Abstract
Xanomeline-trospium (XT) is the first muscarinic-based therapy approved for schizophrenia. It combines M1 and M4 receptor agonism with peripheral antagonism to limit cholinergic side effects. By modulating circuits upstream of dopamine release, XT offers a mechanism that differs from traditional antipsychotics and may address positive, negative, and cognitive symptoms. In July 2025, a consensus panel of clinicians with expertise in treating schizophrenia and real-world experience using XT convened to discuss practical strategies for its use across treatment settings. The panel concluded that XT should be considered early in the course of illness, particularly in first-episode psychosis, because it may alter long-term outcomes while reducing reliance on high-dose dopamine antagonists. Outpatient strategies emphasize individualized titration and proactive management of gastrointestinal side effects to support adherence. Inpatient use allows for more rapid titration and has shown rapid benefits in both positive and negative symptoms, facilitating earlier stabilization and discharge. Cross-titration experience suggests that XT can be dose-sparing when combined with dopamine blockers, reducing the burden of metabolic and motor side effects. These real-world insights highlight XT as a versatile treatment option that expands the therapeutic possibilities for schizophrenia.
J Clin Psychiatry 2025;86(4):hxtachi2509
Published Online: November 7, 2025.
To Cite: Melnick I, Crown EC, Zinzuvadia M, et al. Real-world implementation of xanomeline-trospium in schizophrenia: a consensus panel report. J Clin Psychiatry. 2025;86(4):hxtachi2509.
To Share: https://doi.org/10.4088/JCP.hxtachi2509
© 2025 Physicians Postgraduate Press, Inc.
This Academic Highlights section of The Journal of Clinical Psychiatry presents the highlights of the virtual consensus panel meeting “Real-World Implementation of Xanomeline-Trospium in Schizophrenia: A Consensus Panel” which was held July 13, 2025.
The meeting was chaired by Ilan Melnick, MD, Chief Medical Officer and Staff Psychiatrist Passageway Residence of Dade County; Voluntary Assistant Professor Miller School of Medicine University of Miami; Assistant Professor Florida International University School of Medicine, Miami, Florida. The faculty were Erin C. Crown, MHS, PA-C, Owner and Chief Operating Officer of Future Options Research, LLC; Manish Zinzuvadia, MD, Park Avenue Wellness Center, New York, New York; and Michael M. Halassa, MD, PhD, Professor and Director of Translational Research, Department of Neuroscience; Professor, Department of Psychiatry, Tufts University School of Medicine, Boston, Massachusetts (correspondence: [email protected]; 136 Harrison Ave., Boston, MA 02111)
Financial disclosures appear at the end of the article.
This evidence-based, peer-reviewed Academic Highlights article was prepared by Healthcare Global Village, Inc. The consensus panel meeting on which this manuscript is based was funded by Bristol-Myers Squibb Company, Princeton, NJ. The sponsor had no role in the writing, review, or submission of this manuscript. The authors, with support from Healthcare Global Village, Inc., drafted and submitted the article for peer review. Article processing charges, medical writing, editorial, and other assistance were funded by Healthcare Global Village, Inc. Editorial support was provided by Aric Fader, PhD, Alku, Inc., and P. Benjamin Everett, PhD, Healthcare Global Village, Inc. The opinions expressed herein are those of the faculty and do not necessarily reflect the views of Healthcare Global Village, Inc., the publisher, or the commercial sponsor. This article is distributed by Healthcare Global Village, Inc. for educational purposes only.
Key Clinical Insights from the Expert Panel
- Outpatient initiation should be individualized, with early use of XT offering the potential for long-term benefits and reduced reliance on dopamine-blocking agents.
- Inpatient initiation may provide rapid stabilization, including improvements in negative symptoms, while supporting effective transition planning to outpatient care.
- Cross-titration is best achieved with a structured, class-specific tapering strategy, with proactive counseling and antiemetic support to enhance tolerability.
- XT may be dose-sparing, allowing effective control with lower doses of antipsychotics, thereby reducing risks of movement disorders, metabolic complications, and other long-term sequelae of dopamine blockade.
- Concomitant use of XT with LAIs requires a tailored approach: use of XT in a patient on a maintenance dose of an LAI may allow for a reduction in LAI dose, once the XT dose has been stabilized. This approach may result in better symptom control while minimizing cumulative dopamine receptor burden.
Introduction
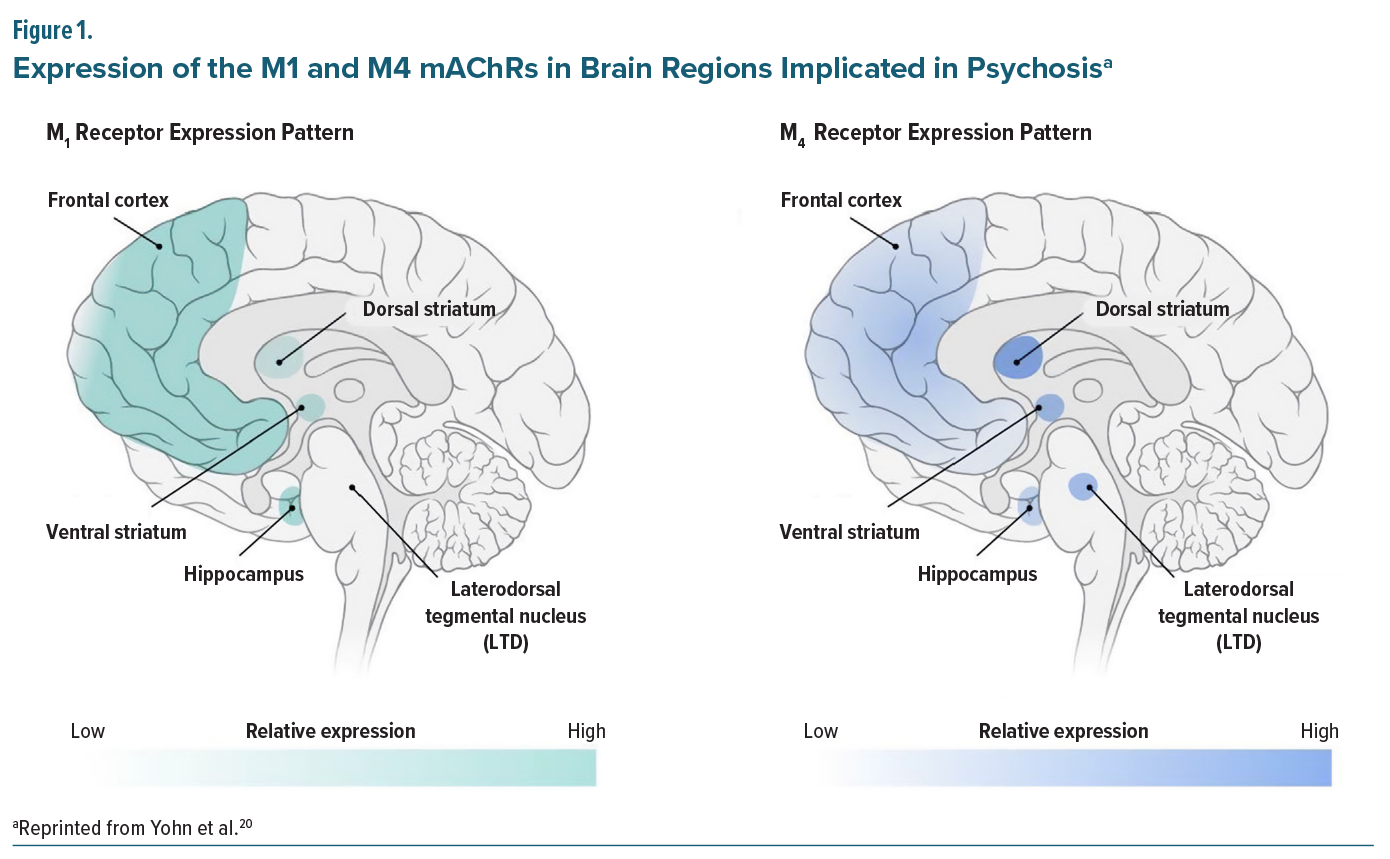
There is a need for early and rapid intervention to prevent symptom recurrence and functional loss among patients with schizophrenia.1,2 Likewise, relapse prevention is key, as relapse may lead to illness progression and treatment refractory schizophrenia.3 Schizophrenia presents with a range of positive symptoms such as hallucinations and delusions as well as negative symptoms including anhedonia, avolition, and affective flattening.4-7 It is also characterized by cognitive impairments, which encompass deficits in memory, attention, executive function, and social cognition.4-6,8-10 While pharmacological management is considered foundational in schizophrenia, currently available antipsychotics primarily treat positive symptoms11; however, some patients experience residual or treatment-resistant positive symptoms, while negative symptoms and cognitive impairment have remained largely intractable to currently available antipsychotic medications.6,7,12-14 As schizophrenia is a chronic neuropsychiatric syndrome requiring lifelong management, adverse effects associated with long-term utilization of antipsychotics can be problematic and become debilitating. Adverse events of concern include extrapyramidal side effects (EPS), tardive dyskinesia, weight gain, metabolic changes, and elevation of prolactin levels, which have recently been linked to an increased risk of breast cancer in women with schizophrenia.15-19The M1 and M4 muscarinic acetylcholine receptors (mAChRs) are predominantly located in brain regions central to psychosis, including the frontal cortex, striatum, and hippocampus. Their distribution and expression patterns suggest distinct but complementary roles in regulating dopamine signaling (Figure 1).20 In the frontal cortex, where M1 receptors are highly expressed, their activation is hypothesized to enhance excitatory-inhibitory balance,21,22 and thereby strengthen top-down control of the dopaminergic midbrain.20,23
