Depression and ischemic heart disease commonly coexist, and each can worsen the course and outcome of the other. Depression is commonly treated with selective serotonin reuptake inhibitors (SSRIs), and ischemic heart disease patients commonly receive aspirin and/or clopidogrel. SSRIs can interact with these antiplatelet treatments in 2 ways. First, SSRIs can increase the risk of bleeding events with antiplatelet therapy; this risk may be diminished by the administration of a proton pump inhibitor. Second, fluoxetine and fluvoxamine may inhibit the metabolic activation of clopidogrel, thereby potentially diminishing the efficacy of clopidogrel. Issues related to these drug interactions are discussed.

- Depression and ischemic heart disease commonly coexist, and each can worsen the course of the other.
- SSRIs (commonly used for depression) can increase the risk of bleeding events with antiplatelet therapy (commonly used for ischemic heart disease); this risk may be diminished by use of a proton pump inhibitor.
- Fluoxetine and fluvoxamine may inhibit the metabolic activation of clopidogrel and thus potentially diminish its efficacy.
Clinical Problem
Mr K is 67 years old. He has been diagnosed with major depressive disorder. He has a history of ischemic heart disease (IHD). What might be the concerns associated with treating his depression with a selective serotonin reuptake inhibitor (SSRI)?
The Relationship Between Depression and Ischemic Heart Disease
Patients with depression are at increased risk of IHD events, and patients who have IHD are at increased risk of depression; the presence of each condition worsens the course and outcome of the other.1 It is important, therefore, for depression to be identified early and treated effectively in patients with IHD.2,3 Given that SSRIs are commonly used antidepressants, clinicians should be aware that SSRIs may have effects beyond antidepressant action in patients with IHD.
Possible Benefits of SSRIs in Patients With Ischemic Heart Disease
SSRIs effectively attenuate depression, including depression that complicates the course of IHD.4 SSRIs may also improve the course of IHD5-10 through multiple mechanisms (Table 1)11-17—this is an important consideration, given that tricyclic antidepressants have been associated with an increased risk of IHD events.18,19
Possible Adverse Interactions of SSRIs in Patients With Ischemic Heart Disease
Patients with IHD are usually prescribed antiplatelet treatment to reduce the risk of future ischemic events. Aspirin and clopidogrel are 2 drugs that are commonly prescribed for this purpose, separately or together. SSRIs may adversely interact with these 2 drugs, as discussed in the sections that follow. Other interactions between SSRIs and cardiovascular drugs will be examined in a future article.
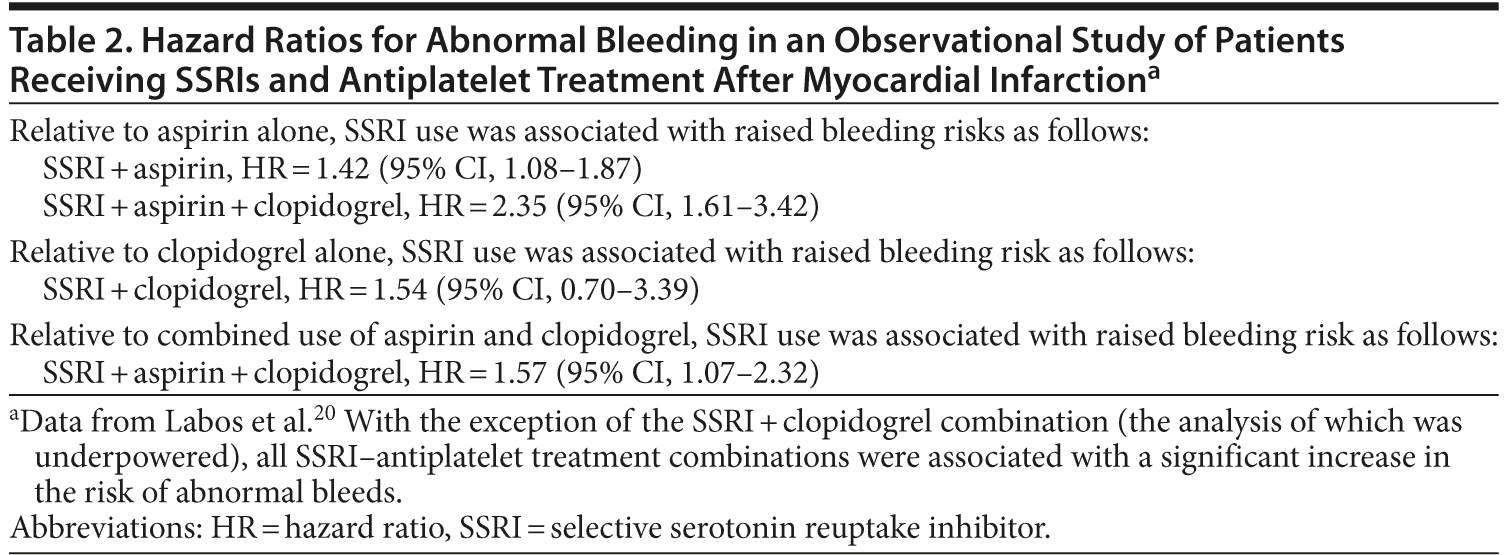
Risk of abnormal bleeding. SSRIs increase the risk of abnormal bleeding through 2 important mechanisms: antiplatelet activity and increased gastric acidity.12 The gastrointestinal (GI) tract is the most common site of SSRI-related bleeding.12 A large body of literature describes an increased risk of abnormal bleeding associated with the combination of SSRIs with aspirin or clopidogrel.12 A recent observational study20 is briefly presented by way of example; this study was specifically conducted in IHD patients.
