ABSTRACT
Schizophrenia and bipolar I disorder (BP-I) are chronic, disabling psychiatric illnesses marked by high morbidity, elevated mortality, and functional deterioration, often exacerbated by poor adherence to oral antipsychotic medications. Long-acting injectable (LAI) antipsychotics were developed to address adherence challenges and have demonstrated clinical benefits including reduced non-adherence and relapse rates, fewer hospitalizations, and improved functioning and quality of life, and reduced mortality risk. Among available LAIs, aripiprazole offers a unique pharmacologic profile as the only partial dopamine agonist available in an LAI formulation. Aripiprazole monohydrate LAI is available as a once monthly and a once-every-two-month formulation. In this consensus panel report, five psychiatric experts convened to evaluate the pharmacokinetic properties, safety, efficacy, and clinical utility of aripiprazole monohydrate LAIs in the treatment of schizophrenia and BP-I. The panel focused particularly on the more recently approved once-every-2-month ready-to-use formulation. This article summarizes the evidence reviewed by the panel and highlights key considerations for optimizing the use of aripiprazole monohydrate LAIs in clinical practice to enhance treatment outcomes in patients with schizophrenia and BP-I.
J Clin Psychiatry 2025;86(2):plunlai2424ah1
Published Online: June 13, 2025.
To Cite: Goldberg JF, Achtyes ED, Correll CU, et al. Optimizing treatment with aripiprazole monohydrate: pharmacokinetic advantages of long-acting injectable formulations, a consensus panel report. J Clin Psychiatry. 2025;86(2):plunlai2424ah1.
To Share: https://doi.org/10.4088/JCP.plunlai2424ah1
© 2025 Physicians Postgraduate Press, Inc.
See more Academic Highlights in this series: Part 2 | Part 3
This Academic Highlights section of The Journal of Clinical Psychiatry presents the highlights of the virtual consensus panel meeting “Optimizing Treatment with Aripiprazole Monohydrate: Pharmacokinetic Advantages of Long-Acting Injectable Formulations, A Consensus Panel Report,” which was held September 9, 2024.
The meeting was chaired by Joseph F. Goldberg, MD, Department of Psychiatry, Icahn School of Medicine at Mount Sinai, New York, New York. The faculty were Eric D. Achtyes, MD, MS, Department of Psychiatry, Western Michigan University Homer Stryker M.D. School of Medicine, Kalamazoo, Michigan; Christoph U. Correll, MD, Department of Psychiatry, Zucker Hillside Hospital, Glen Oaks, New York; Department of Psychiatry and Molecular Medicine, Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Hempstead, New York; Department of Child and Adolescent Psychiatry, Charité Universitätsmedizin, Berlin, Germany; Martha Sajatovic, MD, Department of Psychiatry and Department of Neurology, Case Western Reserve University School of Medicine, Cleveland, Ohio; and Stephen R. Saklad, PharmD, BCPP, Division of Pharmacotherapy and Translational Science, College of Pharmacy, The University of Texas at Austin, San Antonio, Texas.
Financial disclosures appear at the end of the article.
This evidence-based peer-reviewed Academic Highlights was prepared by Healthcare Global Village, Inc. The panel meeting for the development of this article was organized and funded by Otsuka Pharmaceutical Europe Ltd and H. Lundbeck A/S. Both companies also funded article processing charges, medical writing, editorial, and other assistance. Editorial support was provided by Angel Rodriguez, PharmD, MS, BCACP, Banner Medical LLC, and Healthcare Global Village with funding by Otsuka Pharmaceuticals Europe Ltd & H. Lundbeck A/S. The sponsors performed a courtesy review for medical accuracy. The opinions expressed herein are those of the faculty and do not necessarily reflect the views of Healthcare Global Village, Inc., the publisher, or the commercial supporters. This article is distributed by Otsuka Pharmaceutical Europe Ltd and H. Lundbeck A/S for educational purposes only.
Schizophrenia and bipolar I disorder (BP-I) are severe chronic psychiatric disorders associated with significant morbidity, driven by poor health behaviors, cognitive dysfunction, and substantial reductions in functioning and quality of life.1–3 Individuals with schizophrenia experience a markedly reduced life expectancy, estimated at 15 to 20 years shorter than the general population, along with elevated risks of physical comorbidities, substance use disorders, suicide, and accidental injury.1,4–7 The disease course is often characterized by recurrent relapses that interrupt periods of partial or full remission, contributing to progressive deterioration in social and occupational functioning.8 Patients with BP-I face similarly high physical and psychiatric morbidity and mortality risk, contributed to by recurrent episodes of mania and depression that significantly impact quality of life and functioning.4–7 Early symptom recurrence, high relapse rates, and poor adherence to daily oral medications complicate long-term management of the disorder and are associated with higher rates of hospitalization and suicide.2
Antipsychotic use has been identified as a significant factor contributing to reducing all-cause mortality in patients diagnosed with schizophrenia, and antipsychotic adherence versus nonadherence is associated with a significant decrease in death from cardiovascular causes, decreased risk of developing metabolic syndrome, and increased adherence to cardiometabolic medications.1,9–12 Similarly, nonadherence represents a significant barrier to the treatment success of BP-I and schizophrenia and is associated with poor clinical outcomes, functional impairments, impaired quality of life, and increased health-service utilization.13,14 Unfortunately, poor adherence to antipsychotic medications in psychiatric disorders like schizophrenia and BP-I has been estimated to be as high as 40%–50%.14,15 Among patients with BP-I, those prescribed multiple antipsychotic medications experience particularly high rates of rehospitalizations due to difficulties with polypharmacy and medication nonadherence, or irregularities in adherence.16
First developed for the treatment of schizophrenia in the 1960s, LAI antipsychotics were designed to address concerns with nonadherence but were historically viewed as a last resort treatment option.17 Advances in the development of second-generation antipsychotic LAIs have led to the approval of multiple agents, formulations, and dosing frequencies,18,19 which collectively have led to lower risks of hospitalizations or symptom relapse; improved treatment effectiveness, clinical efficacy, safety and tolerability, and patient functioning and quality of life; and reduced mortality risk when compared to oral antipsychotics.1,11,12,20–23 Second-generation LAIs are now recommended for use in patients diagnosed with schizophrenia if they have a history of poor or uncertain adherence or based on patient preference, and are growing in use for the treatment of BP-I.24–28
In this consensus panel meeting, 5 psychiatric experts reviewed the available evidence on the pharmacokinetics, safety, and efficacy of aripiprazole monohydrate LAIs for the treatment of schizophrenia and BP-I, with a special focus on the most recently approved aripiprazole monohydrate once-every-2-month ready-to-use (Ari 2MRTU) 960 mg formulation. This Academic Highlights article summarizes their discussion of the evidence and presents the panel’s conclusions.
Methods
In October 2024, a panel of psychiatrists with expertise in psychopharmacology, the clinical treatment of schizophrenia and BP-I, and antipsychotic prescribing convened to discuss the pharmacokinetic properties, safety, efficacy, and clinical utility of aripiprazole monohydrate LAIs in the treatment of patients living with schizophrenia or BP-I. The focus of the panel was on aripiprazole monohydrate formulations only, as aripiprazole lauroxil, a prodrug of aripiprazole that is also available as an LAI has been reviewed comprehensively in a very recent publication.29 The panel was chaired by Joseph F. Goldberg, MD, and included panelists Eric D. Achtyes, MD; Christoph U. Correll, MD; Martha Sajatovic, MD; and Stephen R. Saklad, PharmD. The consensus panel meeting was held virtually with facilitated discussion. The panel analyzed publicly available clinical trial data and shared their perspectives to reach a consensus on utilization of aripiprazole monohydrate LAIs in treatment considerations for schizophrenia and BP-I. This article presents the consensus findings from the panel discussion.
Impact of LAI Antipsychotics for Schizophrenia and BP-I
LAI antipsychotics are designed to improve adherence by providing sustained therapeutic plasma concentrations between doses. The duration of action between injections depends on the specific formulation, dosage, and pharmacokinetic profile of the individual medication.19 The use of LAI antipsychotics reduces the need for patients to remember taking daily oral medications, allows for less variable plasma concentrations by bypassing hepatic first-pass metabolism, less peak-trough variability, and are associated with reduced risk of breakthrough symptoms.8,30 Patients treated with LAIs are 67% less likely to interrupt treatment compared to those using oral antipsychotics.12 Moreover, increased adherence with LAI antipsychotics is associated with improved adherence to other medications, which significantly supports improved patient outcomes and public health by decreasing the healthcare burden of frequent and extensive psychiatric hospital admissions.10,14,31
Additionally, peak-to-trough plasma concentrations vary widely across antipsychotics and can be affected by differences in dosing and formulation.18,19 Oral medications requiring once or twice daily dosing have larger fluctuations in peak-to-trough concentrations compared to LAIs and may negatively impact clinical response and tolerability. While peak concentration is an indicator of the severity of adverse effects, 32 withdrawal symptoms can be common following missed doses of oral antipsychotics but can also be associated with trough plasma concentrations despite good adherence. 33
The second-generation antipsychotics available in LAI formulations include aripiprazole, olanzapine, paliperidone, and risperidone; among these, only aripiprazole is a partial D2 receptor agonist, while the others are primarily D2 receptor antagonists.34 With a broad range of LAI antipsychotics now available, clinicians and patients benefit from flexible dosing schedules, ranging from biweekly to once every 6 months. 35 These therapies differ in efficacy, safety profiles, and dosing frequency, all of which are driven by each medication’s individual pharmacokinetic and pharmacodynamic properties.18,19 The optimal LAI would offer robust efficacy with a favorable safety and tolerability profile and a convenient dosing regimen.