In contrast, M4 receptors are enriched in subcortical regions, including the striatum and laterodorsal tegmentum. Activation of M4 is proposed to dampen dopaminergic activity within schizophrenia-relevant circuits, particularly the ventral striatum (nucleus accumbens). Together, M1 and M4 receptor activity are thought to converge on cortical and subcortical pathways implicated in psychosis, with M1 signaling more closely linked to cognitive and negative symptoms and M4 signaling to positive symptoms.20,23,24
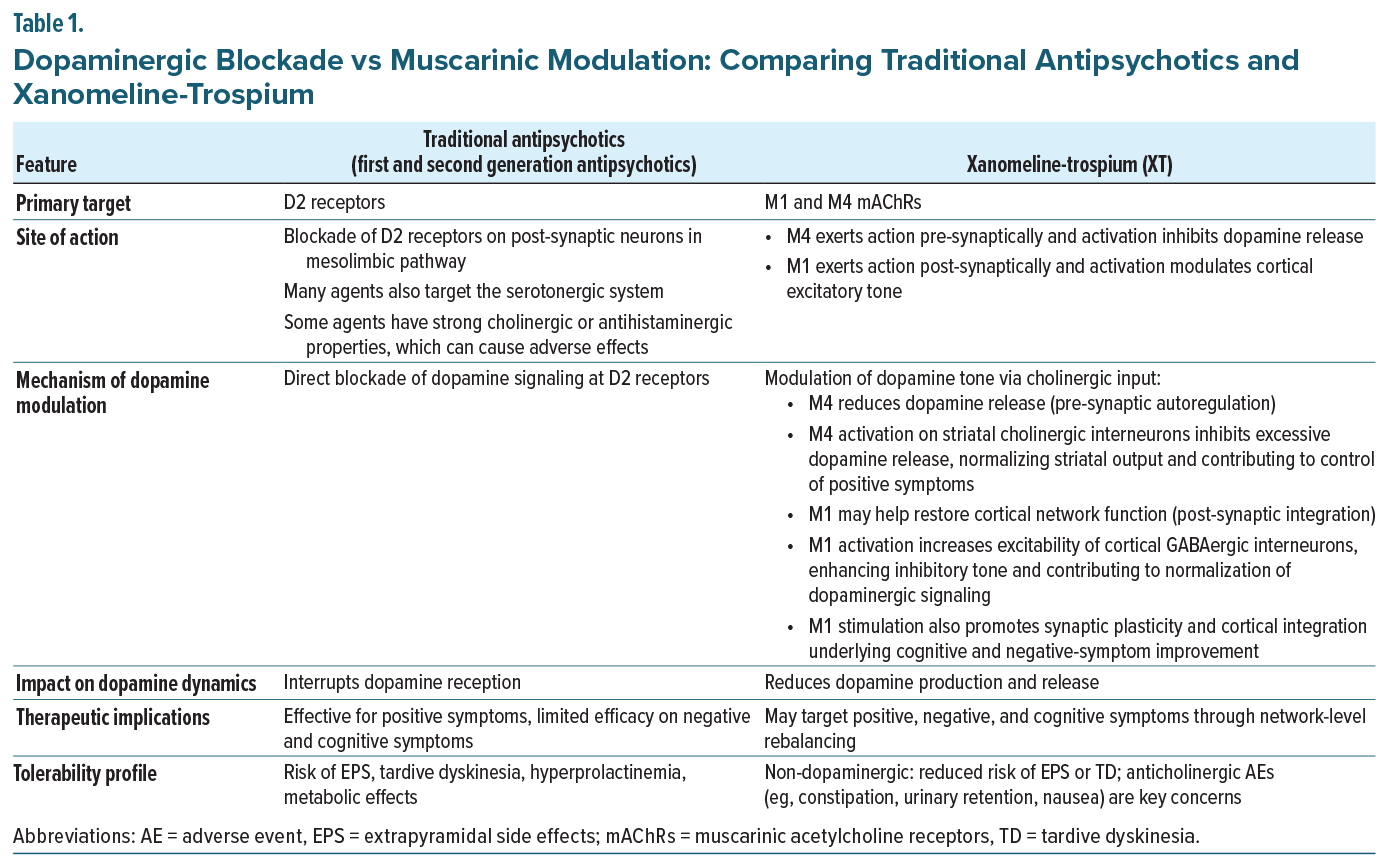
Muscarinic modulation is not a new strategy in schizophrenia treatment. Xanomeline was developed by Eli Lilly in the 1990s for Alzheimer’s disease and schizophrenia, and demonstrated promising efficacy in Phase 2 studies, but its development was halted due to peripheral cholinergic adverse events, which at the time were deemed unmanageable.25 Trospium chloride, an mAChR antagonist developed in Europe in the 1970s for overactive bladder and approved in the US in 2004,26 has a large structure that limits blood-brain barrier penetration, making it an effective peripheral anticholinergic.27 The fixed-dose combination of xanomeline and trospium (XT) is the first FDA-approved treatment for schizophrenia that is not classified as an antipsychotic and therefore does not carry the boxed warning for increased mortality in elderly patients with dementia-related psychosis.28 XT pairs M1/M4-preferring muscarinic receptor agonism with peripherally restricted mAChR antagonism to reduce peripheral cholinergic adverse events (eg, nausea, vomiting, diarrhea), representing a novel mechanism of action that is distinct from all currently available antipsychotics, which act primarily through blockade of the dopamine D2 receptor. Unlike dopamine receptor antagonists or partial agonists, XT modulates presynaptic dopamine via M1 and M4 activity, offering low risk of metabolic, cardiac, or extrapyramidal side effects and demonstrating broad efficacy across positive, negative, cognitive, and general psychopathology symptoms in clinical trials.16,18,29
Clinical research conducted to date has indicated that stimulation of M1 and M4 receptors with XT improves multiple symptoms of schizophrenia, with rapid and sustained improvement of both positive and negative symptoms.30-37 XT’s novel mechanism of action offers new opportunities for patients with schizophrenia and their care teams. Although its novelty may generate some hesitation among clinicians, the recommendations presented herein are grounded in the real-world experience of experts treating schizophrenia across the disease spectrum, from recently diagnosed to long-term patients, and span the inpatient and outpatient settings.
Methods
On July 13, 2025, an expert consensus panel was convened to explore the real-world clinical implementation of XT for the treatment of adult patients with schizophrenia. The panelists were selected for their extensive clinical expertise in managing schizophrenia across the life cycle in both outpatient and inpatient settings, as well as for their depth of knowledge in psychopharmacology. In addition, one panelist (Dr Michael Halassa) brought a unique perspective bridging bench-to-bedside research in the neurobiology of schizophrenia and the development of novel therapeutics. The discussion focused on the novel mechanism of action of XT, its practical role in clinical care, strategies for initiation and dose titration, cross-titration with other agents, and the management of XT-related adverse events. The consensus panel was held virtually with a facilitated discussion guide. The chair was Ilan Melnick, MD, and the participants included Erin C. Crown, MHS, PA-C; Michael M. Halassa, MD, PhD; and Manish Zinzuvadia, MD. This article includes the key consensus findings.
Mechanistic Distinction and Clinical Relevance
At the circuit level, schizophrenia is characterized by a cortical excitatory glutamatergic and inhibitory GABAergic imbalance, which disrupts basic cortical information processing38 and is thought to lead to the core symptoms of schizophrenia.39-42 Traditional antipsychotics are thought to act, primarily, by blocking dopamine D2 receptors, thereby mitigating the downstream effects of excessive dopamine signaling. A major hypothesis in schizophrenia is that hyperdopaminergia within mesolimbic pathways underlies positive symptoms such as hallucinations, delusions, and disorganized thought.43-46 Broader dopaminergic dysregulation, including hypodopaminergic states in frontal cortex, has been implicated in cognitive impairment and negative symptoms.47 Current antipsychotics block dopamine D2 receptors, providing control of positive symptoms for many patients, but fail to address the broader abnormalities that account for the full spectrum of aberrant dopaminergic transmission.48 Conversely, muscarinic modulation may offer an upstream approach; activation of M4 receptors, located subcortically, can reduce presynaptic dopamine release and primarily address positive symptoms, while activation of M1 receptors, enriched in frontal cortical excitatory circuits, may improve negative symptoms and may potentially address cognitive impairment while correcting aberrant dopaminergic signaling via top-down executive control.49,50 As Dr Melnick explained, “M1 and M4 are mostly seen in the brain. Activating M1 and M4 essentially helps reduce dopamine downstream without blocking the D2 receptor.” He expanded on that statement by adding, “If you activate M1, you’re essentially hitting the brake. That brake slows down activity in the ventral tegmental area (VTA), which in turn reduces dopamine release into the striatum.” Detailed comparisons of mechanistic pathways of XT versus traditional antipsychotics are presented in Table 1.