Labos et al20 described a retrospective cohort study of 27,058 patients 50 years and older who received aspirin (n = 14,426); clopidogrel (n = 2,467); aspirin and clopidogrel (n = 9,475); aspirin and an SSRI (n = 406); aspirin, clopidogrel, and an SSRI (n = 239); or clopidogrel and an SSRI (n = 45) after an acute myocardial infarction. There were 1,070 episodes of bleeding recorded across a mean duration of follow-up of 3 years. SSRIs were associated with an increased hazard of bleeding episodes in almost all analyses (Table 2).
The absolute risk of bleeding associated with SSRI use appeared small. Relative to the use of aspirin alone, the combination of an SSRI with aspirin raised the bleeding risk by 0.5 events per 100 patient-years. Relative to the use of aspirin combined with clopidogrel, the combination of an SSRI with aspirin and clopidogrel raised the bleeding risk by 1.5 bleeding events per 100 patient-years. These risks seem trivial at an individual level; however, they are clinically significant at the population level because they suggest that if 200 IHD patients are treated with antiplatelet and SSRI drugs for 1 year, 1 to 3 patients could experience an abnormal bleeding episode. It is also important to note that the patients in this study were followed up until admission for a bleeding episode, admission for recurrent myocardial infarction, death, or the end of the study period. Thus, given that most cases of SSRI-related bleeding occur early during treatment,12 the average of 3 years of follow-up per patient would have considerably diluted the magnitude of the identified risk.
Labos et al20 found that the risks were similar when the analyses were restricted to cases of GI bleeding. Finally, and especially importantly, they found that non-SSRI antidepressants were not associated with an increased risk of bleeding events.
How may this interaction be prevented? Observational studies suggest that the concurrent administration of proton pump inhibitors may decrease the risk of SSRI-related bleeds.12 However, some proton pump inhibitors, such as omeprazole and esomeprazole,21-23 potently inhibit cytochrome P450 (CYP) 2C19, which can diminish the efficacy of clopidogrel if the patient happens to be receiving the drug (as discussed in the next section). In this context, lansoprazole and dexlansoprazole are less potent inhibitors of CYP2C19, and rabeprazole and pantoprazole are weak inhibitors.21-23 The US Food and Drug Administration (FDA) specifically discourages the use of omeprazole in patients who are receiving clopidogrel and suggests a preference for pantoprazole.24
On a separate note, IHD is more common in elderly subjects, and the elderly may be more vulnerable to SSRI-related abnormal bleeding.12
Risk of diminished efficacy of clopidogrel. Clopidogrel is a prodrug that is activated by CYP2C19.25 A systematic review and meta-analysis of 9 studies (pooled N = 9,685) evaluated the influence of the CYP2C19 genotype on outcomes in patients receiving clopidogrel; patients with reduced-function variations of one or both of the CYP2C19 genes had significantly worse cardiovascular or cerebrovascular outcomes.25 This finding suggests that, in patients who receive fluoxetine or fluvoxamine, the efficacy of clopidogrel could be similarly impaired, because both SSRIs inhibit CYP2C19. In November 2009, the FDA issued a warning that clopidogrel should not be combined with various CYP2C19 inhibitors, including fluoxetine and fluvoxamine.26
Concluding Notes: Treatment Considerations
The preceding discussion suggests the following:
- Fluoxetine and fluvoxamine are best avoided in IHD patients who are receiving clopidogrel because both SSRIs may diminish the efficacy of clopidogrel.
