ABSTRACT
β-Blockers (BBs) are prescribed to a wide range of patients with cardiovascular and neuropsychiatric disorders. For more than half a century, BB treatment has been associated with depression as an adverse effect. The evidence in support of this association includes case reports, observational studies, and randomized controlled trials (RCTs). However, a large number of studies that refute the association have also been published. A very large meta-analysis of the psychiatric adverse effects of BBs, as reported in RCTs, was recently published. This meta-analysis found that BBs were not associated with an increased risk of depression or of withdrawal due to depression in comparison with either placebo or active controls. However, BBs were associated with an increased risk of fatigue/tiredness in comparison with placebo as well as in comparison with some groups of active controls. BBs were additionally associated with an increased risk of unusual dreams, relative to placebo. These findings suggest that fatigue/tiredness and unusual dreams may be misinterpreted by patients and clinicians as depression, explaining why BBs have been associated with depression risk. Furthermore, because BBs are commonly prescribed to patients with ischemic heart disease (IHD), and because IHD patients are at increased risk of depression, confounding by indication may explain why some patients treated with BBs later develop depression. These considerations notwithstanding, there are many reasons why the findings of the meta-analysis cannot be taken as reassurance on the subject. As examples, the RCTs in the meta-analysis mostly ascertained depression as a symptom rather than as a clinical diagnosis; and the meta-analysis did not consider risks with specific BBs such as propranolol, which has been strongly associated with the risk of depression in previous studies. In short, the final word, perhaps, remains to be said.
J Clin Psychiatry 2021;82(3):21f14095
To cite: Andrade C. β-blockers and the risk of new-onset depression: meta-analysis reassures, but the jury is still out. J Clin Psychiatry. 2021;82(3):21f14095.
To share: https://doi.org/10.4088/JCP.21f14095
© Copyright 2021 Physicians Postgraduate Press, Inc.
β-Blockers (BBs) comprise a group of about 2 dozen drugs that are competitive antagonists at β-adrenergic receptors. BBs are classified in different ways. For example, propranolol, nadolol, timolol, and pindolol are classical, first generation, nonselective BBs; they block both β1 and β2 adrenoceptors. Atenolol, metoprolol, esmolol, and bisoprolol are newer, second generation, selective blockers of β1 adrenoceptors. Labetalol, nebivolol, and carvedilol are examples of third generation BBs; they have additional properties besides β1-blocking selectivity. For example, nebivolol has vasodilation activity, and labetalol and carvedilol block α1 adrenoceptors in addition to β1 adrenoceptors. Finally, drugs such as propranolol are lipophilic and readily cross the blood-brain barrier, whereas drugs such as atenolol are hydrophilic and have low central nervous system activity.1
Indications
BBs are important in several different contexts in patients with cardiovascular disorders such as ischemic heart disease (IHD), cardiac failure, cardiac arrhythmias, and hypertension.2,3 BBs are also prescribed for many neuropsychiatric indications, with rather discouraging results for most anxiety disorders4 and akathisia5,6 and with good results for performance anxiety,7 essential tremor,8 and migraine prophylaxis.9 BBs have also been used to successfully attenuate lithium tremor.10 Less popularly, BBs have been used in attempts to reduce agitation in dementia11 and aggression in schizophrenia and other psychiatric disorders.12,13
β-Blockers and Depression
BB (pindolol) augmentation of antidepressant drugs has been suggested to accelerate treatment response in depression.14 However, for over half a century, BB treatment has also been associated with new-onset depression.15 A decade ago, Luijendijk and Koolman16 identified 22 observational studies of the association between BBs and incident depression; they described a pattern where the publication of one study identifying a significant association was followed by the publication of several other studies that refuted the association. Surprisingly, a recent population-based observational study that used data extracted from Danish registers found that 4 of 15 BBs were actually associated with a reduced risk of depression.17
One observational, registry-based study has suggested that BBs are associated with an increased risk of suicide18; however, the analysis in this study did not adjust for potential confounders, and a subsequent study found no increase in suicide risk associated with BB treatment but, unexpectedly, an increased suicide risk associated with current use of angiotensin-receptor antagonists.19
In a systematic review and meta-analysis of randomized controlled trials (RCTs), Ko et al20 identified 15 studies conducted in patients (pooled N > 35,000) with cardiovascular disorders. All trials recruited at least 100 patients, each, and all followed patients for at least 6 months, reporting depressive symptom outcomes. The authors found that BBs were associated with a significant increase in sexual dysfunction (number needed to harm [NNH] per year of treatment, 199) and fatigue (NNH per year of treatment, 57), but not with a significant increase in depressive symptoms.