Distinctive Profile of Aripiprazole Monohydrate LAIs
Aripiprazole, brexpiprazole, and cariprazine are distinctive due to their pharmacologic profiles as partial dopamine D2 receptor agonists.36,37 Among the dopamine receptor partial agonists, aripiprazole is the only agent available in an LAI formulation, making its mechanism of action unique within the class of LAIs. Aripiprazole exhibits a complex receptor binding profile, acting as a partial agonist at dopamine D2, D3, and serotonin 5-HT1A receptors and as an antagonist at serotonin 5-HT2A receptors.35,38 Aripiprazole binds to dopamine receptors with a higher affinity than endogenous dopamine, functioning similarly to traditional dopamine antagonists by reducing dopamine activity where it is excessive (eg, dorsal striatum39) while preserving dopamine function in areas where it is needed (eg, the frontal lobe, mesolimbic pathway, sensorimotor striatum, and tuberoinfundibular tract).40 This mechanism helps minimize adverse events like extrapyramidal effects, prolactin elevation, and sexual dysfunction while promoting better functional outcomes and patient motivation.41–43
Aripiprazole is metabolized by cytochrome P450 (CYP) 2D6 and CYP3A4. Oral aripiprazole formulations have a bioavailability of 87% and reach peak plasma concentrations within 3 to 5 hours, with an average elimination half-life of 75 hours.44 The prolonged absorption of the active ingredient from the injection site is an example of “flip-flop” pharmacokinetics where the absorption rate controls the duration of the LAI and the elimination rate controls the peak concentrations. Both aripiprazole monohydrate LAI formulations significantly extend the duration of therapeutic plasma concentrations compared with available oral options. Aripiprazole once-monthly 400 mg (AOM 400) formulation reaches peak plasma concentrations within 4 days following monthly deltoid injections and 5–7 days following monthly gluteal injections, has a half-life of 30 to 46.5 days, and reaches steady-state concentrations by the fourth dose, regardless of the injection site.19
The aripiprazole 2-month ready-to-use (Ari 2MRTU) 960 mg formulation was developed to extend the therapeutic plasma concentrations achieved by AOM 400 mg from 1 month to 2 months, primarily by increasing the administered dose to allow for every-other-month dosing.35,45 Ari 2MRTU 960 has linear pharmacokinetics in the approved dose range. Steady-state aripiprazole exposures were reached by the fourth dose. Plasma exposures at steady state were compared between Ari 2MRTU 960 and AOM 400. The average plasma concentrations of aripiprazole were 263 ng/mL for Ari 2MRTU 960 and 280 ng/mL for AOM 400, well above the targeted minimum predicted threshold of 95 ng/mL. The Cmax of aripiprazole was 342 ng/mL and 344 ng/mL for Ari 2MRTU 960 and AOM 400, respectively. The median steady-state terminal elimination half-life was 29.4 days for both Ari 2MRTU 960 and Ari 2MRTU 720.35,46,47
The target dose of the 2-month formulation is 960 mg for most patients, while a 720 mg dose is available in the case of tolerability concerns, in patients who are known CYP2D6 poor metabolizers, or in patients who are taking concomitant strong inhibitors of CYP3A4 or CYP2D6.35,46
Safety of Aripiprazole Monohydrate LAIs
Traditional antipsychotics carry a risk of adverse events, including drug-induced parkinsonism (DIP), weight gain, prolactin elevations, sexual and motor adverse events, sedation, insomnia, and withdrawal effects.8 Tolerability for most medications, including antipsychotic drugs, decreases with age, starting around age 50, with neurological effects, including DIP, becoming more prevalent. As a result, aging patients are at an increased risk of fractures and osteoporosis-related fragility fractures due to physical restlessness, physical aggression, and increased fall risk from medication-related sedation, psychomotor impairment, bradykinesia, or postural hypotension.48 Adverse effects have been identified as a priority for patients diagnosed with schizophrenia or BP-I when deciding whether to take a prescribed medication, with the majority of patients reporting weight gain, physical restlessness, and somnolence as important adverse events of current treatments.49
The oral formulation of aripiprazole has long been considered well-tolerated and favorable compared to a number of other atypical antipsychotics, as it has a minimal propensity for clinically significant weight gain and metabolic disruption.50,51 A systematic review of the efficacy and safety of aripiprazole in the treatment of people with schizophrenia found that aripiprazole showed similar efficacy to that of both first-generation and second-generation antipsychotic medications but was associated with significantly lower weight gain, disruptions in glucose, triglyceride and cholesterol concentrations when compared to other antipsychotics including risperidone and olanzapine.52 Additionally, aripiprazole was associated with fewer reports of DIP or extrapyramidal effects and decreased use of antiparkinsonian medications when compared to risperidone.52
Beyond its favorable cardiometabolic profile, aripiprazole is less sedating compared with other antipsychotics, due to its lack of histaminergic activity, and its partial dopamine agonism avoids prolactin elevations often noted with other antipsychotics.48,53 This prolactin-sparing characteristic of aripiprazole may avoid sexual adverse events caused by prolactin elevation and may reduce the risk of hip fractures associated with long-term hyperprolactinemia, compared to other antipsychotics.48,54
LAI antipsychotics offer several clinical advantages over oral formulations, including improved tolerability profiles, reduced risk of drug-drug interactions, and more consistent plasma concentrations, which may enhance treatment adherence and therapeutic outcomes.8,22,23 The panel highlighted that since many adverse events associated with antipsychotics are peak-trough variation-related, LAIs may be associated with lower rates of adverse events as they provide flattened peak-trough variability and more consistent plasma drug concentrations.19 For instance, LAIs may help mitigate withdrawal symptoms common with missed doses of oral antipsychotics.33 Although late-emerging adverse events can theoretically occur with any LAI antipsychotic, the panel noted they are rarely observed in clinical practice with aripiprazole monohydrate LAIs due to the tolerability of the medication. Weight gain was the only noted exception, as this generally cumulative adverse effect can also potentially be driven by behavioral factors like improved patient motivation and well-being.55 Tardive dyskinesia, a potentially irreversible movement disorder associated with long-term antipsychotic use, is also a consideration, though the risk is significantly lower with second-generation agents like aripiprazole compared to first-generation antipsychotics.56
In the treatment of both schizophrenia and BP-I, AOM 400 has demonstrated a similar safety profile to oral aripiprazole.33,57,58 In the comparative study of AOM 400 and Ari 2MRTU 960, adverse events were similar between both formulations, with the most frequently reported being weight gain and injection-site pain, and no new safety findings were reported. 45
Panel Consensus Statement #1
“Reduced Side Effects and Enhanced Functionality: Aripiprazole monohydrate LAIs are associated with fewer adverse motor and metabolic effects compared to first-generation antipsychotic formulations and some second-generation antipsychotics, due to the partial dopamine agonist mechanism, stable plasma concentrations, lack of withdrawal, and improved adherence. Aripiprazole monohydrate LAIs support improved social and occupational functionality, offering benefits in quality of life.”