An additional possibility regarding XT’s circuit mechanism of action may be its impact on prefrontal cortex (PFC) function. Prefrontal cortex hypofunction (hypofrontality) in schizophrenia is well-documented via functional neuroimaging,51,52 and its origins have been variously attributed to neurodevelopmental mechanisms such as excessive synaptic pruning in adolescence.53,54 Because the PFC is a major source of “top-down” executive control,55 its reduced functional output is thought to drive widespread circuit dysregulation, including the aberrant dopaminergic activity described above. Importantly, the PFC also plays a central role in motivational drive, suggesting that M1-mediated activation may contribute to the notable effects of XT on negative symptoms in schizophrenia, as discussed later in this article.
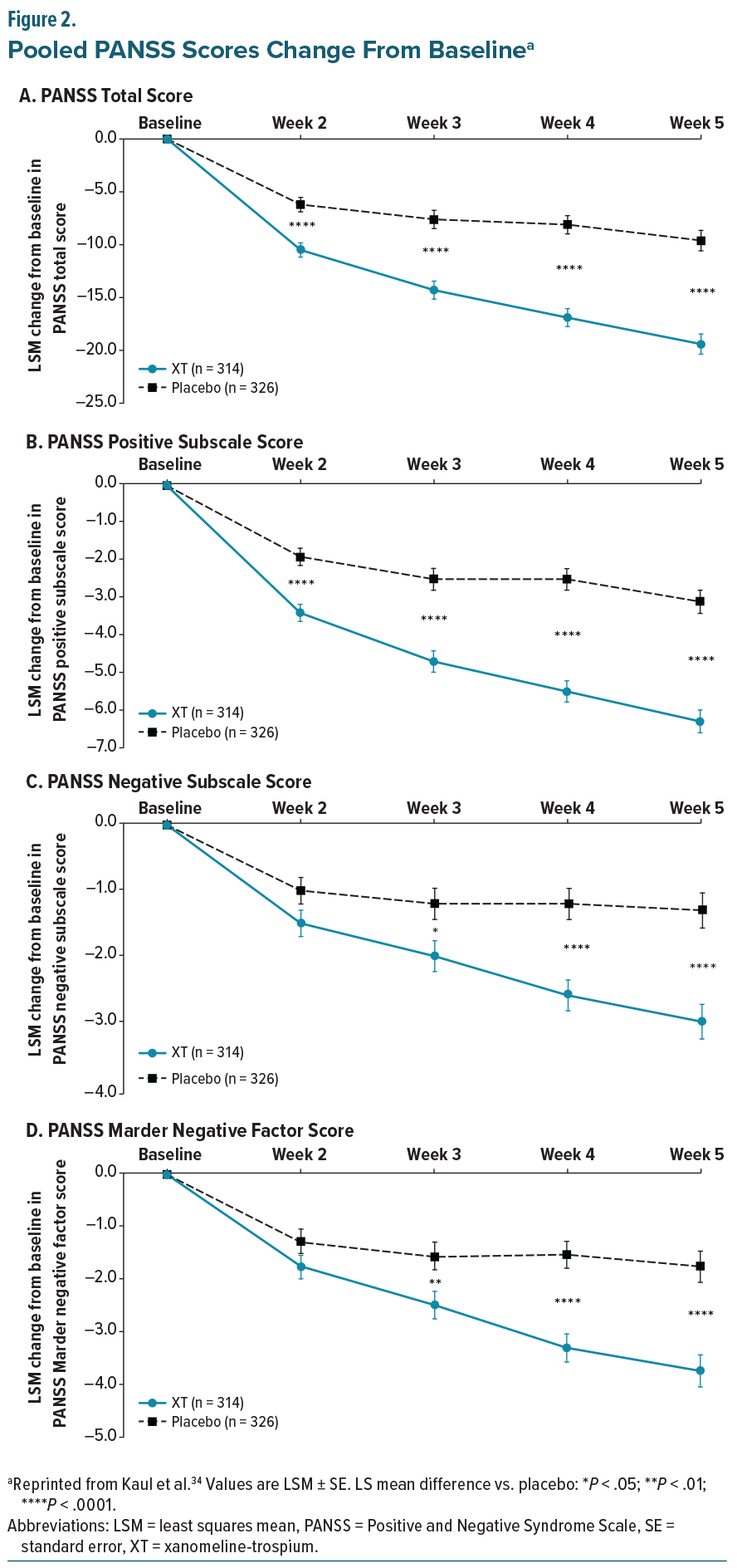
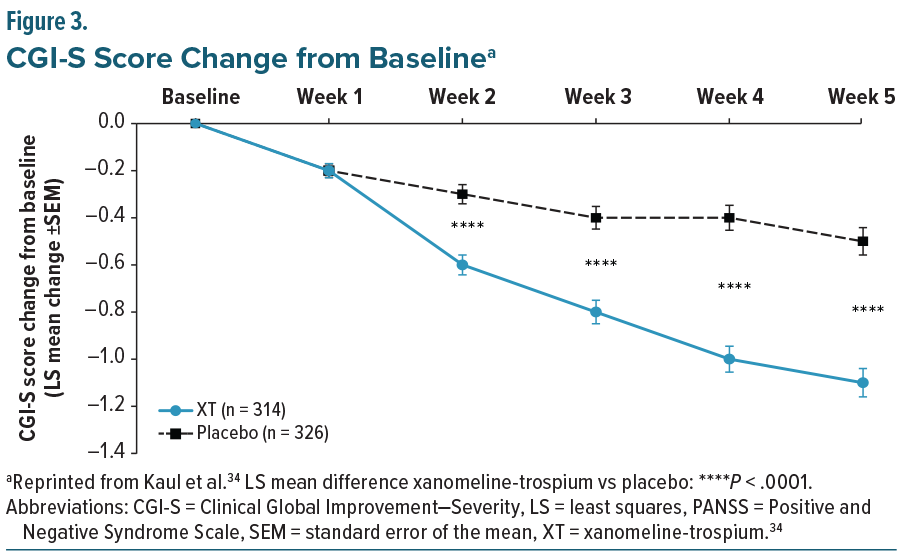
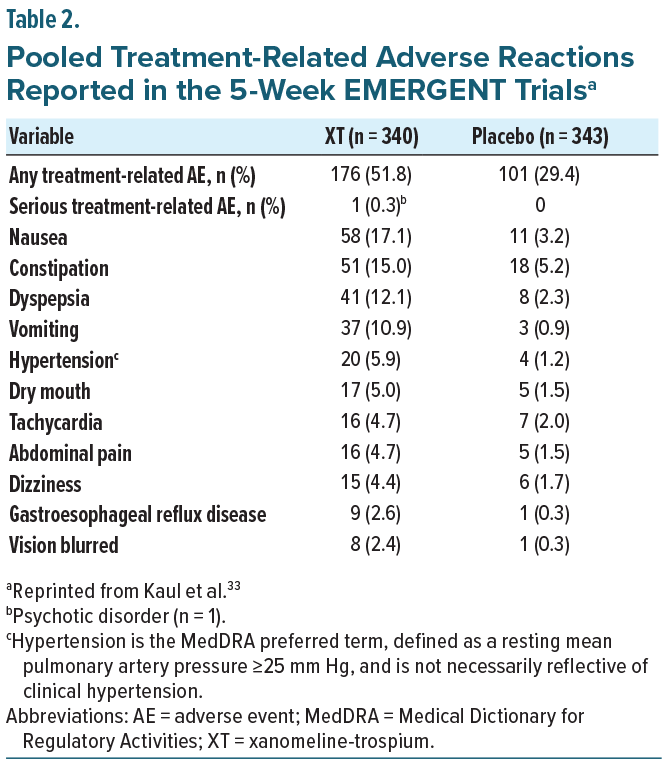
The EMERGENT trials (EMERGENT-1 [NCT03697252], EMERGENT-2 [NCT04659161], and EMERGENT-3 [NCT04738123]) were randomized, double-blind, placebo-controlled, 5-week inpatient studies designed to evaluate the safety and efficacy of XT in adults aged 18–60 years with schizophrenia who had experienced an acute exacerbation of positive symptoms requiring hospitalization within the prior 2 months. The primary endpoint was the change from baseline in the Positive and Negative Syndrome Scale (PANSS) total score, with secondary endpoints including PANSS positive, PANSS negative, PANSS Marder negative factor, and Clinical Global Impression–Severity (CGI-S) scores.33-35 Post-hoc pooled efficacy results demonstrated that XT was associated with statistically significant improvements in PANSS total and positive subscale scores compared with placebo, with separation evident at the first assessment and continuing to increase through the end of the study (Figure 2).34 Reductions in PANSS negative symptoms, including the Marder negative factor, emerged more gradually, with numerical differences appearing early but not reaching statistical significance until week 3 (Figure 2). As with PANSS total and positive subscales, these improvements continued to diverge from placebo over time.34 CGI-S scores also showed statistically significant separation by week 2, with progressive improvement maintained through study completion (Figure 3).34 Taken together, these findings indicate that XT provides both early antipsychotic efficacy and more gradual benefits on negative symptoms, supporting its potential to address multiple symptom domains in schizophrenia.