A Recent Meta-Analysis
The relationship between BB treatment and the risk of depression has at last been investigated in a comprehensive, PRISMA-compliant, PROSPERO-registered meta-analysis. In this meta-analysis,21 the authors searched electronic databases (including clinical trial registries), reference lists, and other sources for double-blind, parallel group RCTs of non-topical BB treatments, used in monotherapy or as add-on therapy for any nonpsychiatric indication in adolescents and adults. RCTs were included only if they were at least 2 weeks in duration. The risk of depression and other carefully and well-operationalized psychiatric adverse events was compared between BB and control groups using random effects models. The risk of fatigue/tiredness was examined as a positive control because this adverse effect is known to be associated with BBs.
The review identified 285 RCTs of 24 BBs. These included 200 RCTs with an active comparator arm, 73 RCTs with a placebo control arm, and 12 RCTs with both active and placebo control arms. In these RCTs, 288 BB samples were compared with active drugs and 87 were compared with placebo in a total of 361 unique BB samples (pooled N = 53,533). The most common active comparators were α1-blockers, calcium channel blockers, renin-angiotensin system blockers, and thiazide and thiazide-like diuretics.
Most of the RCTs had been conducted for the indication arterial hypertension (197 trials, N = 32,042); other frequent indications included myocardial infarction, heart failure, and angina pectoris. Migraine was the commonest noncardiovascular indication (19 RCTs; N = 1,313). Only 53 RCTs had used an active method to assess adverse events. As many as 157 RCTs had industry support. Only 151 of the 285 RCTs could be included in the various quantitative analyses.
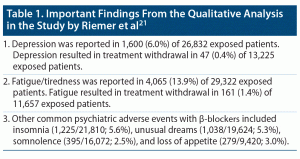
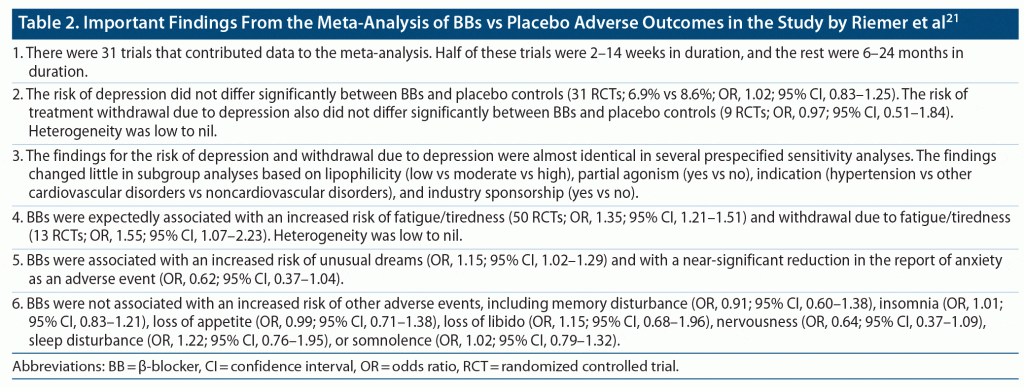
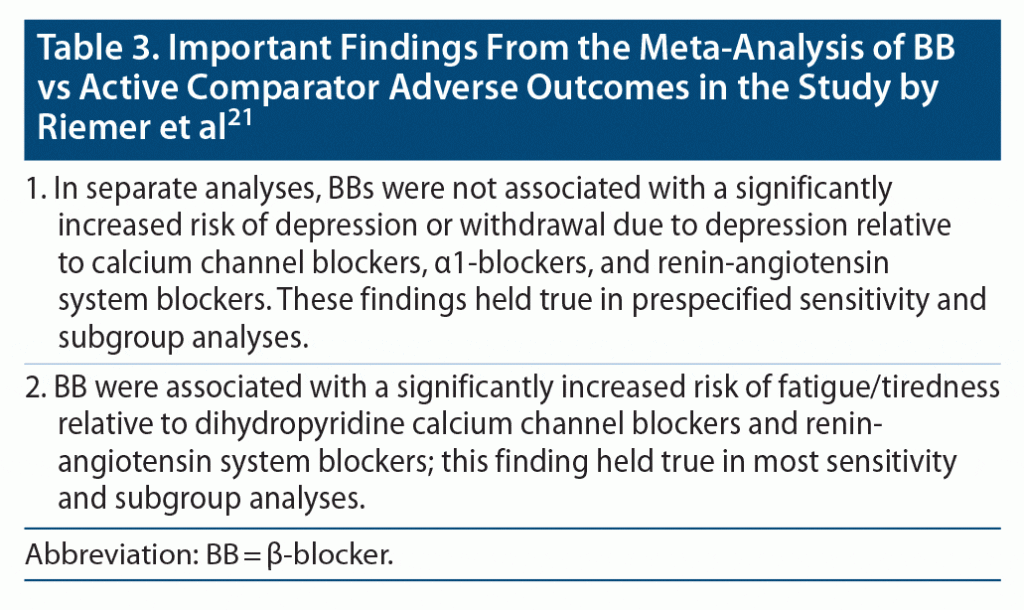
Important findings from the meta-analysis,21 published in the main paper and in a 110-page supplement, are presented in Table 1, Table 2, and Table 3. In summary, BBs were not associated with an increased risk of depression or withdrawal due to depression relative to either placebo or active controls. However, BBs were associated with an increased risk of fatigue/tiredness relative to placebo as well as relative to some groups of active controls. BBs were also associated with an increased risk of unusual dreams, relative to placebo.
Interpretation of the Results
On the surface, this very large meta-analysis21 of placebo arm and active arm parallel group RCTs provides strong evidence for the absence of risk of new-onset depression following initiation of BB treatment. So, what is the reason for the longstanding clinical concern that BB treatment is associated with an increased risk of developing depression?