Efficacy of Aripiprazole Monohydrate LAIs
The efficacy of AOM 400 was established in placebo-controlled clinical trials for both schizophrenia and BP-I.33,57,59 Additionally, several studies of patients diagnosed with schizophrenia and BP-I have demonstrated that AOM 400 is as effective as oral aripiprazole in reducing relapses, hospitalizations, and mortality rates.58,60–63 Compared to oral antipsychotic medication regimens, aripiprazole monohydrate LAIs reduce the need for additional psychotropic medications, stabilizing patients and minimizing polypharmacy, both of which are associated with fewer adverse events and improved adherence.19,64 Despite improved adherence compared to oral therapies, adherence challenges can persist with LAIs due to a variety of factors, including cognitive impairment/medication forgetting and psychiatric comorbidities such as substance use disorders, which can lead to delayed or missed doses.65,66 Therefore, flexibility in the timing of dose administration for of either aripiprazole monohydrate LAI is a critical advantage in supporting its efficacy.35
The efficacy of Ari 2MRTU 960 was initially evaluated utilizing a bridging non-inferiority strategy that compared the pharmacokinetic profile of Ari 2MRTU 960 to AOM 400 in clinically stable patients diagnosed with schizophrenia or BP-I. Patients were enrolled to receive Ari 2MRTU 960 mg every 56 days or AOM 400 mg every 28 days for a 32-week treatment period. Mean plasma concentrations of aripiprazole were similar and comparable between the two formulations, with trough plasma concentrations remaining above the minimum efficacy threshold of 95 ng/mL at all points in the study following the initiation protocol. Efficacy assessments at week 32 found minimal differences between treatment groups and that trial subjects remained stable throughout the study treatment period.45 Two secondary analyses of this study were then conducted for patients diagnosed with schizophrenia and BP-I as separate subgroups, with findings mirroring those of the combined study, indicating similar efficacy between Ari 2MRTU 960 and AOM 400 for each patient population studied.67–70
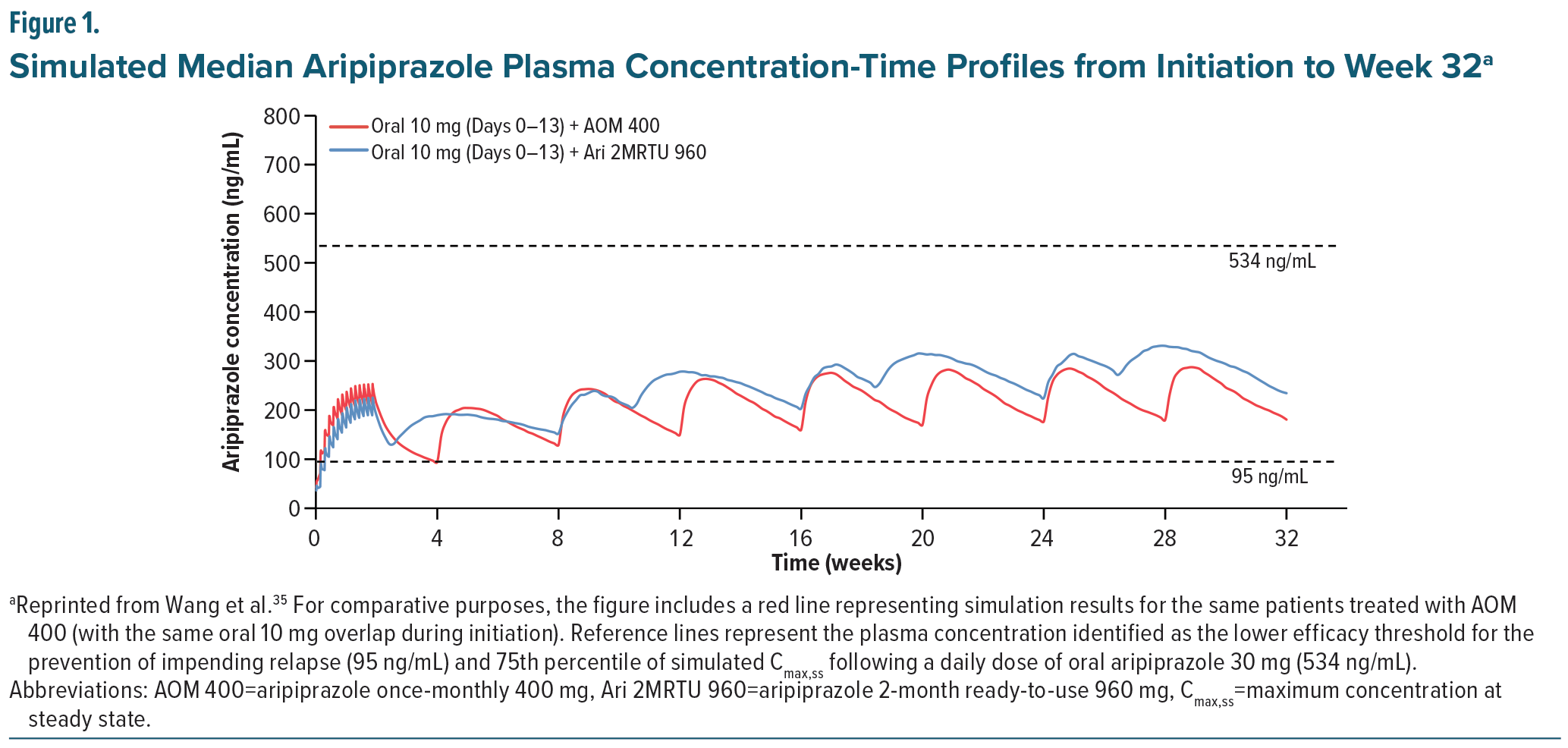
Efficacy data from the Ari 2MRTU 960 non-inferiority study were incorporated into a previously validated population-based pharmacokinetic model to characterize its pharmacokinetics and explore aspects of its dosing using multiple simulations.35,45 In the tested models, plasma concentrations of AOM 400 mg and Ari 2MRTU 960 mg were comparable from initiation to week 32. As shown in Figure 1, simulations demonstrated an Ari 2MRTU 960 pharmacokinetic exposure profile comparable to that of AOM 400 but with more consistent plasma drug concentrations and a longer dosing interval.35 Additionally, the Ari 2MRTU 960 formulation offers additional patient flexibility as patients have a 2-week window in which to get their next injection once steady-state plasma concentrations have been reached; however the panel emphasized that patients and providers should be diligent about scheduling injections as close to every 60 days as possible.46
Panel Consensus Statement #2
“Improved Adherence and Outcomes Through LAIs: LAIs, including aripiprazole monohydrate LAIs, address challenges of poor adherence in patients diagnosed with schizophrenia and BP-I. The consistent and comparable pharmacokinetic profiles of AOM 400 and Ari 2MRTU 960 ensure smoother plasma concentrations, similar safety profiles, and the benefit of reducing relapses, hospitalizations, and mortality rates compared to oral medications.”
Initiation and Management of ARI 2MRTU
Pharmacokinetic Interactions
As aripiprazole is primarily metabolized by CYP2D6 and 3A4, drug-drug interactions or drug-gene interactions involving these enzymes exist.71 While AOM 400 is the recommended maintenance dose for monthly administration, doses can be adjusted (eg, to 300 mg or 200 mg) based on individual metabolism, the presence of enzyme inhibitors, pharmacogenetic factors, or tolerability issues. If both CYP2D6 and CYP3A4 inhibitors are present, the dose can be reduced to as low as 160 mg monthly.
The FDA labeling of Ari 2MRTU advises utilizing the 720 mg dose in patients who are poor CYP2D6 metabolizers, or in those taking concomitant medications that are strong CYP2D6 and CYP3A4 inhibitors.72 Patients taking concomitant medications which inhibit both CYP2D6 and CYP3A4 should avoid use of Ari 2MRTU. The panel discussion highlighted the low likelihood of encountering a patient requiring dose adjustment based on their CYP enzyme polymorphism and concluded that routine pharmacogenetic testing before initiating aripiprazole therapy is not generally necessary, regardless of initiating either the oral or LAI formulations of aripiprazole monohydrate.
Initiation Strategy
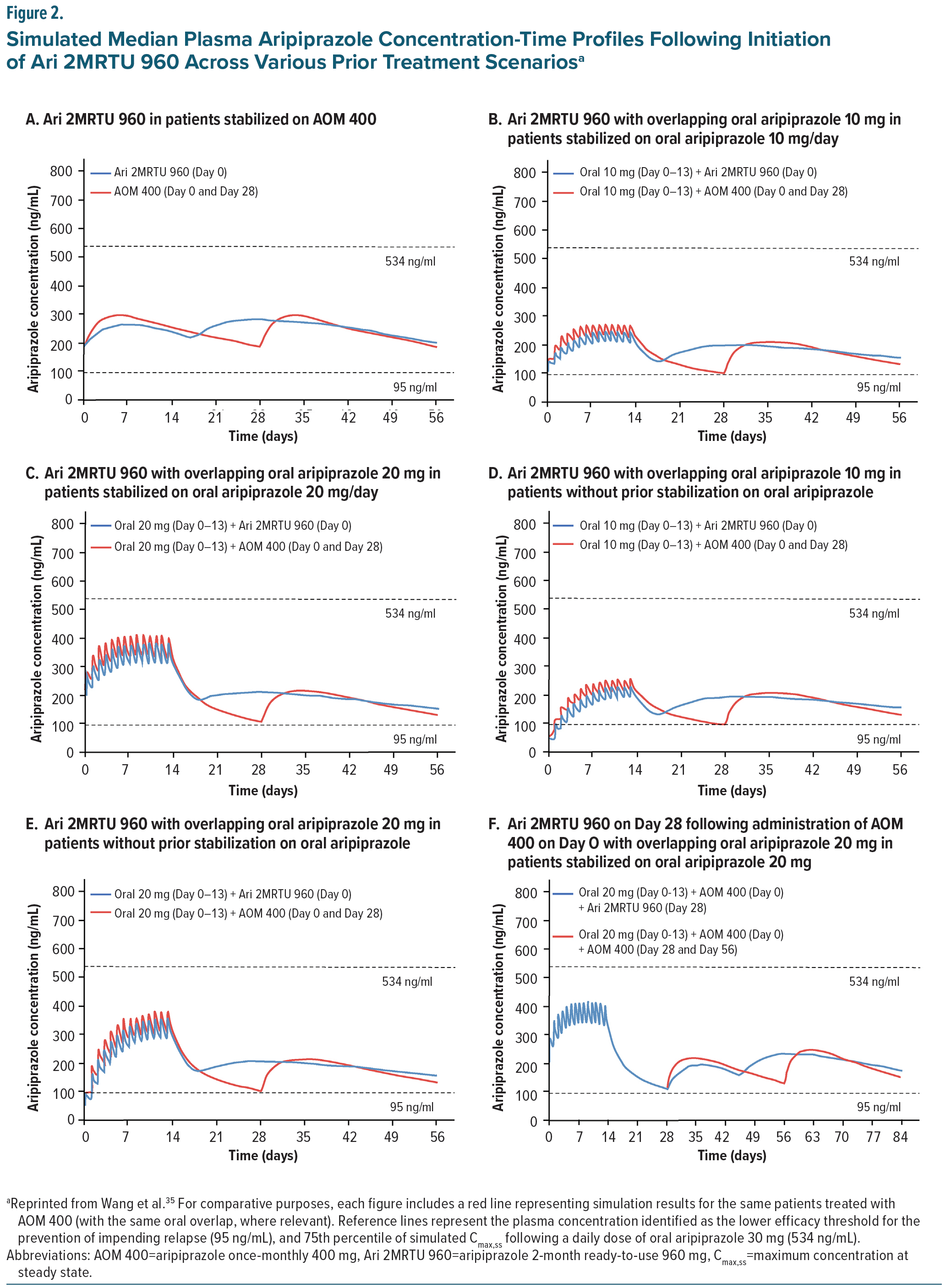