A separate pooled analysis of the EMERGENT 1–3 trials examined the safety and tolerability of XT in the same patients. Throughout the 5-week studies, discontinuation rates were similar between groups (27.6% XT vs 22.7% placebo), though treatment-emergent adverse events were more common with XT (67.9% vs 51.3%), largely gastrointestinal in nature, and generally mild or moderate (Table 2). Subgroup analyses revealed no clinically meaningful differences, and rates of EPS, somnolence, and weight gain remained low in both groups.33 It is important to note that in the EMERGENT trials, XT was taken on an empty stomach, as trospium chloride is not well absorbed when taken with food, potentially attenuating the peripheral anticholinergic effects of trospium. Nausea and vomiting can be problematic if XT is administered after a patient has eaten.56
Following the completion of EMERGENT-1, -2, and -3, two 52-week long-term, open-label extension trials, EMERGENT-4 (NCT04659174)37 and EMERGENT-5 (NCT04820309),36,56 were conducted. EMERGENT-4 included eligible patients who had completed EMERGENT 1–3 regardless of if they had been randomized to XT or placebo, while EMERGENT-5 was designed to demonstrate the long-term safety and efficacy of XT in patients who were naïve to XT therapy. Analysis from EMERGENT-4 demonstrated that improvements in PANSS total score with XT were evident by week 2 and sustained through week 52, with 68.6% and 37.1% of participants achieving ≥30% and ≥50% improvement from baseline in their PANSS scores, respectively. Among those who continued XT from the double-blind study, 73.7% achieved ≥30% improvement in PANSS scores, compared with 62.5% who were switched from placebo in the parent study to XT, while CGI-S improvements of at least 1 point were observed in 82.9% of the overall population by week 52. At that time, 42.9% of participants achieved CGI-S scores ≤3, indicating mild illness severity.
Safety findings indicated 53% of participants experienced at least 1 treatment-emergent adverse event (TEAE), most of which were mild to moderate in nature and consistent with the muscarinic activity of XT. The most frequent anticholinergic events were dry mouth (17.8%), constipation (9.9%), and dyspepsia (8.6%), while common procholinergic events included nausea (10.5%), vomiting (6.6%), and diarrhea (5.9%); these typically arose within the first 2 weeks, were self-limited, and rarely led to discontinuation. Serious TEAEs occurred in 3.6%–7.4% of participants.
Panel Consensus Statement #1
“Xanomeline-Trospium (XT) Should Be Considered Early in the Treatment Course for Appropriate Patients.” XT demonstrates efficacy in managing both positive and negative symptoms of schizophrenia and may support functional improvement and cognitive preservation. These benefits are especially relevant in first-episode psychosis and early-stage illness, where early intervention may alter the trajectory of disease progression. Because schizophrenia requires lifelong treatment, initiating therapy with a well-tolerated agent like xanomeline-trospium may reduce the burden of long-term complications commonly associated with antipsychotic therapy and support sustained functional recovery.
Expert Strategies for Initiation and Titration of XT Monotherapy in Outpatients
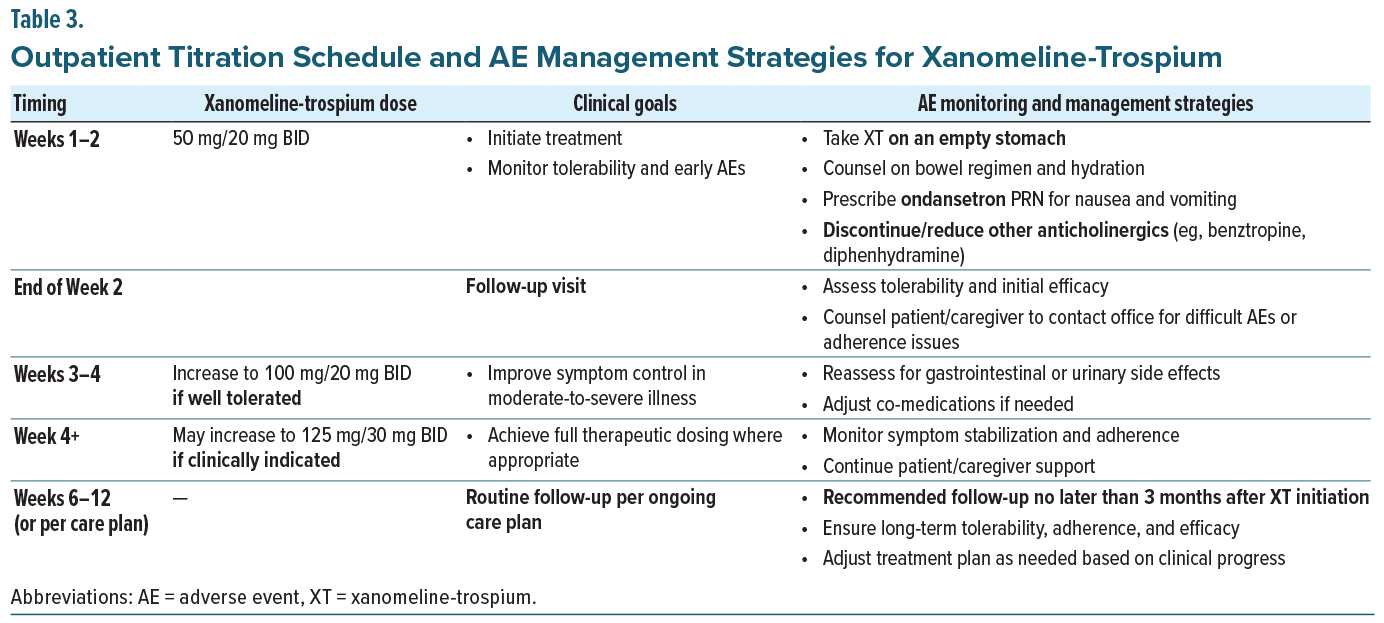
Xanomeline-trospium is available in 3 fixed dosed combinations, a starting dose of 50 mg/20 mg and 2 maintenance doses, 100 mg/20 mg and 125 mg/30 mg. All doses should be taken twice daily in the absence of food. The panelists reached consensus that outpatient initiation of XT should begin with the starting dose of 50 mg/20 mg twice daily for 1 to 2 weeks, with efficacy and tolerability assessed at the first follow-up, ideally in person to closely monitor for symptom improvement and side effects. Dose titration should then proceed with careful clinical monitoring: if the medication is well tolerated, the dose may be increased to 100 mg/20 mg, with follow-up scheduled according to the patient’s care plan, typically within 2–12 weeks. If XT continues to be well tolerated, the dose may be further increased to 125 mg/30 mg, again with follow-up scheduled per the patient’s care plan (see Table 3 for a detailed initiation plan). The panel emphasized the importance of timely initiation, recommending that patients fill their prescription immediately after the appointment so treatment can begin that same evening. In their collective experience, the panelists noted that the majority of patients stabilize on 100 mg/20 mg–125 mg/30 mg twice daily; however, a minority of patients, particularly younger individuals or those earlier in their illness course, have achieved good control on the starting dose of 50 mg/20 mg. It is worth noting that in the EMERGENT trials, all participants were titrated to the maximum dose of 125 mg/30 mg, though dose reduction to 100 mg/20 mg was permitted if tolerability concerns arose.35
Because gastrointestinal adverse events such as nausea and vomiting are among the most distressing tolerability issues, the panel recommended that all patients be co-prescribed a 14-day supply of ondansetron 4 mg at initiation, with instructions to re-dose after 30 minutes if symptoms persist. This proactive approach was viewed as an effective strategy to facilitate adherence and reduce the risk of early discontinuation. Patients should also be counseled to take XT on an empty stomach, as food can interfere with trospium chloride absorption and increase the likelihood of gastrointestinal side effects. Based on their clinical experience, the panel agreed that by using this strategy, nausea and vomiting have not been a major concern when initiating XT. They further recommended avoiding other antiemetics, particularly dopamine receptor antagonists, which may interfere with other psychotropic medications.
Cognitive impairment often begins during the prodromal phase of schizophrenia and can be present for 5–10 years before the onset of psychosis.57,58 Given this trajectory, the panelists agreed that XT represents a good option for early initiation because it provides control of both positive and negative symptoms while also offering potential benefits for cognition. In clinical practice, the panelists noted that efficacy was observed relatively quickly in the outpatient setting, often within 1 week, particularly for negative symptoms and cognitive impairment. Ms Crown stated, “Often, I don’t need to go beyond 100 mg/20 mg; even 50 mg/20 mg BID can be sufficient to control symptoms, especially in early psychosis.” She further observed, “My patients come in after 1 week of XT therapy, and it really helps their thoughts become more organized and clearer. I see tangible pro-cognitive effects with this treatment.”