There are 2 explanations. One is that it is well known that IHD and depression are each associated with an increased risk of the other,22,23 and IHD is an important indication for the initiation of BB treatment. So, confounding by indication may explain clinical observations of new-onset depression after initiation of BBs in patients with IHD. The second explanation is that tiredness/fatigue and unusual dreams, which Riemer et al21 found to be significantly more common with BBs, are common symptoms of depression and may be misinterpreted and misreported as depression.
Riemer et al21 wrote in their abstract that insomnia and sleep disorder were other psychiatric adverse events that were possibly related to BB therapy. Whereas neither adverse event emerged as a significant finding in the main quantitative analyses for either placebo- or active-controlled comparisons, there were some significant or near-significant associations with BB treatment in sensitivity and subgroup analyses. So, insomnia and sleep disorder may additionally contribute to the possibility of nonspecific adverse events being misinterpreted as depression.
Critical Comments
Although this meta-analysis21 is an important milestone for research in the field, it is not without limitations. The first limitation is that the meta-analysis included a large number of short-term trials, some of which (eg, the 2- to 4-week trials) may have been too short to allow for the emergence of depression after treatment with BBs. These trials could therefore have biased the research findings toward the null hypothesis. A second limitation is that in most of the RCTs, investigators did not elicit adverse events systematically but merely recorded adverse events that were reported; because such an approach could underestimate the incidence of adverse events, the findings of the meta-analysis could, therefore, again have been biased toward the null hypothesis.
A third and important limitation is that there is no report of whether depression as a clinical diagnosis was formally recorded as an adverse outcome in any of the RCTs; in fact, knowing how RCTs are usually conducted, it is unlikely that this was done. So, whereas we know that depression as a symptom or possibly a syndrome (as operationalized in the Supplementary Materials, Table S4, by the authors of the meta-analysis) was not more common with BBs, we have no way of knowing whether depression as a clinical diagnosis occurred more frequently with BBs or not. The real question is whether patients receiving BBs suffer clinically relevant depression, such as a major depressive episode, and not whether they complain of depression as a symptom.