The initiation strategy of AOM 400 and Ari 2MRTU 960 varies depending on the clinical context and needs of the patient and clinician. Due to the long absorption half-life of the aripiprazole monohydrate LAIs, patients can begin oral and injectable therapy simultaneously and discontinue the oral component once steady-state levels are achieved. 35 Multiple initiation strategies exist and may be equally appropriate and effective.18 The transition rate from oral aripiprazole will also be affected by the severity of the disease and the care environment (eg, inpatient vs outpatient; good vs limited social support).18 Multiple treatment strategies were modeled based on real-world clinical scenarios (Figure 2).35 Some clinicians may prefer several weeks of oral aripiprazole to confirm tolerability and establish efficacy, while others favor a faster transition, particularly in acute care settings where rapid stabilization is critical. Regardless of the approach, all modeled strategies, whether transitioning directly from AOM 400 to Ari 2MRTU 960 (Figure 2A) or first establishing tolerability with various doses of oral aripiprazole (Figure 2B–E), resulted in aripiprazole plasma concentrations well above the minimum therapeutic threshold of 95 ng/mL. Figure 2F illustrates an additional strategy, initiating both AOM 400 and oral aripiprazole on day 0, then transitioning to Ari 2MRTU 960 on day 28.35
AOM 400 was initially approved with a single injection start strategy, where patients received a single injection of AOM 400 into a gluteal or deltoid muscle and continued oral aripiprazole (10 mg or 20 mg) for 14 days to achieve therapeutic plasma concentrations. More recently, a two-injection start (TIS) strategy has been approved in the United States, Canada, and Europe, allowing initiation without the 14-day oral supplementation period. 73,74 The TIS strategy may be particularly advantageous for patients at risk for adherence-related relapse or suboptimal treatment outcomes.75,76 In patients with established tolerability to oral aripiprazole, the TIS strategy involves 2 separate injections of AOM 400 administered at different sites (gluteal or deltoid), along with a single 20 mg oral dose of aripiprazole. 75 The TIS strategy achieves therapeutic plasma concentrations without the need for additional oral dosing, potentially reducing adherence-related undertreatment and relapse during initiation, decreasing the length of acute hospital stays, minimizing the burden on caregivers to supervise oral medication, and expanding treatment options for patients and providers. 75 A recently published survey76 of physicians (62.8%) and nurses (29.2%) in Germany, Italy, and the United Kingdom who had prescribed or administered AOM 400-TIS at least 3 times in patients with schizophrenia identified the most common reasons for initiating AOM 400 with the TIS strategy as a history of poor adherence (85.1%), relapse (59.6%), and high hospitalization rates (48.9%). The primary prescribing goals reported were improving adherence (70.2%), preventing relapse (69.1%), and enhancing patient quality of life (62.8%). Most healthcare providers agreed or strongly agreed that AOM 400-TIS was easy to administer (79.8%) and had a safety and tolerability profile comparable to the 1-injection start regimen (69.1%), with the majority expressing satisfaction with patient outcomes (84.0%).76
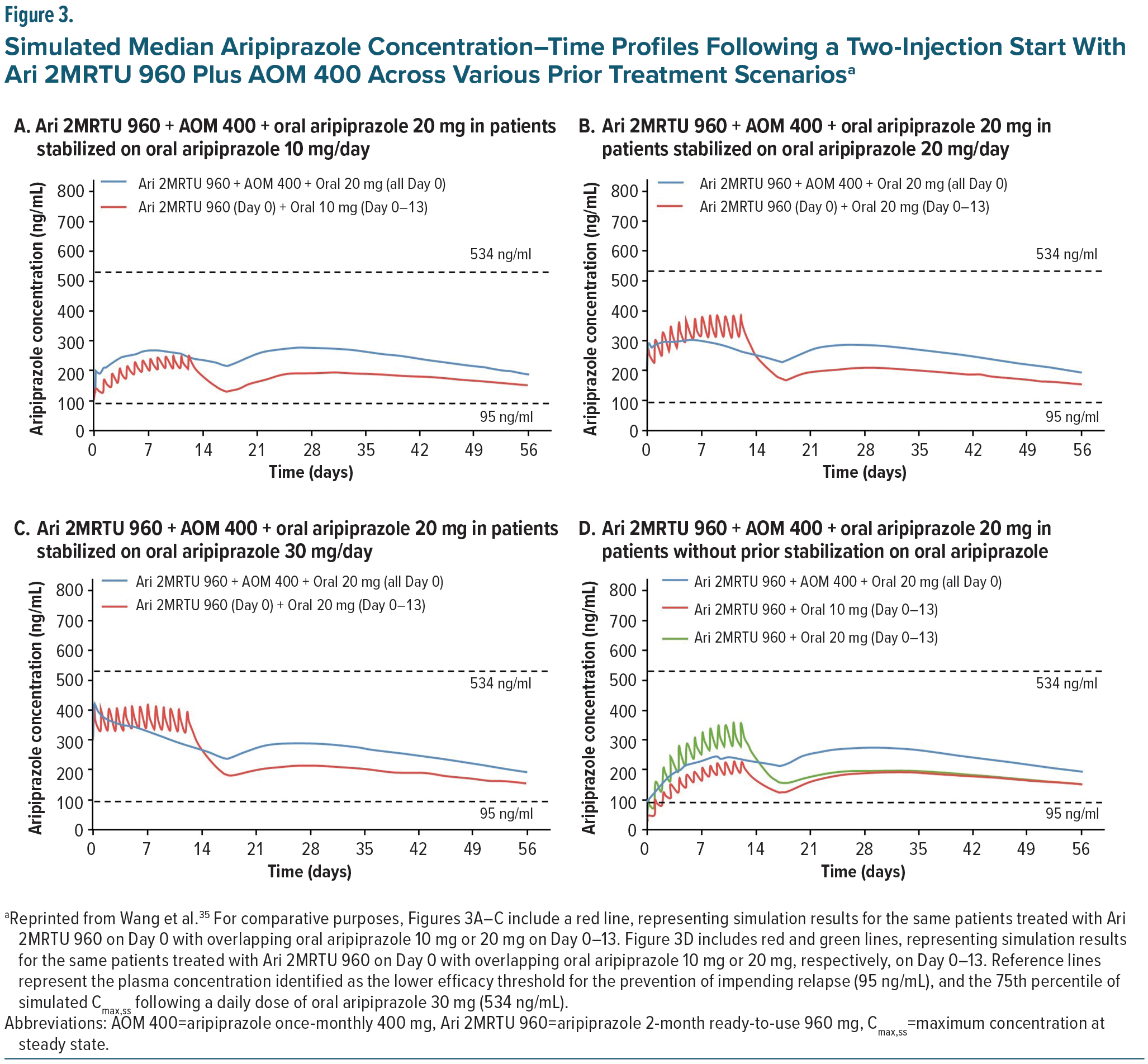
For Ari 2MRTU 960, multiple treatment initiation strategies were evaluated by Wang et al using population-based pharmacokinetic modeling.35 As shown in Figure 3, Ari 2MRTU 960 reaches therapeutic plasma concentrations (above 95 ng/mL) from the first dose, regardless of initiation strategy, ensuring early efficacy without waiting for full steady-state (~5 half-lives). Additionally, across all treatment initiation scenarios evaluated, Ari 2MRTU 960 resulted in plasma concentrations of aripiprazole similar to those achieved with AOM 400. Of the dosing strategy scenarios evaluated, patients who received a TIS strategy consisting of a single dose of oral aripiprazole 20 mg in combination with Ari 2MRTU 960 mg gluteal injection and AOM 400 mg deltoid or gluteal injection on day 1 had more stable plasma concentrations of aripiprazole, compared with the 3 other start strategies analyzed (Figure 3).35
Plasma Concentration Monitoring
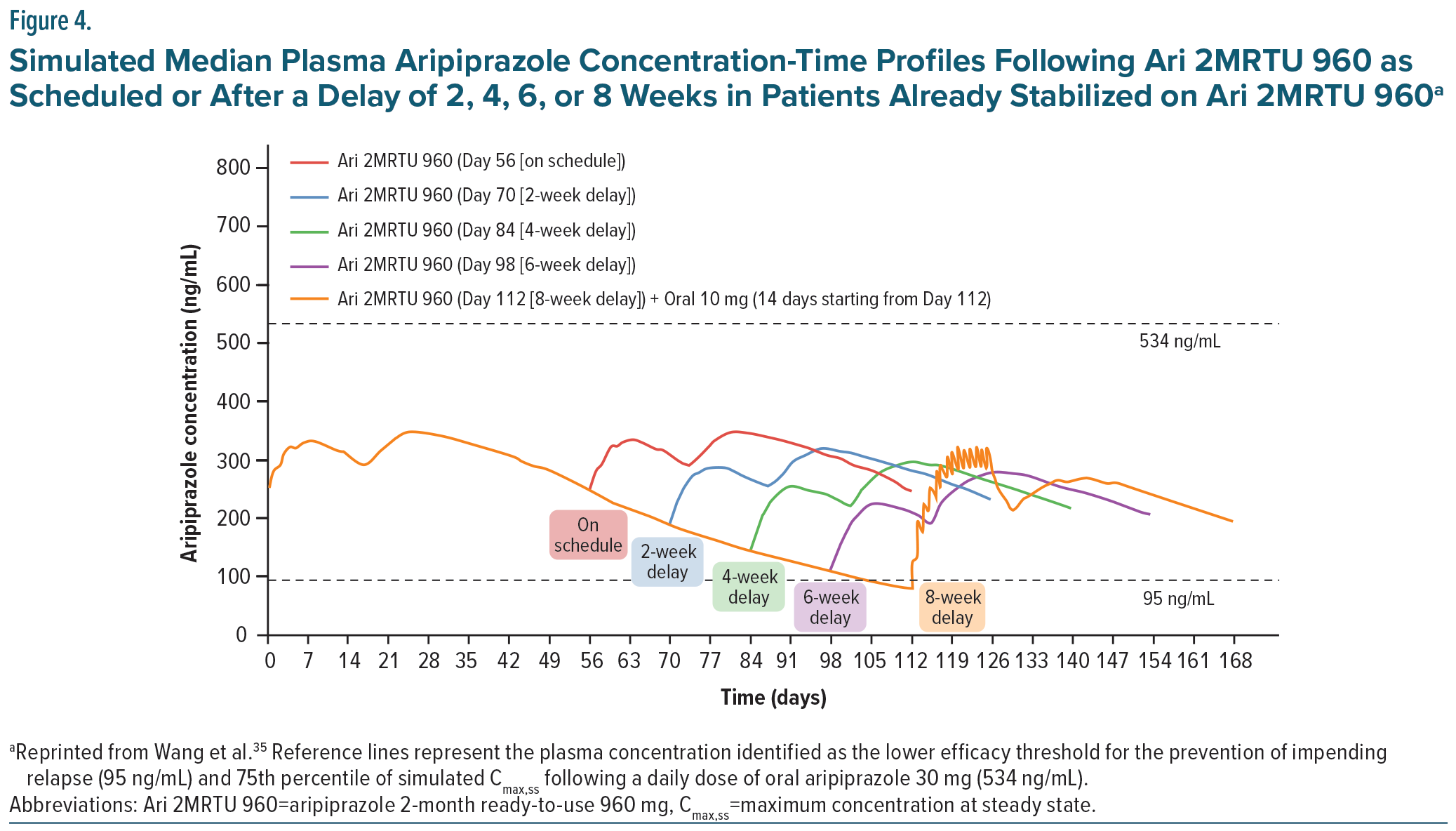
Plasma concentrations achieved with Ari 2MRTU 960 have demonstrated more consistent efficacy with an improved risk-benefit profile compared to AOM 400, offering patients the potential for improved sustained efficacy, with every-other-month injections compared to once monthly injections.35 In patients receiving AOM 400, injections must be received within a window of 2 days each month to maintain consistent, steady-state plasma concentrations. 58 However, in patients taking Ari 2MRTU 960, this window can be increased to 2 weeks when needed and still maintain therapeutic plasma concentrations well above the 95 ng/mL threshold (Figure 4). This allows far more patient flexibility in scheduling their injections, which should translate to improved plasma concentration stability and medication adherence.35 However, such delays can accumulate and should not be repeated, as they can lead to diluted blood concentrations and breakthrough psychosis.77 In one study, using a population-based pharmacokinetic model, missed/delayed doses were simulated to assess the effects of imperfect adherence with every 2-month dosing interval and demonstrated that imperfect adherence with up to a 6-week delay was able to maintain therapeutic concentrations of aripiprazole.35 The dosing recommendations in the FDA label state that if more than 8 weeks and less than 14 weeks have elapsed since the last injection, the next dose of Ari 2MRTU 960 should be administered as soon as possible and then the once-every-2-month schedule should be resumed. If a patient is beyond 14 weeks since their last injection, Ari 2MRTU 960 should be reinitiated.46