The panel noted that a careful review of all medications, including prescriptions, over-the-counter medicines, and dietary supplements, is important when initiating any new therapy. Management of anticholinergic burden is particularly important when considering XT, as trospium is a potent peripherally acting anticholinergic. The panelists emphasized reducing or eliminating unnecessary anticholinergic agents whenever possible. For example, many patients with schizophrenia use diphenhydramine for sleep, which can substantially increase anticholinergic load; the panel recommended discontinuing it in favor of a more appropriate sleep aid, such as an orexin-receptor blocking agent (eg, suvorexant). Other sources of cholinergic burden, such as medications for bladder control, COPD, Alzheimer’s disease, and Parkinson’s disease, should also be carefully reviewed. Management of urinary retention is especially important, as it is typically treated with anticholinergic agents. Keeping this in mind, the panelists further noted that XT may not be an appropriate option for men over 50 years of age with a history of benign prostatic hyperplasia, which may be associated with urinary retention.59
Panel Consensus Statement #2
“Initiation and Titration Should Be Individualized Based on Symptom Management and Tolerability.” Outpatient initiation of XT should begin at 50 mg/20 mg twice daily for 1 to 2 weeks. Dose titration should include close clinical monitoring to assess efficacy and manage emergent adverse effects. The panel recommends prophylactic prescription of ondansetron to mitigate nausea and vomiting. While some individuals demonstrate adequate symptom control at the 100 mg/20 mg dose, others may require full titration to 125 mg/30 mg to achieve maximum therapeutic benefit.
Initiation and Titration Strategies in the Inpatient Setting
In addition to its utility in the outpatient setting, XT has demonstrated the ability to produce rapid symptom improvement in hospitalized patients. Often, patients admitted for inpatient care are acutely decompensated, frequently having discontinued their medications prior to hospitalization. Because of the decompensated nature of the patient, rapid control of symptoms is crucial for decreasing the risk of harm to the patient, healthcare staff, and other individuals that may come into contact with the patient.60,61 In clinical practice, the panelists have noted meaningful changes in negative symptoms and overall illness severity. As Dr Halassa noted, “After a few doses at 50 mg/20 mg, I see marked improvement in negative symptoms,” underscoring the rapid onset of efficacy observed in the inpatient setting. Because inpatient care is typically brief and requires urgent symptom control, gradual titration is less practical. Instead, Dr Halassa recommended a faster dosing strategy, similar to that used in the EMERGENT trials, with escalation to 100 mg/20 mg–125 mg/30 mg by day 2 or 3 of hospitalization.62
The inpatient setting provides several advantages for initiation of XT. Adverse events can be more readily managed given the availability of 24-hour nursing support and daily prescriber oversight. To address gastrointestinal adverse events, care must be taken to ensure dosing on an empty stomach. To ensure proactive management of cholinergic side effects, especially nausea and vomiting, the panel again recommended concomitant ondansetron at initiation. This proactive strategy was viewed as effective for reducing the likelihood of early discontinuation and is facilitated by the structured nature of inpatient care, where supportive medications can be administered promptly and consistently. Many patients are far into their illness course and are typically on high doses of second-generation antipsychotics at the time of admission. In Dr Halassa’s experience, and consistent with the literature, these individuals are frequently on complex polypharmacy regimens.12 He frequently uses XT in combination with an atypical antipsychotic to achieve sufficient stabilization for discharge. Importantly, his clinical experience suggests that XT may be antipsychotic dose-sparing. Dr Halassa noted that the combination of XT with a lower dose of an atypical antipsychotic has provided effective symptom control while reducing reliance on higher traditional antipsychotic doses. This approach has the potential to be a safer long-term strategy, as many of the adverse effects associated with antipsychotics, such as metabolic burden, extrapyramidal symptoms, and tardive dyskinesia, are dose related.16–18,29
Panel Consensus Statement #3
“Most Clinically Relevant Adverse Events Associated with Xanomeline-Trospium Are Anticholinergic; Proactive Management Is Key.” Monitor for urinary retention, dry mouth, and constipation. Discontinue or minimize additive burden from other anticholinergic agents.
Recent real-world data reported by Dr. Halassa63 further support XT’s observed inpatient efficacy. In a retrospective analysis of 49 hospitalized patients with chronic, treatment-resistant psychotic disorders, approximately half demonstrated clinically meaningful improvement following initiation of XT. Computational clustering and discriminant analyses identified negative symptoms and a history of stimulant use as the strongest predictors of response, whereas intellectual disability was negatively predictive. These findings echo the panel’s clinical experience that XT produces brightening of affect and social engagement in patients with prominent negative symptoms, while suggesting its potential role in stimulant-associated psychosis and its limited utility in neurodevelopmental subgroups. Collectively, this work reinforces XT’s therapeutic potential as a mechanistically distinct treatment option for acutely decompensated inpatients, particularly those with negative-symptom–dominant presentations.
Inpatient care is also an opportunity to prepare for a smooth transition to outpatient management. Discharge planning should include collaboration with case managers, social workers, therapists, and nursing staff to ensure continuity of care and reinforcement of adherence strategies. Patients and their care team should receive counseling on practical aspects of treatment, including adherence to fasting requirements and recognition of common side effects. The panel agreed that every patient should have a follow-up appointment scheduled with their regular psychiatrist prior to discharge to maintain treatment momentum and support long-term stability.
Switching, Adding, and Cross-Titration
Side effect burden is one of the most commonly cited reasons for medication discontinuation and nonadherence in patients with serious mental illness.14,64,65 When side effects become intolerable, a clinician-guided approach to medication discontinuation or down-titration is strongly preferred over abrupt discontinuation, which may be associated with dopamine hypersensitivity and rebound psychosis, thus worsening clinical outcomes.66-68 Because of its novel mechanism of action and favorable tolerability profile, which is not associated with metabolic dysregulation, weight gain, prolactin elevation, or EPS,16,18,29,69 XT offers an appropriate alternative during cross-titration for patients whose recovery is limited by antipsychotic side effects.
For many patients, particularly those further along in the course of their illness, treatment of schizophrenia remains a polypharmacy endeavor.12,70 It is important to note that in clinical development, XT was studied as a monotherapy; currently, there is little information in the literature on combining an antipsychotic with XT. That said, in cases where a polypharmacy approach is necessary, the panel noted based on their clinical experience that XT is an ideal choice as an add-on therapy due to its distinct mechanism of action and differentiated side effect profile. Cross-titration may be considered when patients remain symptomatic despite adherence to antipsychotic therapy, as those with longer illness duration often continue to experience breakthrough positive or negative symptoms or persistent cognitive impairment, which can hinder functional recovery.71,72 In these cases, the panel recommends discontinuation of current antipsychotic therapy while initiating XT. The patient should be closely monitored during initiation. If decompensation occurs, the antipsychotic should be reintroduced. Importantly, the panel noted that in their clinical experience with XT, that it may be antipsychotic dose-sparing in this setting. Further, discussion emphasized that the combination of XT at 100 mg/20 mg–125 mg/30 mg and a low dose of an atypical antipsychotic has often provided enhanced symptom control, resulting in better outcomes for the patient. As Dr Melnick observed, “We’ve noticed that we’ve been able to lower the dose of antipsychotics for many of our patients that are on a maintenance dose of XT.” This strategy has the potential to reduce the long-term risks of antipsychotic side effects including movement disorders and metabolic complications, among others.16,18,29,69
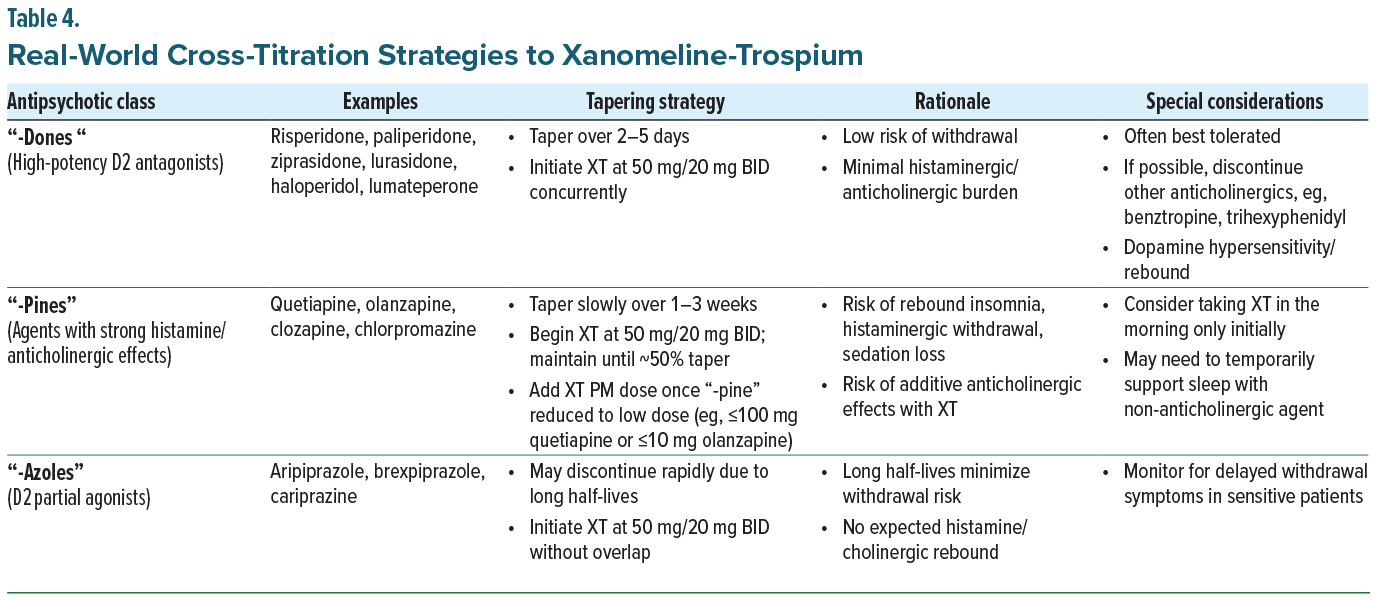
Regardless of whether the rationale for cross-titration is side effect burden or unresolved symptoms, the panel emphasized a structured, clinician-guided approach to medication discontinuation or down-titration, which is dependent on the class of antipsychotic the patient is currently taking (Table 4). In clinical practice, patients should begin the starting dose of XT, 50 mg/20 mg, the same evening they are seen in clinic, while tapering the existing antipsychotic according to its pharmacologic class. For example, “-dones” (eg, risperidone, paliperidone) can often be tapered over several days with minimal withdrawal risk, whereas “-pines” (eg, quetiapine, olanzapine, clozapine) require a slower taper over 1 to 3 weeks to prevent rebound sedation or insomnia. Dopamine D2 receptor partial agonists (“-azoles”), such as aripiprazole, brexpiprazole, or cariprazine, may generally be discontinued more quickly given their long half-lives. Patient counseling remains critical during this transition. Patients should be instructed to take XT on an empty stomach to optimize trospium absorption and reassured that side effects tend to wane with continued use.33 Prophylactic prescription of ondansetron remains warranted to mitigate nausea and vomiting, which panelists noted tend to be more frequent early in treatment. As always, close clinical follow-up is recommended to ensure tolerability and guide adjustments during the overlap period. The recommended tapering strategies are summarized in Table 4.