Perhaps the most important limitation is that we do not know what the findings with commonly used BBs are. These BBs include, for example, propranolol, atenolol, and metoprolol. Why is this important? Consider, for example, the interesting findings in Tables S15 and S19 in the supplementary materials21 that propranolol was associated with depression in 858/3800 (22.6%) patients and with fatigue/tiredness in 1683/4651 (36.2%) patients. Was the strikingly high incidence of depression with propranolol greater than the incidence of depression in the comparator arms? We do not know the answer.
It is important to know whether the risks are different for different BBs because different BBs have different pharmacokinetic and pharmacodynamic properties and may be preferred in different clinical contexts. Returning to propranolol, for example, in a systematic review and meta-analysis24 of 9 RCTs (pooled N = 1,175) of propranolol compared with labetalol, oxprenolol, enalapril, atenolol, diltiazem, hydrochlorothiazide, and ketanserin for the treatment of hypertension, depression was found to occur significantly more frequently in propranolol-treated patients than in control patients (Mantel-Haenszel test, P = .039; mistakenly stated as P = .39 by the authors). This is definitely grounds for concern, and an issue that was not addressed by Riemer et al.21
The last word surely remains to be said. In this context, it has perhaps appropriately been stated that information from case reports should be carefully considered when RCTs have not been adequately designed to detect adverse outcomes.25
Parting Notes: β-Blockers and Bronchoconstriction
BBs are used with caution or are avoided in patients with pulmonary disease, especially bronchial asthma, because of the risk of respiratory adverse events, especially bronchoconstriction.26 However, an analysis of linked data from the UK Clinical Practice Research Datalink found that cardioselective BBs, unlike nonselective BBs, were not associated with the risk of moderate or severe exacerbation of asthma in patients with cardiovascular disease and comorbid asthma.27 Furthermore, in a systematic review and multiple treatment comparison meta-analysis of 23 observational studies and 14 RCTs, Gulea et al28 found that only propranolol was associated with reduced forced expiratory volume during the first second (FEV1); otherwise, relative to no treatment, BB treatment was actually associated with a reduced risk of acute exacerbation of chronic obstructive pulmonary disease events (hazard ratio, 0.77; 95% CI, 0.70–0.85). A qualitative analysis of the data in this study suggested no detrimental effect of BBs on quality of life, all-cause hospitalization, and mortality outcomes. It therefore appears that cardioselective BBs may be safe and may even improve outcomes in patients with comorbid cardiovascular and pulmonary disease; however, the BBs will need to be introduced in low doses and uptitrated gradually, and patients will need to be carefully monitored, especially during the initial weeks.26
Published online: June 1, 2021.
 Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Department of Clinical Psychopharmacology and Neurotoxicology, National Institute of Mental Health and Neurosciences, Bangalore, India ([email protected]).
Financial disclosure and more about Dr Andrade.
References (28)

- Westfall TC, Macarthur H, Westfall DP. Adrenergic agonists and antagonists. In: Brunton LL, Hilal-Dandan R, Knollmann BC, eds. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 13th ed. New York, NY: McGraw Hill; 2018:191–223.
- DiNicolantonio JJ, Fares H, Niazi AK, et al. β-Blockers in hypertension, diabetes, heart failure and acute myocardial infarction: a review of the literature. Open Heart. 2015;2(1):e000230. PubMed CrossRef
- Dézsi CA, Szentes V. The real role of β-blockers in daily cardiovascular therapy. Am J Cardiovasc Drugs. 2017;17(5):361–373. PubMed CrossRef
- Steenen SA, van Wijk AJ, van der Heijden GJMG, et al. Propranolol for the treatment of anxiety disorders: systematic review and meta-analysis. J Psychopharmacol. 2016;30(2):128–139. PubMed CrossRef