Due to sustained plasma concentrations with missed or delayed doses, the consensus panel does not recommend routine plasma drug monitoring for most patients. Monitoring should be reserved for patients on chronic medication regimens that interfere with CYP3A4 or CYP2D6 or in a patient who is a known hypo- or hyper-metabolizer, or those with lack of efficacy at normally effective doses or adverse effects at lower doses as dose adjustments may be needed in these select patient populations.
Patient-Centered Strategies to Support Sustained LAI Adherence
Beyond reducing dosing frequency compared to oral antipsychotics, LAIs may improve adherence in part by reducing or eliminating stigma associated with taking daily medication. A systematic review found that negative attitudes toward medication, including stigma, contributed to nonadherence in 30.5% of included studies.78
A recent qualitative study79 explored patient, caregiver, and prescriber preferences for unbranded LAI dosing frequency and treatment goals in schizophrenia. When asked about the potential of an LAI administered once every 2 months compared to once-monthly treatment, both patients living with schizophrenia and caregivers expressed positive views, perceiving the extended dosing interval as less burdensome. Prescribers also noted potential benefits of every-other-month injections, such as greater flexibility, less injection-related discomfort, and a greater perceived sense of normalcy due to receiving fewer injections. However, prescribers also cited potential drawbacks to LAIs generally, including limited ability to adjust dosing in response to tolerability issues and the possibility of needing oral supplementation if efficacy was not sustained. 79
LAIs may inherently provide more consistent patient-provider contact, as each injection requires a trained healthcare professional to administer the LAI, allowing for closer monitoring of adherence and adverse effects. This interaction may mitigate the risk of treatment interruptions due to unrecognized efficacy issues. Additionally, expanding LAI administration to non-providers, such as pharmacists in outpatient clinics or at pharmacies, may further enhance flexibility and access to care.80–82 While interactions with nursing or other staff who administer LAI injections can be helpful, it is important to note that visits with clinical staff who provide other recovery-oriented services should not be decreased simply because LAI administration allows for clinical contacts to be spaced further apart.
Additionally, a recently published Delphi consensus focused on functional recovery in patients with first-episode psychosis or early-phase schizophrenia emphasized that LAI antipsychotics can support functional recovery by increasing adherence, reducing disease burden, and preventing functional decline. 83 Earlier use of LAIs has been shown to improve outcomes in multiple studies84-87 and has been advocated as good clinical practice.26,88,89
By improving adherence and reducing dosing frequency, Ari 2MRTU 960 allows patients and providers to shift focus from medication management to broader aspects of recovery, such as psychosocial interventions that are critical to functional recovery and enhanced life engagement.90 Fewer injection appointments can provide more opportunities for targeted, non-pharmacologic interventions.23
Additionally, the flexibility of Ari 2MRTU 960’s dosing schedule supports sustained antipsychotic adherence by reducing the need for strict follow-up windows, which can improve patient quality of life.35 Despite these advantages, maintaining consistent follow-up appointments is essential to avoid fluctuations in plasma concentrations and ensure therapeutic stability. Thus, clinicians must remain vigilant in scheduling follow-up injection appointments to support consistent positive outcomes.
Panel Consensus Statement #3
“Flexibility and Patient-Centered Care: The varied dosing intervals of aripiprazole monohydrate LAIs cater to patient preferences and lifestyles, enhancing convenience and reducing the burden of daily medication. This flexibility supports treatment adherence, especially in patients who do not want to disclose to those in their social environment that they are taking antipsychotic medications and who wish to avoid the stigma associated with visible medication use.”
Conclusion
AOM 400 and Ari 2MRTU 960 address multiple challenges faced by patients diagnosed with schizophrenia or BP-I requiring antipsychotic therapy, allowing for sustained plasma concentrations and sustained efficacy with lower rates of relapses and hospitalizations. Compared to oral aripiprazole monohydrate and AOM 400, Ari 2MRTU 960 allows for more flexible dosing intervals based on its pharmacokinetic profile, giving patients more preference and convenience in their dosing schedule. Lastly, aripiprazole monohydrate LAIs are associated with fewer adverse motor and metabolic effects compared to older antipsychotic formulations, further supporting patient adherence and quality of life.
Financial Disclosures
All authors received an honorarium for attendance of the meeting.