Panel Consensus Statement #4
“Cross-Titration with Existing Antipsychotics Is Often Necessary in Clinical Practice.” While XT is approved as for monotherapy in adults with schizophrenia, many patients benefit from cross-titration strategies. High-potency D2 antagonists, such as risperidone, can be tapered more rapidly than antipsychotics with strong histaminergic or anticholinergic activity, such as quetiapine or clozapine. Partial agonists like aripiprazole and cariprazine may not require tapering due to the longer half-lives of the class.
Special Considerations
An additional consideration in patients with schizophrenia is the use of long-acting injectable (LAI) antipsychotics. LAIs offer several advantages, including reduced pill burden, improved adherence to concomitant medications, and lower risks of symptom recurrence and hospitalization.73-76 As noted previously, in clinical practice, the faculty typically taper or discontinue other antipsychotics while initiating XT. The panelists noted that many patients, possibly as many as 50%, are well managed with XT 100 mg/20 mg in combination with a low dose of an atypical antipsychotic. This approach is particularly relevant for patients who had previously been maintained on high doses of antipsychotics yet continued to experience unresolved symptoms and/or cognitive impairments. In this scenario, the panel recommends initiating the XT concomitantly with the LAI. If symptoms improve once the patient is at a maintenance dose of XT, down-titration of the LAI dose can be considered through shared-decision making with the patient and their family and/or care support team.
Discussion
The consensus panel highlighted that the introduction of XT represents an important advance in the treatment of schizophrenia. Unlike traditional antipsychotics that act via dopamine receptor antagonism or partial agonism, XT targets muscarinic M1 and M4 receptors, providing a different molecular mechanism of action and likely distinct circuit-level impact. This distinction offers clinicians new flexibility in patient management across treatment settings, from outpatient care to inpatient stabilization and cross-titration in patients already on antipsychotics.
For outpatients, initiation of XT should be individualized based on symptom burden, tolerability, and prior treatment history. Early use of XT in patients with first-episode psychosis or early in the illness course may support symptomatic improvement while minimizing the long-term risks associated with antipsychotics. Outpatient initiation requires careful titration, close monitoring, and patient counseling to reinforce fasted dosing, adherence, and tolerability optimization. Concomitant prescription of ondansetron is recommended to proactively address the potential of procholinergic side effects.
In contrast, the inpatient setting often involves patients who have discontinued medication and present with acute exacerbations or have long-standing illness with complex polypharmacy. Here, the panelists noted that XT can demonstrate rapid efficacy, including improvements in positive as well as negative symptoms, which may facilitate earlier stabilization and discharge planning. Importantly, inpatient initiation must also anticipate the transition to outpatient follow-up, with coordination among the broader care team to ensure continuity of treatment.
Cross-titration represents a third critical scenario, relevant for patients who remain symptomatic despite adherence to antipsychotic therapy or who are experiencing intolerable side effects. The panel agreed that XT is an attractive option in these circumstances because it can be added to ongoing antipsychotics, with the potential to reduce antipsychotic dose requirements over time. A structured, class-specific tapering approach is recommended to minimize withdrawal effects, with careful patient counseling on dosing without food and proactive use of ondansetron to manage early gastrointestinal effects. LAIs require particular consideration: rather than discontinuation, dose reduction may be the more appropriate strategy once a maintenance dose of XT has been achieved, maintaining or improving efficacy while reducing the cumulative burden of dopamine receptor blockade.
Taken together, these strategies underscore the versatility of XT across clinical scenarios. Whether used as a first-line treatment in early illness, as an inpatient option for acute stabilization, or as an adjunct to ongoing antipsychotic therapy, XT broadens the therapeutic toolkit available to clinicians. Its unique mechanism of action not only addresses persistent symptoms that remain refractory to dopamine-based treatments but also provides the potential for improved long-term safety by reducing reliance on higher antipsychotic doses. Future research and ongoing real-world experience will continue to refine best practices for initiation, titration, and maintenance of XT, but the consensus of this panel is that XT should be considered an important addition to the therapeutic landscape of schizophrenia.
Panel Consensus Statement #5
“XT may allow for antipsychotic dose reduction while maintaining or improving efficacy, potentially mitigating the risk of cumulative, long-term adverse effects.” The panel agreed that the novel mechanism of action of XT provides the opportunity to achieve symptom control at lower doses of concomitant antipsychotics. This dose-sparing effect is particularly important because many adverse effects associated with dopamine receptor blockade are dose dependent and accumulate with long-term use. Given that schizophrenia is a chronic condition requiring lifelong pharmacologic treatment, the potential to maintain efficacy while reducing reliance on higher doses of dopamine antagonists represents a meaningful advance for long-term safety and tolerability.
Conclusions
Xanomeline-trospium represents a novel and clinically meaningful addition to the treatment of schizophrenia. Across outpatient, inpatient, and cross-titration settings, the expert panel emphasized its versatility, tolerability, and potential to address persistent symptoms while reducing reliance on high-dose dopamine antagonists. By expanding therapeutic options, XT offers clinicians a new pathway toward improving both short- and long-term outcomes for patients living with schizophrenia.
The strategies outlined in this report reflect the consensus of experienced clinicians who, collectively and at the time of the consensus panel meeting, had treated approximately 200 patients with XT. As such, these recommendations are based on early clinical experience and should be interpreted with appropriate caution. Limitations include the absence of randomized head-to-head comparisons with standard antipsychotics, lack of long-term safety and functional outcome data, and the potential for practice patterns to evolve as familiarity with XT grows. Future research, including prospective real-world evidence studies and pragmatic trials, will be critical to validate these approaches, clarify optimal patient selection, and define best practices for initiation, titration, and maintenance of XT in routine clinical care.
Treatment with XT represents a paradigm shift in schizophrenia management by modulating the muscarinic acetylcholine receptor system. This unique mechanism of action, supported by both randomized, placebo-controlled, clinical trial data and panelist experience, indicates that XT provides effective control of positive, negative, and cognitive symptoms with an acceptable and manageable side effect profile. The experts who participated in this consensus panel have extensive experience treating patients with schizophrenia, and they used that knowledge to reach agreements and provide recommendations that offer practical, early insights for clinicians, including how they can integrate XT into their treatment armamentarium for patients with schizophrenia. All members of the panel agreed that both continued education and practice-based guidance are crucial for broader adoption of XT in order to improve treatment of schizophrenia.
Financial Disclosures
Dr Halassa declares no conflicts and received no financial compensation for participation in the consensus panel meeting. All other authors received an honorarium from Bristol Myers Squibb for participation in the panel meeting.
Dr Melnick has received honoraria for speaking/teaching from AbbVie, Alkermes, Axsome, Boehringer Ingelheim, Bristol Myers Squibb, Eisai, Idorsia, Indivior, Intra-Cellular Therapies, Janssen, Lilly, Lundbeck, Merck, Neurocrine, Otsuka, Sage, Sunovion, Takeda, Teva, Vanda; and has received advisory board fees from Abbvie, Alkermes, Axsome, Boehringer Ingelheim, Bristol Myers Squibb, Eisai, Idorsia, Indivior, Intra-Cellular Therapies, Janssen, Lilly, Lundbeck, Merck, Neurocrine, Otsuka, Sage, Sunovion, Takeda, Teva. Ms Crown has received honoraria for speaking/teaching from Axsome Therapeutics, Boehringer-Ingelheim, Bristol-Myers Squibb, Intra-Cellular Therapies, Johnson & Johnson Pharmaceuticals, Karuna Therapeutics, Lundbeck, Neurocrine Biosciences, Otsuka, Supernus Pharmaceuticals, and Takeda; has received consulting fees from Psychiatric Times. Dr Zinzuvadia has received honoraria for speaking/teaching from AbbVie, Alkermes, Axsome, Bristol Myers Squibb, Luye, Otsuka, and Teva.