- Wells BG, Cold JA, Marken PA, et al. A placebo-controlled trial of nadolol in the treatment of neuroleptic-induced akathisia. J Clin Psychiatry. 1991;52(6):255–260. PubMed
- Lima AR, Bacalcthuk J, Barnes TR, et al. Central action beta-blockers versus placebo for neuroleptic-induced acute akathisia. Cochrane Database Syst Rev. 2004;(4):CD001946. PubMed
- Davidson JRT. Pharmacotherapy of social anxiety disorder: what does the evidence tell us? J Clin Psychiatry. 2006;67(suppl 12):20–26. PubMed
- Shanker V. Essential tremor: diagnosis and management. BMJ. 2019;366:l4485. PubMed CrossRef
- Silberstein SD, Holland S, Freitag F, et al; Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78(17):1337–1345. PubMed CrossRef
- Gelenberg AJ, Jefferson JW. Lithium tremor. J Clin Psychiatry. 1995;56(7):283–287. PubMed
- Kunik ME, Yudofsky SC, Silver JM, et al. Pharmacologic approach to management of agitation associated with dementia. J Clin Psychiatry. 1994;55(suppl):13–17. PubMed
- Allan ER, Alpert M, Sison CE, et al. Adjunctive nadolol in the treatment of acutely aggressive schizophrenic patients. J Clin Psychiatry. 1996;57(10):455–459. PubMed CrossRef
- Goedhard LE, Stolker JJ, Heerdink ER, et al. Pharmacotherapy for the treatment of aggressive behavior in general adult psychiatry: a systematic review. J Clin Psychiatry. 2006;67(7):1013–1024. PubMed CrossRef
- Portella MJ, de Diego-Adeliño J, Ballesteros J, et al. Can we really accelerate and enhance the selective serotonin reuptake inhibitor antidepressant effect? a randomized clinical trial and a meta-analysis of pindolol in nonresistant depression. J Clin Psychiatry. 2011;72(7):962–969. PubMed CrossRef
- Waal HJ. Propranolol-induced depression. BMJ. 1967;2(5543):50. PubMed CrossRef
- Luijendijk HJ, Koolman X. The incentive to publish negative studies: how beta-blockers and depression got stuck in the publication cycle. J Clin Epidemiol. 2012;65(5):488–492. PubMed CrossRef
- Kessing LV, Rytgaard HC, Ekstrøm CT, et al. Antihypertensive drugs and risk of depression: a nationwide population-based study. Hypertension. 2020;76(4):1263–1279. PubMed CrossRef
- Sørensen HT, Mellemkjaer L, Olsen JH. Risk of suicide in users of beta-adrenoceptor blockers, calcium channel blockers and angiotensin converting enzyme inhibitors. Br J Clin Pharmacol. 2001;52(3):313–318. PubMed CrossRef
- Callréus T, Agerskov Andersen U, Hallas J, et al. Cardiovascular drugs and the risk of suicide: a nested case-control study. Eur J Clin Pharmacol. 2007;63(6):591–596. PubMed CrossRef
- Ko DT, Hebert PR, Coffey CS, et al. Beta-blocker therapy and symptoms of depression, fatigue, and sexual dysfunction. JAMA. 2002;288(3):351–357. PubMed CrossRef
- Riemer TG, Villagomez Fuentes LE, Algharably EAE, et al. Do beta-blockers cause depression? systematic review and meta-analysis of psychiatric adverse events during beta-blocker therapy. Hypertension. 2021;77(5):1539–1548. PubMed CrossRef
- Glassman AH, Shapiro PA. Depression and the course of coronary artery disease. Am J Psychiatry. 1998;155(1):4–11. PubMed CrossRef
- Ziegelstein RC. Depression in patients recovering from a myocardial infarction. JAMA. 2001;286(13):1621–1627. PubMed CrossRef
- Patten SB. Propranolol and depression: evidence from the antihypertensive trials. Can J Psychiatry. 1990;35(3):257–259. PubMed CrossRef
- Steffensmeier JJ, Ernst ME, Kelly M, et al. Do randomized controlled trials always trump case reports? a second look at propranolol and depression. Pharmacotherapy. 2006;26(2):162–167. PubMed CrossRef
- Lipworth B, Wedzicha J, Devereux G, et al. Beta-blockers in COPD: time for reappraisal. Eur Respir J. 2016;48(3):880–888. PubMed CrossRef
- Morales DR, Lipworth BJ, Donnan PT, et al. Respiratory effect of beta-blockers in people with asthma and cardiovascular disease: population-based nested case control study. BMC Med. 2017;15(1):18. PubMed CrossRef
- Gulea C, Zakeri R, Alderman V, et al. Beta-blocker therapy in patients with COPD: a systematic literature review and meta-analysis with multiple treatment comparison. Respir Res. 2021;22(1):64. PubMed CrossRef
This PDF is free for all visitors!