi:10.1016/0022-3956(75)90026-6
28. Ehrensperger MM, Berres M, Taylor KI, et al. Early detection of Alzheimer’s disease with a total score of the German CERAD. J Int Neuropsychol Soc. 2010;16(5):910-920. PubMed doi:10.1017/S1355617710000822
29. Savitz J, Drevets WC. Bipolar and major depressive disorder: neuroimaging the developmental-degenerative divide. Neurosci Biobehav Rev. 2009;33(5):699-771. PubMed doi:10.1016/j.neubiorev.2009.01.004
30. Stuhrmann A, Suslow T, Dannlowski U. Facial emotion processing in major depression: a systematic review of neuroimaging findings. Biol Mood Anxiety Disord. 2011;1(1):10. PubMed doi:10.1186/2045-5380-1-10
31. Elfenbein HA, Marsh AA, Ambady N, et al. Emotional intelligence and the recognition of emotion from facial expressions. In: Feldman Barrett L, Salovey P, eds. The Wisdom in Feeling: Psychological Processes in Emotional Intelligence. New York, NY: Guilford Press; 2002:37-59.
Editor’s Note: We encourage authors to submit papers for consideration as a part of our Focus on Geriatric Psychiatry section. Please contact Jordan F. Karp, MD, at [email protected], or Gary W. Small, MD, at [email protected].
This PDF is free for all visitors!