- If an SSRI is prescribed to an IHD patient who is receiving aspirin or clopidogrel, the concurrent prescription of a proton pump inhibitor that does not significantly inhibit CYP2C19 (eg, rabeprazole, pantoprazole) could reduce the risk of GI bleeding without diminishing the efficacy of clopidogrel.
J Clin Psychiatry 2012;73(12):e1475-e1477 (doi:10.4088/JCP.12f08248)
© Copyright 2012 Physicians Postgraduate Press, Inc.
 Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Department of Clinical Psychopharmacology and Neurotoxicology, National Institute of Mental Health and Neurosciences, Bangalore, India ([email protected]).
Financial disclosure and more about Dr Andrade.
REFERENCES
1. Glassman AH, Shapiro PA. Depression and the course of coronary artery disease. Am J Psychiatry. 1998;155(1):4-11. PubMed
2. Santangelo A, Testa׬ M, Barbagallo P, et al. Use of specific serotonin reuptake inhibitors (SSRIs) (sertraline or citalopram) in the treatment of depression reduces the cardiovascular risk in the elderly: evidence from a Sicilian population > 80 years recovered in the assisted sanitary residences (RSA). Arch Gerontol Geriatr. 2009;48(3):350-352. PubMed doi:10.1016/j.archger.2008.02.014
3. Davidson KW, Rieckmann N, Clemow L, et al. Enhanced depression care for patients with acute coronary syndrome and persistent depressive symptoms: Coronary Psychosocial Evaluation Studies randomized controlled trial. Arch Intern Med. 2010;170(7):600-608. PubMed doi:10.1001/archinternmed.2010.29
4. Pizzi C, Rutjes AWS, Costa GM, et al. Meta-analysis of selective serotonin reuptake inhibitors in patients with depression and coronary heart disease. Am J Cardiol. 2011;107(7):972-979. PubMed doi:10.1016/j.amjcard.2010.11.017
5. Sauer WH, Berlin JA, Kimmel SE. Selective serotonin reuptake inhibitors and myocardial infarction. Circulation. 2001;104(16):1894-1898. PubMed doi:10.1161/hc4101.097519
6. Sauer WH, Berlin JA, Kimmel SE. Effect of antidepressants and their relative affinity for the serotonin transporter on the risk of myocardial infarction. Circulation. 2003;108(1):32-36. PubMed doi:10.1161/01.CIR.0000079172.43229.CD
7. Schlienger RG, Fischer LM, Jick H, et al. Current use of selective serotonin reuptake inhibitors and risk of acute myocardial infarction. Drug Saf. 2004;27(14):1157-1165. PubMed doi:10.2165/00002018-200427140-00006
8. Taylor CB, Youngblood ME, Catellier D, et al. ENRICHD Investigators. Effects of antidepressant medication on morbidity and mortality in depressed patients after myocardial infarction. Arch Gen Psychiatry. 2005;62(7):792-798. PubMed doi:10.1001/archpsyc.62.7.792
9. Tiihonen J, Lönnqvist J, Wahlbeck K, et al. Antidepressants and the risk of suicide, attempted suicide, and overall mortality in a nationwide cohort. Arch Gen Psychiatry. 2006;63(12):1358-1367. PubMed doi:10.1001/archpsyc.63.12.1358
10. Ziegelstein RC, Meuchel J, Kim TJ, et al. Selective serotonin reuptake inhibitor use by patients with acute coronary syndromes. Am J Med. 2007;120(6):525-530. PubMed doi:10.1016/j.amjmed.2006.10.026
11. Serebruany VL. Selective serotonin reuptake inhibitors and increased bleeding risk: are we missing something? Am J Med. 2006;119(2):113-116. PubMed doi:10.1016/j.amjmed.2005.03.044
12. Andrade C, Sandarsh S, Chethan KB, et al. Serotonin reuptake inhibitor antidepressants and abnormal bleeding: a review for clinicians and a reconsideration of mechanisms. J Clin Psychiatry. 2010;71(12):1565-1575. PubMed doi:10.4088/JCP.09r05786blu