Dr Goldberg has received consulting fees from Genomind; has received honoraria for speaking/teaching from Abbvie, Alkermes, Axsome, Bristol-Myers Squibb, and Intracellular Therapies; has received advisory board fees from Luye Pharmaceuticals, Merck, Neurelis, Neuroma, Otsuka, Sunovion, and Supernus; and has received royalties from American Psychiatric Publishing, and Cambridge University Press. Dr Achtyes has received consulting fees from Clinical Care Options, Boehringer-Ingelheim, VML Health, CMEology, CME Outfitters, Otsuka/Lundbeck, and TotalCME; has received grant/research support from Teva, InnateVR, Boehringer-Ingelheim, Neurocrine Biosciences, Karuna/Bristol Myers Squibb, Janssen, Alkermes, Takeda; and has received advisory board fees from Indivior and Alkermes. Dr Correll has stock options in Cardio Diagnostics, Kuleon Biosciences, LB Pharma, Medlink, Mindpax, Quantic, and Terran; has received consulting fees from AbbVie, Acadia, Adock Ingram, Alkermes, Allergan, Angelini, Aristo, Biogen, Boehringer-Ingelheim, Bristol-Myers Squibb, Cardio Diagnostics, Cerevel, CNX Therapeutics, Compass Pathways, Darnitsa, Delpor, Denovo, Eli Lilly, Gedeon Richter, Hikma, Holmusk, IntraCellular Therapies, Jamjoom Pharma, Janssen/J&J, Karuna, LB Pharma, Lundbeck, MedInCell, MedLink, Merck, Mindpax, Mitsubishi Tanabe Pharma, Maplight, Mylan, Neumora Therapeutics, Neurocrine, Neurelis, Newron, Noven, Novo Nordisk, Otsuka, PPD Biotech, Recordati, Relmada, Reviva, Rovi, Sage, Saladax, Sanofi, Seqirus, SK Life Science, Sumitomo Pharma America, Sunovion, Sun Pharma, Supernus, Tabuk, Takeda, Teva, Terran, Tolmar, Vertex, Viatris and Xenon; has received grant/research support from Boehringer-Ingelheim, Janssen, and Takeda; has received honoraria for speaking/teaching from AbbVie, Angelini, Aristo, Boehriger-Ingelheim, Cerevel, Damitsa, Gedeon Richter, Hikma, IntraCellular Therapies, Janssen/J&J, Karuna, Lundbeck, Mitsubishi Tanabe Pharma, Mylan, Otsuka, Recordati, Seqirus, Sunovion, Tabuk, Takeda, and Viatris; has received advisory board fees from AbbVie, Allergan, Angelini, Boehringer Ingelheim, Bristol-Myers Squibb, Cerevel, Compass, Gedeon Richter, Janssen/J&J, Karuna, LB Pharma, Lundbeck, MedInCell, Merck, Neurelis, Neurocrine, Newron, Novo Nordisk, Otsuka, Recordati, Rovi, Sage, Seqirus, Life Science, Sunovion, Supernus, Teva, Vertex, and Viatris; and has received royalties from UpToDate. Dr Sajatovic has received research grants within past 3 years from Neurelis, Intra-Cellular, Merck, Otsuka, and Alkermes; has received consulting fees in the past year from Otsuka, Lundbeck, Janssen, Teva, and Medscape; has received royalties in the past year from Springer Press, Johns Hopkins University Press, Oxford Press, and UpToDate; and has received compensation for preparation of/participation in CME activities in the past year from American Physician’s Institute (CMEtoGo), American Epilepsy Society, and Clinical Care Options. Dr Saklad has received consulting fees from Alkermes, Genomind, Janssen, Karuna, Lundbeck, and Otsuka and has received honoraria for speaking/teaching from Otsuka PsychU, Neurocrine, Teva, and Texas Society of Health System Pharmacists.
References (90)

- Correll CU, Bitter I, Hoti F, et al. Factors and their weight in reducing life expectancy in schizophrenia. Schizophr Res. Dec 2022;250:67–75. https://doi.org/10.1016/j.schres.2022.10.019
- Serra G, Koukopoulos A, De Chiara L, et al. Early clinical predictors and correlates of long-term morbidity in bipolar disorder. European Psychiatry. 2017;43:35–43. https://doi.org/10.1016/j.eurpsy.2017.02.480
- Zhong Y, Chen Y, Su X, et al. Global, regional and national burdens of bipolar disorders in adolescents and young adults: a trend analysis from 1990 to 2019. Gen Psychiatr. 2024;37(1):e101255. https://doi.org/10.1136/gpsych-2023-101255
- Chan JKN, Correll CU, Wong CSM, et al. Life expectancy and years of potential life lost in people with mental disorders: a systematic review and meta-analysis. EClinicalMedicine. Nov 2023;65:102294. https://doi.org/10.1016/j.eclinm.2023.102294
- Correll CU, Solmi M, Veronese N, et al. Prevalence, incidence and mortality from cardiovascular disease in patients with pooled and specific severe mental illness: a large-scale meta-analysis of 3,211,768 patients and 113,383,368 controls. World Psychiatry. Jun 2017;16(2):163–180. https://doi.org/10.1002/wps.20420
- Vancampfort D, Correll CU, Galling B, et al. Diabetes mellitus in people with schizophrenia, bipolar disorder and major depressive disorder: a systematic review and large scale meta-analysis. World Psychiatry. Jun 2016;15(2):166–74. https://doi.org/10.1002/wps.20309
- Vancampfort D, Stubbs B, Mitchell AJ, et al. Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder: a systematic review and meta-analysis. World Psychiatry. Oct 2015;14(3):339–47. https://doi.org/10.1002/wps.20252
- Biagi E, Capuzzi E, Colmegna F, et al. Long-acting injectable antipsychotics in schizophrenia: literature review and practical perspective, with a focus on aripiprazole once-monthly. Adv Ther. May 2017;34(5):1036–1048. https://doi.org/10.1007/s12325-017-0507-x
- Taipale H, Tanskanen A, Mehtälä J, et al. 20-year follow-up study of physical morbidity and mortality in relationship to antipsychotic treatment in a nationwide cohort of 62,250 patients with schizophrenia (FIN20). World Psychiatry. Feb 2020;19(1):61–68. https://doi.org/10.1002/wps.20699
- Solmi M, Tiihonen J, Lähteenvuo M, et al. Antipsychotics use is associated with greater adherence to cardiometabolic medications in patients with schizophrenia: results from a nationwide, within-subject design study. Schizophr Bull. Jan 21 2022;48(1):166–175. https://doi.org/10.1093/schbul/sbab087
- Correll CU, Solmi M, Croatto G, et al. Mortality in people with schizophrenia: a systematic review and meta-analysis of relative risk and aggravating or attenuating factors. World Psychiatry. Jun 2022;21(2):248–271. https://doi.org/10.1002/wps.20994
- Rubio JM, Taipale H, Tanskanen A, et al. Long-term continuity of antipsychotic treatment for schizophrenia: a nationwide study. Schizophr Bull. Oct 21 2021;47(6):1611–1620. https://doi.org/10.1093/schbul/sbab063
- Chakrabarti S. Medication non-adherence in bipolar disorder: Review of rates, demographic and clinical predictors. World J Meta-Anal. Aug 26 2017;5(4):103–23. https://doi.org/10.13105/wjma.v5.i4.103
- Kane JM, Kishimoto T, Correll CU. Non-adherence to medication in patients with psychotic disorders: epidemiology, contributing factors and management strategies. World Psychiatry. Oct 2013;12(3):216–26. https://doi.org/10.1002/wps.20060
- Forte A, Baldessarini RJ, Tondo L, et al. Long-term morbidity in bipolar-I, bipolar-II, and unipolar major depressive disorders. J Affect Disord. 2015/06/01/ 2015;178:71–78. doi:https://doi.org/10.1016/j.jad.2015.02.011
- Golden JC, Goethe JW, Woolley SB. Complex psychotropic polypharmacy in bipolar disorder across varying mood polarities: A prospective cohort study of 2712 inpatients. J Affect Disord 2017/10/15/ 2017;221:6–10. doi:https://doi.org/10.1016/j.jad.2017.06.005
- Johnson DAW. Historical perspective on antipsychotic long-acting injections. Br J Psychiatry. 2009;195(S52):s7-s12. https://doi.org/10.1192/bjp.195.52.s7
- Højlund M, Correll CU. Switching to long-acting injectable antipsychotics: pharmacological considerations and practical approaches. Exp Opinion Pharmacotherapy. 2023;24(13):1463–1489. https://doi.org/10.1080/14656566.2023.2228686
- Correll CU, Kim E, Sliwa JK, et al. Pharmacokinetic characteristics of long-acting injectable antipsychotics for schizophrenia: an overview. CNS Drugs. 2021;35(1):39–59. https://doi.org/10.1007/s40263-020-00779-5
- Kishimoto T, Hagi K, Kurokawa S, et al. Long-acting injectable versus oral antipsychotics for the maintenance treatment of schizophrenia: a systematic review and comparative meta-analysis of randomised, cohort, and pre-post studies. Lancet Psychiatry. May 2021;8(5):387–404. https://doi.org/10.1016/s2215-0366(21)00039-0
- Rubio JM, Schoretsanitis G, John M, et al. Psychosis relapse during treatment with long-acting injectable antipsychotics in individuals with schizophrenia-spectrum disorders: an individual participant data meta-analysis. Lancet Psychiatry. Sep 2020;7(9):749–761. https://doi.org/10.1016/s2215-0366(20)30264-9
- Haddad PM, Correll CU. Long-acting antipsychotics in the treatment of schizophrenia: opportunities and challenges. Expert Opin Pharmacother. Mar 2023;24(4):473–493. https://doi.org/10.1080/14656566.2023.2181073
- Correll CU, Citrome L, Haddad PM, et al. The use of long-acting injectable antipsychotics in schizophrenia: evaluating the evidence. J Clin Psychiatry. 2016;77(suppl 3):1–24. https://doi.org/10.4088/JCP.15032su1
- Schoretsanitis G, Kane JM, Correll CU, et al. Predictors of lack of relapse after random discontinuation of oral and long-acting injectable antipsychotics in clinically stabilized patients with schizophrenia: a re-analysis of individual participant data. Schizophr Bull. Mar 1 2022;48(2):296–306. https://doi.org/10.1093/schbul/sbab091
- Sajatovic M, Ross R, Legacy SN, et al. Initiating/maintaining long-acting injectable antipsychotics in schizophrenia/schizoaffective or bipolar disorder - expert consensus survey part 2. Neuropsychiatr Dis Treat. 2018;14:1475–1492. https://doi.org/10.2147/ndt.S167485
- Sajatovic M, Ross R, Legacy SN, et al. Identifying patients and clinical scenarios for use of long-acting injectable antipsychotics - expert consensus survey part 1. Neuropsychiatr Dis Treat. 2018;14:1463–1474. https://doi.org/10.2147/ndt.S167394
- Kessing LV. Should long-acting injectable antipsychotics be used earlier and more frequent in bipolar disorder, type I? Nordic J Psychiatry. 2022/10/03 2022;76(7):487–488. https://doi.org/10.1080/08039488.2021.2008000
- Correll CU, Martin A, Patel C, et al. Systematic literature review of schizophrenia clinical practice guidelines on acute and maintenance management with antipsychotics. Schizophrenia (Heidelb). Feb 24 2022;8(1):5. https://doi.org/10.1038/s41537-021-00192-x
- Citrome L, Correll CU, Cutler AJ, et al. Aripiprazole lauroxil: development and evidence-based review of a long-acting injectable atypical antipsychotic for the treatment of schizophrenia. Neuropsychiatr Dis Treat. 2025;21:575–596. https://doi.org/10.2147/NDT.S499367
- Hasan A, Falkai P, Wobrock T, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 2: update 2012 on the long-term treatment of schizophrenia and management of antipsychotic-induced side effects. World J Biol Psychiatry. Feb 2013;14(1):2–44. https://doi.org/10.3109/15622975.2012.739708
- Bell Lynum K, Awasthi S, Huang S, et al. The impact of aripiprazole once-monthly initiation and persistence on concomitant psychiatric medications in adults diagnosed with bipolar I disorder: a retrospective analysis of claims data from the United States. Poster presented at: Psych Congress; October 29–November 2, 2024, Boston, Massachusetts.