References (76)

- Correll CU, Martin A, Patel C, et al. Systematic literature review of schizophrenia clinical practice guidelines on acute and maintenance management with antipsychotics. Schizophrenia (Heidelb). Feb 24 2022;8(1):5. doi.org/10.1038/s41537-021-00192-x
- Keepers GA, Fochtmann LJ, Anzia JM, et al. The American Psychiatric Association Practice Guideline for the Treatment of Patients With Schizophrenia. Am J Psychiatry. 2020;177(9):868–872. doi.org/10.1176/appi.ajp.2020.177901
- Emsley R, Chiliza B, Asmal L. The evidence for illness progression after relapse in schizophrenia. Schizophr Res. 2013;148(1–3):117–21. doi.org/10.1016/j.schres.2013.05.016
- American Psychiatric Association. Schizophrenia spectrum and other psychotic disorders. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, Text Revision. American Psychiatric Association; 2022.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
- Carbon M, Correll CU. Thinking and acting beyond the positive: the role of the cognitive and negative symptoms in schizophrenia. CNS Spectr. Dec 2014;19 Suppl 1:38–52; quiz 35–7, 53. doi.org/10.1017/S1092852914000601
- Correll CU, Schooler NR. Negative symptoms in schizophrenia: a review and clinical guide for recognition, assessment, and treatment. Neuropsychiatr Dis Treat. 2020;16:519–534. doi.org/10.2147/NDT.S225643
- Millan MJ, Agid Y, Brune M, et al. Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat Rev Drug Discov. 2012;11(2):141–68. doi.org/10.1038/nrd3628
- Anticevic A, Halassa MM. The thalamus in psychosis spectrum disorder. Front Neurosci. 2023;17:1163600. doi.org/10.3389/fnins.2023.1163600
- Halassa MM, Frank MJ, Garety P, et al. Developing algorithmic psychiatry via multi-level spanning computational models. Cell Rep Med. 2025;6(5):102094. doi.org/10.1016/j.xcrm.2025.102094
- Haddad PM, Correll CU. Long-acting antipsychotics in the treatment of schizophrenia: opportunities and challenges. Expert Opin Pharmacother. 2023;24(4):473–493. doi.org/10.1080/14656566.2023.2181073
- Lahteenvuo M, Tiihonen J. Antipsychotic polypharmacy for the management of schizophrenia: evidence and recommendations. Drugs. 2021;81(11):1273–1284. doi.org/10.1007/s40265-021-01556-4
- Bowie CR, Harvey PD. Cognition in schizophrenia: impairments, determinants, and functional importance. Psychiatr Clin North Am. 2005;28(3):613–33, 626. doi.org/10.1016/j.psc.2005.05.004
- Doane MJ, Raymond K, Saucier C, et al. Unmet needs with antipsychotic treatment in schizophrenia and bipolar I disorder: patient perspectives from qualitative focus groups. BMC Psychiatry. 2023;23(1):245. doi.org/10.1186/s12888-023-04746-4
- Andrade C. Schizophrenia, antipsychotic drugs, and risk of breast cancer. J Clin Psychiatry. 2025;86(3):25f15960.
- Burschinski A, Schneider-Thoma J, Chiocchia V, et al. Metabolic side effects in persons with schizophrenia during mid- to long-term treatment with antipsychotics: a network meta-analysis of randomized controlled trials. World Psychiatry. 2023;22(1):116–128. doi.org/10.1002/wps.21036
- Carbon M, Hsieh CH, Kane JM, et al. Tardive dyskinesia prevalence in the period of second-generation antipsychotic use: a meta-analysis. J Clin Psychiatry. 2017;78(3):e264-e278. doi.org/10.4088/JCP.16r10832
- Huhn M, Nikolakopoulou A, Schneider-Thoma J, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet. 2019;394(10202):939–951. doi.org/10.1016/S0140-6736(19)31135-3
- Pillinger T, Howes OD, Correll CU, et al. Antidepressant and antipsychotic side-effects and personalised prescribing: a systematic review and digital tool development. Lancet Psychiatry. 2023;10(11):860–876. doi.org/10.1016/S2215-0366(23)00262-6
- Yohn SE, Weiden PJ, Felder CC, et al. Muscarinic acetylcholine receptors for psychotic disorders: bench-side to clinic. Trends Pharmacol Sci. 2022;43(12):1098–1112. doi.org/10.1016/j.tips.2022.09.006
- Howes OD, Shatalina E. Integrating the neurodevelopmental and dopamine hypotheses of schizophrenia and the role of cortical excitation-inhibition balance. Biol Psychiatry. 2022;92(6):501–513. doi.org/10.1016/j.biopsych.2022.06.017
- Mishra W, Kheradpezhouh E, Arabzadeh E. Activation of M1 cholinergic receptors in mouse somatosensory cortex enhances information processing and detection behaviour. Commun Biol. 2024;7(1):3. doi.org/10.1038/s42003-023-05699-w
- Yohn SE, Harvey PD, Brannan SK, et al. The potential of muscarinic M(1) and M(4) receptor activators for the treatment of cognitive impairment associated with schizophrenia. Front Psychiatry. 2024;15:1421554. doi.org/10.3389/fpsyt.2024.1421554
- Saint-Georges Z, MacDonald J, Al-Khalili R, et al. Cholinergic system in schizophrenia: a systematic review and meta-analysis. Mol Psychiatry. 2025;30(7):3301–3315. doi.org/10.1038/s41380-025-03023-y
- Bodick NC, Offen WW, Levey AI, et al. Effects of xanomeline, a selective muscarinic receptor agonist, on cognitive function and behavioral symptoms in Alzheimer disease. Arch Neurol. 1997;54(4):465–73. doi.org/10.1001/archneur.1997.00550160091022
- Sanctura [package insert]. Allergan; 2011. Accessed August 20, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/021595s006lbl.pdf
- Madersbacher H, Rovner E. Trospium chloride: the European experience. Expert Opin Pharmacother. 2006;7(10):1373–80. doi.org/10.1517/14656566.7.10.1373
- COBENFY (xanomeline and trospium chloride) capsules, for oral use [package insert]. Bristol-Myers Squibb; 2024.
- Pillinger T, McCutcheon RA, Vano L, et al. Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: a systematic review and network meta-analysis. Lancet Psychiatry. 2020;7(1):64–77. doi.org/10.1016/S2215-0366(19)30416-X
- Dean B, Scarr E. Muscarinic M1 and M4 receptors: hypothesis driven drug development for schizophrenia. Psychiatry Res. 2020;288:112989. doi.org/10.1016/j.psychres.2020.112989
- Horan WP, Targum SD, Claxton A, et al. Efficacy of KarXT on negative symptoms in acute schizophrenia: a post hoc analysis of pooled data from 3 trials. Schizophr Res. 2024;274:57–65. doi.org/10.1016/j.schres.2024.08.001
- Horan WP, Sauder C, Harvey PD, et al. The impact of xanomeline and trospium chloride on cognitive impairment in acute schizophrenia: replication in pooled data from two phase 3 trials. Am J Psychiatry. 2025;182(3):297–306. doi.org/10.1176/appi.ajp.20240076
- Kaul I, Claxton A, Sawchak S, et al. Safety and tolerability of xanomeline and trospium chloride in schizophrenia: pooled results from the 5-week, randomized, double-blind, placebo-controlled EMERGENT trials. J Clin Psychiatry. 2025;86(1)doi:10.4088/JCP.24m15497
- Kaul I, Sawchak S, Claxton A, et al. Efficacy of xanomeline and trospium chloride in schizophrenia: pooled results from three 5-week, randomized, double-blind, placebo-controlled, EMERGENT trials. Schizophrenia (Heidelb). 2024;10(1):102. doi.org/10.1038/s41537-024-00525-6
- Kaul I, Sawchak S, Walling DP, et al. Efficacy and safety of xanomeline-trospium chloride in schizophrenia: a randomized clinical trial. JAMA Psychiatry. 2024;81(8):749–756. doi.org/10.1001/jamapsychiatry.2024.0785
- Kaul I, Claxton A, Sauder C, et al. Long-term safety, tolerability, and efficacy of xanomeline and trospium chloride in people with schizophrenia: results from the 52-week, open-label EMERGENT-5 trial. Presented at: Nevada Psychiatric Association 30th Annual Psychopharmacology Update; February 13, 2025; Las Vegas, Nevada.
- Kaul I, Cohen EA, Miller AC, et al. Maintenance of efficacy of KarXT (xanomeline and trospium) in schizophrenia. Poster presented at: Annual Congress of the Schizophrenia International Research Society; April 3–7, 2024; Florence, Italy.