13. Serebruany VL, Glassman AH, Malinin AI, et al, Sertraline AntiDepressant Heart Attack Randomized Trial Study Group. Platelet/endothelial biomarkers in depressed patients treated with the selective serotonin reuptake inhibitor sertraline after acute coronary events: the Sertraline AntiDepressant Heart Attack Randomized Trial (SADHART) Platelet Substudy. Circulation. 2003;108(8):939-944. PubMed doi:10.1161/01.CIR.0000085163.21752.0A
14. Serebruany VL, Glassman AH, Malinin AI, et al. Selective serotonin reuptake inhibitors yield additional antiplatelet protection in patients with congestive heart failure treated with antecedent aspirin. Eur J Heart Fail. 2003;5(4):517-521. PubMed doi:10.1016/S1388-9842(03)00005-9
15. Lekakis J, Ikonomidis I, Papoutsi Z, et al. Selective serotonin re-uptake inhibitors decrease the cytokine-induced endothelial adhesion molecule expression, the endothelial adhesiveness to monocytes and the circulating levels of vascular adhesion molecules. Int J Cardiol. 2010;139(2):150-158. PubMed doi:10.1016/j.ijcard.2008.10.010
16. Pizzi C, Mancini S, Angeloni L, et al. Effects of selective serotonin reuptake inhibitor therapy on endothelial function and inflammatory markers in patients with coronary heart disease. Clin Pharmacol Ther. 2009;86(5):527-532. PubMed doi:10.1038/clpt.2009.121
17. Tseng YL, Chiang ML, Huang TF, et al. A selective serotonin reuptake inhibitor, citalopram, inhibits collagen-induced platelet aggregation and activation. Thromb Res. 2010;126(6):517-523. PubMed doi:10.1016/j.thromres.2010.09.017
18. Cohen HW, Gibson G, Alderman MH. Excess risk of myocardial infarction in patients treated with antidepressant medications: association with use of tricyclic agents. Am J Med. 2000;108(1):2-8. PubMed doi:10.1016/S0002-9343(99)00301-0
19. Rosenberg LB, Whang W, Shimbo D, et al. Exposure to tricyclic antidepressants is associated with an increased risk of incident CHD events in a population-based study. Int J Cardiol. 2010;145(1):124-125. PubMed doi:10.1016/j.ijcard.2009.06.036
20. Labos C, Dasgupta K, Nedjar H, et al. Risk of bleeding associated with combined use of selective serotonin reuptake inhibitors and antiplatelet therapy following acute myocardial infarction. CMAJ. 2011;183(16):1835-1843. PubMed doi:10.1503/cmaj.100912
21. Li XQ, Andersson TB, Ahlström M, et al. Comparison of inhibitory effects of the proton pump-inhibiting drugs omeprazole, esomeprazole, lansoprazole, pantoprazole, and rabeprazole on human cytochrome P450 activities. Drug Metab Dispos. 2004;32(8):821-827. PubMed doi:10.1124/dmd.32.8.821
22. Fontes-Carvalho R, Albuquerque A, Araújo C, et al. Omeprazole, but not pantoprazole, reduces the antiplatelet effect of clopidogrel: a randomized clinical crossover trial in patients after myocardial infarction evaluating the clopidogrel-PPIs drug interaction. Eur J Gastroenterol Hepatol. 2011;23(5):396-404. PubMed doi:10.1097/MEG.0b013e3283460110
23. Zvyaga T, Chang SY, Chen C, et al. Evaluation of six proton pump inhibitors as inhibitors of various human cytochromes P450: focus on cytochrome P450 2C19. Drug Metab Dispos. 2012;40(9):1698-1711. PubMed doi:10.1124/dmd.112.045575
24. Food and Drug Administration. Information on clopidogrel bisulfate (marketed as Plavix). http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm190836.htm. Updated October 27, 2010. Accessed October 31, 2012.
25. Mega JL, Simon T, Collet JP, et al. Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta-analysis. JAMA. 2010;304(16):1821-1830. PubMed doi:10.1001/jama.2010.1543
26. Food and Drug Administration. Update to the labeling of clopidogrel bisulfate (marketed as Plavix) to alert healthcare professionals about a drug interaction with omeprazole (marketed as Prilosec and Prilosec OTC). http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm190787.htm. Updated November 17, 2009. Accessed October 31, 2012.
This PDF is free for all visitors!