- Sheehan JJ, Reilly KR, Fu DJ, et al. Comparison of the peak-to-trough fluctuation in plasma concentration of long-acting injectable antipsychotics and their oral equivalents. Innov Clin Neurosci. Jul 2012;9(7–8):17–23.
- Kane JM, Sanchez R, Perry PP, et al. Aripiprazole intramuscular depot as maintenance treatment in patients with schizophrenia: a 52-week, multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2012;73(5):617–24. https://doi.org/10.4088/JCP.11m07530
- Citrome L. Long-acting injectable antipsychotics: what, when, and how - CORRIGENDUM. CNS Spectr. Dec 2022;27(6):764. https://doi.org/10.1017/s1092852921000778
- Wang Y, Harlin M, Larsen F, et al. population pharmacokinetics and dosing simulations for aripiprazole 2-month ready-to-use long-acting injectable in adult patients with schizophrenia or bipolar I disorder. Clin Pharmacol Drug Dev. Jun 2024;13(6):631–643. https://doi.org/10.1002/cpdd.1397
- Mailman RB, Murthy V. Third generation antipsychotic drugs: partial agonism or receptor functional selectivity? Curr Pharm Des. 2010;16(5):488–501. https://doi.org/10.2174/138161210790361461
- Vasiliu O. Third-generation antipsychotics in patients with schizophrenia and non-responsivity or intolerance to clozapine regimen: What is the evidence? Review. Frontiers in Psychiatry. November 29, 2022;13:10.3389/fpsyt.2022.1069432
- Winans E. Aripiprazole. Am J Health-System Pharmacy. 2003;60(23):2437–2445. https://doi.org/10.1093/ajhp/60.23.2437
- McCutcheon R, Beck K, Jauhar S, et al. Defining the Locus of Dopaminergic Dysfunction in Schizophrenia: A Meta-analysis and Test of the Mesolimbic Hypothesis. Schizophr Bull. Oct 17 2018;44(6):1301–1311. https://doi.org/10.1093/schbul/sbx180
- Correll CU, Abi-Dargham A, Howes O. Emerging Treatments in Schizophrenia. J Clin Psychiatry. 2022;83(1):SU21024IP1 https://doi.org/10.4088/JCP.SU21024IP1
- Gründer G, Kungel M, Ebrecht M, et al. Aripiprazole: pharmacodynamics of a dopamine partial agonist for the treatment of schizophrenia. Pharmacopsychiatry. Feb 2006;39 Suppl 1:S21–5. https://doi.org/10.1055/s-2006-931485
- de Bartolomeis A, Tomasetti C, Iasevoli F. Update on the Mechanism of Action of Aripiprazole: Translational Insights into Antipsychotic Strategies Beyond Dopamine Receptor Antagonism. CNS Drugs. Sep 2015;29(9):773–99. https://doi.org/10.1007/s40263-015-0278-3
- Taylor D, Chithiramohan R, Grewal J, et al. Dopamine partial agonists: a discrete class of antipsychotics. Int J Psychiatry Clin Pract. Sep 2023;27(3):272–284. https://doi.org/10.1080/13651501.2022.2151473
- Kim Y, Jeon JY, Chung YC, et al. Pharmacokinetics of a New Orally Disintegrating Tablet Formulation of Aripiprazole 15 mg Administered Without Water in Healthy Middle-aged Korean Subjects. Clin Ther. Dec 1 2015;37(12):2772–9. https://doi.org/10.1016/j.clinthera.2015.10.006
- Harlin M, Yildirim M, Such P, et al. A Randomized, Open-Label, Multiple-Dose, Parallel-Arm, Pivotal Study to Evaluate the Safety, Tolerability, and Pharmacokinetics of Aripiprazole 2-Month Long-Acting Injectable in Adults With Schizophrenia or Bipolar I Disorder. CNS Drugs. Apr 2023;37(4):337–350. https://doi.org/10.1007/s40263-023-00996-8
- ABILIFY ASIMTUFII (aripiprazole) extended-release injectable suspension, for intramuscular use. Package insert. Otsuka America Pharmaceutical, Inc; 2025.
- Harlin M, Chepke C, Larsen F, et al. Aripiprazole Plasma Concentrations Delivered from Two 2-Month Long-Acting Injectable Formulations: An Indirect Comparison. Neuropsychiatr Dis Treat. 2023;19:1409–1416. https://doi.org/10.2147/NDT.S412357
- Andrade C. Prolactin-Raising and Prolactin-Sparing Antipsychotic Drugs and the Risk of Fracture and Fragility Fracture in Patients With Schizophrenia, Dementia, and Other Disorders. J Clin Psychiatry. 2023;84(1):23f14790. https://doi.org/10.4088/JCP.23f14790
- Achtyes E, Simmons A, Skabeev A, et al. Patient preferences concerning the efficacy and side-effect profile of schizophrenia medications: a survey of patients living with schizophrenia. BMC Psychiatry. Sep 12 2018;18(1):292. https://doi.org/10.1186/s12888-018-1856-y
- Pae CU. A review of the safety and tolerability of aripiprazole. Expert Opin Drug Saf. May 2009;8(3):373–86. https://doi.org/10.1517/14740330902835493
- Naber D, Hansen K, Forray C, et al. Qualify: a randomized head-to-head study of aripiprazole once-monthly and paliperidone palmitate in the treatment of schizophrenia. Schizophr Res. Oct 2015;168(1–2):498–504. https://doi.org/10.1016/j.schres.2015.07.007
- Ribeiro ELA, de Mendonça Lima T, Vieira MEB, et al. Efficacy and safety of aripiprazole for the treatment of schizophrenia: an overview of systematic reviews. Eur J Clin Pharmacol. Oct 2018;74(10):1215–1233. https://doi.org/10.1007/s00228-018-2498-1
- Stroup TS, Gray N. Management of common adverse effects of antipsychotic medications. World Psychiatry. Oct 2018;17(3):341–356. https://doi.org/10.1002/wps.20567
- Papola D, Ostuzzi G, Thabane L, et al. Antipsychotic drug exposure and risk of fracture: a systematic review and meta-analysis of observational studies. Int Clin Psychopharmacol. Jul 2018;33(4):181–196. https://doi.org/10.1097/yic.0000000000000221
- Mallikaarjun S, Kane JM, Bricmont P, et al. Pharmacokinetics, tolerability and safety of aripiprazole once-monthly in adult schizophrenia: An open-label, parallel-arm, multiple-dose study. Schizophr Res. 2013/10/01/ 2013;150(1):281–288. doi:https://doi.org/10.1016/j.schres.2013.06.041
- Solmi M, Pigato G, Kane JM, et al. Clinical risk factors for the development of tardive dyskinesia. J Neurol Sci. Jun 15 2018;389:21–27. https://doi.org/10.1016/j.jns.2018.02.012
- Calabrese JR, Jin N, Johnson B, et al. Aripiprazole once-monthly as maintenance treatment for bipolar I disorder: a 52-week, multicenter, open-label study. Int J Bipolar Disord. Jun 10 2018;6(1):14. https://doi.org/10.1186/s40345-018-0122-z
- Fleischhacker WW, Sanchez R, Perry PP, et al. Aripiprazole once-monthly for treatment of schizophrenia: double-blind, randomised, non-inferiority study. Br J Psychiatry. Aug 2014;205(2):135–44. https://doi.org/10.1192/bjp.bp.113.134213
- Calabrese JR, Sanchez R, Jin N, et al. Efficacy and Safety of Aripiprazole Once-Monthly in the Maintenance Treatment of Bipolar I Disorder: A Double-Blind, Placebo-Controlled, 52-Week Randomized Withdrawal Study. J Clin Psychiatry. 2017;78(3):324–331. https://doi.org/10.4088/JCP.16m11201
- Hamina A, Taipale H, Lieslehto J, et al. Comparative Effectiveness of Antipsychotics in Patients With Schizophrenia Spectrum Disorder. JAMA Network Open. 2024;7(10):e2438358. https://doi.org/10.1001/jamanetworkopen.2024.38358
- Vita G, Tavella A, Ostuzzi G, et al. Efficacy and safety of long-acting injectable <i>versus</i> oral antipsychotics in the treatment of patients with early-phase schizophrenia-spectrum disorders: a systematic review and meta-analysis. Therapeutic Advances in Psychopharmacology. 2024;14doi:10.1177/20451253241257062
- Woo YS, Jeong JH, Kang H, et al. Preventive effect of aripiprazole once-monthly on relapse into mood episodes in bipolar disorder: A multicenter, one-year, retrospective, mirror image study. J Affect Disord. Apr 15 2024;351:381–386. https://doi.org/10.1016/j.jad.2024.01.257
- Bell Lynum KS, Castro CF, Zhang Z, et al. Aripiprazole once-monthly for the treatment of adult patients with earlier-stage bipolar I disorder: a post hoc analysis of data from a double-blind, placebo-controlled, 52-week randomized withdrawal trial. Int J Bipolar Disord. Oct 27 2024;12(1):37. https://doi.org/10.1186/s40345-024-00358-3
- Weinstock LM, Gaudiano BA, Epstein-Lubow G, et al. Medication burden in bipolar disorder: a chart review of patients at psychiatric hospital admission. Psychiatry Res. Apr 30 2014;216(1):24–30. https://doi.org/10.1016/j.psychres.2014.01.038
- Kane JM, McEvoy JP, Correll CU, et al. Controversies Surrounding the Use of Long-Acting Injectable Antipsychotic Medications for the Treatment of Patients with Schizophrenia. CNS Drugs. Nov 2021;35(11):1189–1205. https://doi.org/10.1007/s40263-021-00861-6
- Weiden PJ, Buckley PF. Reducing the burden of side effects during long-term antipsychotic therapy: the role of “switching” medications. J Clin Psychiatry. 2007;68(Suppl 6):14–23.