- Yizhar O, Fenno LE, Prigge M, et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477(7363):171–8. doi.org/10.1038/nature10360
- Uliana DL, Lisboa JRF, Gomes FV, et al. The excitatory-inhibitory balance as a target for the development of novel drugs to treat schizophrenia. Biochem Pharmacol. 2024;228:116298. doi.org/10.1016/j.bcp.2024.116298
- Anticevic A, Cole MW, Repovs G, et al. Connectivity, pharmacology, and computation: toward a mechanistic understanding of neural system dysfunction in schizophrenia. Front Psychiatry. 2013;4:169. doi.org/10.3389/fpsyt.2013.00169
- Pettine WW, Louie K, Murray JD, et al. Excitatory-inhibitory tone shapes decision strategies in a hierarchical neural network model of multi-attribute choice. PLoS Comput Biol. 2021;17(3):e1008791. doi.org/10.1371/journal.pcbi.1008791
- Yang GJ, Murray JD, Repovs G, et al. Altered global brain signal in schizophrenia. Proc Natl Acad Sci U S A. 2014;111(20):7438–43. doi.org/10.1073/pnas.1405289111
- Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry. 2003;160(1):13–23. doi.org/10.1176/appi.ajp.160.1.13
- Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III—the final common pathway. Schizophr Bull. 2009;35(3):549–62. doi.org/10.1093/schbul/sbp006
- McCutcheon R, Beck K, Jauhar S, et al. Defining the locus of dopaminergic dysfunction in schizophrenia: a meta-analysis and test of the mesolimbic hypothesis. Schizophr Bull. 2018;44(6):1301–1311. doi.org/10.1093/schbul/sbx180
- McCutcheon RA, Abi-Dargham A, Howes OD. Schizophrenia, dopamine and the striatum: from biology to symptoms. Trends Neurosci. 2019;42(3):205–220. doi.org/10.1016/j.tins.2018.12.004
- Howes OD, Nour MM. Dopamine and the aberrant salience hypothesis of schizophrenia. World Psychiatry. 2016;15(1):3–4. doi.org/10.1002/wps.20276
- McCutcheon RA, Krystal JH, Howes OD. Dopamine and glutamate in schizophrenia: biology, symptoms and treatment. World Psychiatry. 2020;19(1):15–33. doi.org/10.1002/wps.20693
- Dencker D, Thomsen M, Wortwein G, et al. Muscarinic acetylcholine receptor subtypes as potential drug targets for the treatment of schizophrenia, drug abuse and Parkinson’s disease. ACS Chem Neurosci. 2012;3(2):80–89. doi.org/10.1021/cn200110q
- Foster DJ, Jones CK, Conn PJ. Emerging approaches for treatment of schizophrenia: modulation of cholinergic signaling. Discov Med. 2012;14(79):413–20.
- Frankle WG, Lerma J, Laruelle M. The synaptic hypothesis of schizophrenia. Neuron. 2003;39(2):205–16. doi.org/10.1016/s0896-6273(03)00423-9
- Glahn DC, Ragland JD, Abramoff A, et al. Beyond hypofrontality: a quantitative meta-analysis of functional neuroimaging studies of working memory in schizophrenia. Hum Brain Mapp. 2005;25(1):60–9. doi.org/10.1002/hbm.20138
- Keshavan MS, Anderson S, Pettegrew JW. Is schizophrenia due to excessive synaptic pruning in the prefrontal cortex? The Feinberg hypothesis revisited. J Psychiatr Res. 1994;28(3):239–65. doi.org/10.1016/0022-3956(94)90009-4
- Selemon LD, Zecevic N. Schizophrenia: a tale of two critical periods for prefrontal cortical development. Transl Psychiatry. 2015;5(8):e623. doi.org/10.1038/tp.2015.115
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi.org/10.1146/annurev.neuro.24.1.167
- Citrome L. Understanding the tolerability profile of xanomeline-trospium combination for the treatment of schizophrenia. J Clin Psychiatry. 2025;86(1):doi:10.4088/JCP.25com15775
- Howes OD, Fusar-Poli P, Bloomfield M, et al. From the prodrome to chronic schizophrenia: the neurobiology underlying psychotic symptoms and cognitive impairments. Curr Pharm Des. 2012;18(4):459–65. doi.org/10.2174/138161212799316217
- Giuliano AJ, Li H, Mesholam-Gately RI, Sorenson SM, et al. Neurocognition in the psychosis risk syndrome: a quantitative and qualitative review. Curr Pharm Des. 2012;18(4):399–415. doi.org/10.2174/138161212799316019
- Price MZ, Price RL. Early outpatient clinical experience with xanomeline and trospium chloride for schizophrenia: a case report. Front Psychiatry. 2025;16:1630574. doi.org/10.3389/fpsyt.2025.1630574
- Pacciardi B, Mauri M, Cargioli C, et al. Issues in the management of acute agitation: how much current guidelines consider safety? Front Psychiatry. 2013;4:26. doi.org/10.3389/fpsyt.2013.00026
- Poranen J, Koistinaho A, Tanskanen A, et al. Twenty-year medication use trends in first-episode bipolar disorder. Acta Psychiatr Scand. 2022;146(6):583–593. doi.org/10.1111/acps.13504
- Kaul I, Sawchak S, Correll CU, et al. Efficacy and safety of the muscarinic receptor agonist KarXT (xanomeline-trospium) in schizophrenia (EMERGENT-2) in the USA: results from a randomised, double-blind, placebo-controlled, flexible-dose phase 3 trial. Lancet. 2024;403(10422):160–170. doi.org/10.1016/S0140-6736(23)02190-6
- Halassa MM. Preliminary real-world predictors of response to muscarinic targeting in psychosis. Nat Mental Health. Published online November 6, 2025. doi:10.1038/s44220-025-00529-w.
- Dibonaventura M, Gabriel S, Dupclay L, et al. A patient perspective of the impact of medication side effects on adherence: results of a cross-sectional nationwide survey of patients with schizophrenia. BMC Psychiatry. 2012;12:20. doi.org/10.1186/1471-244x-12-20
- Velligan DI, Weiden PJ, Sajatovic M, et al. Strategies for addressing adherence problems in patients with serious and persistent mental illness: recommendations from the expert consensus guidelines. J Psychiatr Pract. 2010;16(5):306–24. doi.org/10.1097/01.pra.0000388626.98662.a0
- Ostuzzi G, Vita G, Bertolini F, et al. Continuing, reducing, switching, or stopping antipsychotics in individuals with schizophrenia-spectrum disorders who are clinically stable: a systematic review and network meta-analysis. Lancet Psychiatry. 2022;9(8):614–624. doi.org/10.1016/S2215-0366(22)00158-4
- Yin J, Barr AM, Ramos-Miguel A, et al. Antipsychotic induced dopamine supersensitivity psychosis: a comprehensive review. Curr Neuropharmacol. 2017;15(1):174–183. doi.org/10.2174/1570159x14666160606093602
- Chouinard G, Samaha AN, Chouinard VA, et al. Antipsychotic-induced dopamine supersensitivity psychosis: pharmacology, criteria, and therapy. Psychother Psychosom. 2017;86(4):189–219. doi.org/10.1159/000477313
- 69. Carbon M, Kane JM, Leucht S, et al. Tardive dyskinesia risk with first- and second-generation antipsychotics in comparative randomized controlled trials: a meta-analysis. World Psychiatry. 2018;17(3):330–340. doi.org/10.1002/wps.20579
- Tiihonen J, Taipale H, Mehtala J, et al. Association of antipsychotic polypharmacy vs monotherapy with psychiatric rehospitalization among adults with schizophrenia. JAMA Psychiatry. 2019;76(5):499–507. doi.org/10.1001/jamapsychiatry.2018.4320
- Kane JM, Leucht S, Carpenter D, et al, Expert Consensus Panel for Optimizing Pharmacologic Treatment of Psychotic Disorders. The expert consensus guideline series. Optimizing pharmacologic treatment of psychotic disorders. Introduction: methods, commentary, and summary. J Clin Psychiatry. 2003;64 Suppl 12:5–19.
- Takeuchi H, Kantor N, Uchida H, et al. Immediate vs gradual discontinuation in antipsychotic switching: a systematic review and meta-analysis. Schizophr Bull. 2017;43(4):862–871. doi.org/10.1093/schbul/sbw171
- Bell Lynum K, Awasthi S, Huang S, et al. The impact of aripiprazole once-monthly initiation and persistence on concomitant psychiatric medications in adults: diagnosed with bipolar I disorder: a retrospective analysis of claims data from the United States. Presented at: Psych Congress; October 29–November 2, 2024; Boston, Massachusetts.
- Ostuzzi G, Bertolini F, Tedeschi F, et al. Oral and long-acting antipsychotics for relapse prevention in schizophrenia-spectrum disorders: a network meta-analysis of 92 randomized trials including 22,645 participants. World Psychiatry. 2022;21(2):295–307. doi.org/10.1002/wps.20972
- Vgontzas AN, Paschalidou A, Simos PG, et al. Impact of long-acting injectable antipsychotics vs. oral medication on relapses of patients with psychosis and bipolar disorder. Psychiatry Res. 2024;332:115676. doi.org/10.1016/j.psychres.2023.115676
- Yan T, Greene M, Chang E, et al. Medication adherence and discontinuation of aripiprazole once-monthly 400 mg (AOM 400) versus oral antipsychotics in patients with schizophrenia or bipolar I disorder: a real-world study using US claims data. Adv Ther. 2018;35(10):1612–1625. doi.org/10.1007/s12325-018-0785-y
This PDF is free for all visitors!