- Citrome L, Such P, Yild irim M, et al. Safety and Efficacy of Aripiprazole 2-Month Ready-to-Use 960 mg: Secondary Analysis of Outcomes in Adult Patients With Schizophrenia in a Randomized, Open-label, Parallel-Arm, Pivotal Study. J Clin Psychiatry. 2023;84(5):23m14873 https://doi.org/10.4088/JCP.23m14873
- McIntyre RS, Such P, Yildirim M, et al. Safety and efficacy of aripiprazole 2-month ready-to-use 960 mg: secondary analysis of outcomes in adult patients with bipolar I disorder in a randomized, open-label, parallel-arm, pivotal study. Curr Med Res Opin. Jul 2023;39(7):1021–1030. https://doi.org/10.1080/03007995.2023.2219155
- Citrome L, Such P, Yildirim M, et al. Plain Language Summary of Publication: A comparison of once-monthly and once-every-2-months injectable formulations of aripiprazole in people with schizophrenia. Ther Adv Psychopharmacol. 2024;14:20451253241286319. https://doi.org/10.1177/20451253241286319
- McIntyre RS, Such P, Yildirim M, et al. Use of an injection of aripiprazole given once every 2 months (Abilify Asimtufii(®)) in people with bipolar I disorder: a Plain Language Summary of Publication. Ther Adv Psychopharmacol. 2024;14:20451253241278830. https://doi.org/10.1177/20451253241278830
- Suzuki T, Nagai G, Mihara K, et al. CYP1A2*F Polymorphism Contributes at Least Partially to the Variability of Plasma Levels of Dehydroaripiprazole, an Active Metabolite of Aripiprazole, in Schizophrenic Patients. Drug Metabol Bioanal Lett. 2024;17(1):7–12.
- ABILIFY. Package insert. Accessed January 16, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/021436s041,021713s032,021729s024,021866s026lbl.pdf
- ABILIFY MAINTENA (aripiprazole) for extended-release injectable suspension, for intramuscular use. Package insert. Otsuka America Pharmaceutical, Inc; 2025.
- Abilify Maintena. European Medicines Agency. Updated April 7, 2024. Accessed March 28, 2025. https://www.ema.europa.eu/en/medicines/human/EPAR/abilify-maintena
- Wang Y, Wang X, Harlin M, et al. An alternative start regimen with aripiprazole once-monthly in patients with schizophrenia: population pharmacokinetic analysis of a single-day, two-injection start with gluteal and/or deltoid intramuscular injection. Curr Med Res Opin. Nov 2021;37(11):1961–1972. https://doi.org/10.1080/03007995.2021.1965974
- Fagiolini A, Leopold K, Pappa S, et al. Survey on the Initiation of Aripiprazole Once-Monthly via a Two-Injection Start in Adult Patients with Schizophrenia: Experience of European Healthcare Professionals. Adv Ther. Apr 2025;42(4):1935–1949. https://doi.org/10.1007/s12325-025-03130-w
- Correll CU, Sliwa JK, Najarian DM, et al. Practical considerations for managing breakthrough psychosis and symptomatic worsening in patients with schizophrenia on long-acting injectable antipsychotics. CNS Spectr. Aug 2019;24(4):354–370. https://doi.org/10.1017/S1092852918001098
- Velligan DI, Sajatovic M, Hatch A, et al. Why do psychiatric patients stop antipsychotic medication? A systematic review of reasons for nonadherence to medication in patients with serious mental illness. Patient Prefer Adherence. 2017;11:449–468. https://doi.org/10.2147/ppa.S124658
- Pappa S, Yildirim M, Loomer S, et al. Exploring Patient, Caregiver, and Prescriber Preferences for an Injectable Antipsychotic Administered Every 2 Months for the Maintenance Treatment of Schizophrenia: A Multicenter Qualitative Interview Study Conducted in Europe. Patient Prefer Adherence. 2025;19:1179–1195. https://doi.org/10.2147/PPA.S520160
- Chang HH, Vaughn LM, Liu D. Rural ambulatory care pharmacists providing in-clinic and home visit services improve adherence to long-acting injectable antipsychotics. Ment Health Clin. Jun 2024;14(3):229–232. https://doi.org/10.9740/mhc.2024.06.229
- Lin C, Strauss R, Hong J, et al. Impact of a pharmacist-administered long-acting injectable antipsychotic service in a supermarket-based community pharmacy on medication adherence. JACCP: Journal of the American College of Clinical Pharmacy. 2019;2(4):343–348. doi:https://doi.org/10.1002/jac5.1159
- Murphy AL, Suh S, Gillis L, Morrison J, et al. Pharmacist Administration of Long-Acting Injectable Antipsychotics to Community-Dwelling Patients: A Scoping Review. Pharmacy (Basel). Feb 27 2023;11(2)doi:10.3390/pharmacy11020045
- Gorwood P, Yildirim M, Madera-McDonough J, et al. Assessment of functional recovery in patients with schizophrenia, with a focus on early-phase disease: results from a Delphi consensus and narrative review. BMC Psychiatry. Apr 17 2025;25(1):398. https://doi.org/10.1186/s12888-025-06725-3
- Correll CU, Benson C, Emond B, et al. Comparison of clinical outcomes in patients with schizophrenia following different long-acting injectable event-driven initiation strategies. Schizophrenia (Heidelb). Feb 11 2023;9(1):9. https://doi.org/10.1038/s41537-023-00334-3
- Kane JM, Schooler NR, Marcy P, et al. Effect of Long-Acting Injectable Antipsychotics vs Usual Care on Time to First Hospitalization in Early-Phase Schizophrenia: A Randomized Clinical Trial. JAMA Psychiatry. Dec 1 2020;77(12):1217–1224. https://doi.org/10.1001/jamapsychiatry.2020.2076
- Wei Y, Yan VKC, Kang W, et al. Association of Long-Acting Injectable Antipsychotics and Oral Antipsychotics With Disease Relapse, Health Care Use, and Adverse Events Among People With Schizophrenia. JAMA Netw Open. Jul 1 2022;5(7):e2224163. https://doi.org/10.1001/jamanetworkopen.2022.24163
- Vita G, Tavella A, Ostuzzi G, et al. Efficacy and safety of long-acting injectable versus oral antipsychotics in the treatment of patients with early-phase schizophrenia-spectrum disorders: a systematic review and meta-analysis. Ther Adv Psychopharmacol. 2024;14:20451253241257062. https://doi.org/10.1177/20451253241257062
- McCutcheon RA, Pillinger T, Varvari I, et al. INTEGRATE: international guidelines for the algorithmic treatment of schizophrenia. Lancet Psychiatry. Mar 31 2025;doi:10.1016/S2215–0366(25)00031–8
- Arango C, Fagiolini A, Gorwood P, et al. Delphi panel to obtain clinical consensus about using long-acting injectable antipsychotics to treat first-episode and early-phase schizophrenia: treatment goals and approaches to functional recovery. BMC Psychiatry. Jun 21 2023;23(1):453. https://doi.org/10.1186/s12888-023-04928-0
- Correll CU, Ismail Z, McIntyre RS, et al. Patient Functioning and Life Engagement: Unmet Needs in Major Depressive Disorder and Schizophrenia. J Clin Psychiatry. 2022;83(4):LU21112AH1 https://doi.org/10.4088/JCP.LU21112AH1
This PDF is free for all visitors!