ABSTRACT
Objective: To examine the efficacy and safety of paliperidone palmitate once-monthly (PP1M) versus oral antipsychotics (OAPs) in Black/African American patients with schizophrenia and a history of criminal justice system involvement.
Methods: This was a post hoc analysis of a 15-month prospective, randomized, open-label, parallel-group, multicenter US study conducted from May 2010 to December 2013 that examined a subpopulation of Black/African American patients with schizophrenia (Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition criteria). The primary objective was to compare time to first treatment failure in patients treated with PP1M versus OAPs. Secondary objectives were to compare time to first institutionalization (psychiatric hospitalization or arrest/incarceration) and mean number of treatment failure events and institutionalizations over 15 months in PP1M-treated and OAP-treated patients.
Results: The intention-to-treat population included 275 Black/African American patients (PP1M, n = 145; OAPs, n = 130). Median time to first treatment failure was not reached for PP1M-treated patients and was 270 days for OAP-treated patients; hazard ratio (HR) was 1.39 (95% CI, 0.97–1.99; P = .075). Median time to first institutionalization was not reached for PP1M-treated patients and was 304 days for OAP-treated patients; HR was 1.49 (95% CI, 1.01–2.19; P = .043). Mean numbers of treatment failure events and institutionalizations were lower with PP1M than OAPs. The safety profile of PP1M was consistent with that of previous PP1M studies.
Conclusions: In a Black/African American subpopulation of patients with schizophrenia and prior criminal justice system involvement, PP1M reduced the number of treatment failures, thereby reducing the number of psychiatric hospitalizations and/or arrests/incarcerations compared with daily OAPs.
Trial Registration: ClinicalTrials.gov identifier: NCT01157351
J Clin Psychiatry 2021;82(2):20m13356
To cite: Bell Lynum KS, Henderson DC, Wright HJ, et al. Treatment effect with paliperidone palmitate compared with oral antipsychotics in Black/African American patients with schizophrenia and a history of criminal justice system involvement: a post hoc analysis of the PRIDE study. J Clin Psychiatry. 2021;82(2):20m13356.
To share: https://doi.org/10.4088/JCP.20m13356
© Copyright 2021 Physicians Postgraduate Press, Inc.
aJanssen Scientific Affairs, LLC, Titusville, New Jersey
bDepartment of Psychiatry, Boston University School of Medicine and Boston Medical Center, Boston, Massachusetts
cDepartment of Psychology, Temple University, Philadelphia, Pennsylvania
dJanssen Research and Development, LLC, Titusville, New Jersey
‡Affiliation at the time the analysis was conducted.
*Corresponding author: Karimah S. Bell Lynum, PharmD, MBA, BCPP, Janssen Scientific Affairs, LLC, 1125 Trenton-Harbourton Rd, Titusville, NJ 08560 ([email protected]).
Schizophrenia is a chronic, serious mental illness that typically leads to social and occupational dysfunction.1 The prevalence of the disease in adults in the United States is estimated to be between 0.25% and 0.64%.2 Black/African American individuals are diagnosed with schizophrenia at a higher rate than the general population, although this could be due in part to misdiagnosis arising from clinical bias and sociological factors.3 In the United States, people with serious mental illness, including those with schizophrenia, are more likely to be incarcerated than residing in a psychiatric hospital or mental health residential facility4 and therefore may not receive adequate treatment for their condition. Of concern is that African American patients with schizophrenia are overrepresented in the US inmate population compared with other ethnic groups with schizophrenia.5,6
Oral antipsychotic (OAP) therapy is the mainstay treatment for schizophrenia, and OAP medications need to be taken consistently to maintain long-term symptom control. However, approximately half of patients with schizophrenia are reported to be nonadherent to antipsychotic medication,7 often owing to poor insight into their own illness.8 Treatment nonadherence increases the likelihood of relapse, which can lead to hospitalization9–11 and increases the risk of violent and nonviolent offenses.12,13 In addition, without sustained control of psychotic symptoms, patients with schizophrenia and a history of criminal justice system involvement are at greater risk of repeated cycles of hospitalization11,14 and incarceration,5 known as the “revolving door” phenomenon.5,6 Long-acting injectable (LAI) antipsychotic medications provide extended therapeutic plasma concentrations15,16 and eliminate the need for daily OAPs, which may improve treatment adherence and reduce the risk of relapse.17–19
Paliperidone palmitate once-monthly (PP1M) is a LAI indicated for the treatment of schizophrenia in adults. The PRIDE (Paliperidone Palmitate Research In Demonstrating Effectiveness) study examined the efficacy of PP1M in delaying time to first relapse compared with OAPs in patients with schizophrenia and a history of contact with the criminal justice system, demonstrating that PP1M was superior to OAPs in delaying time to first treatment failure in these patients.20
It is increasingly recognized that racial disparities exist and must be addressed in the medical community.21 Given that African Americans are generally underrepresented in clinical trials,22 including those assessing psychiatric medications,23 a notable characteristic of the PRIDE study was that Black/African American patients represented 62.1% of the total study population.20 This post hoc subgroup analysis of the PRIDE study examined the efficacy and safety of PP1M versus oral OAP treatment in delaying time to first treatment failure in a high-risk population of Black/African American patients diagnosed with schizophrenia and with a history of contact with the criminal justice system. The aim of the present analysis was to provide further insights on the benefit-risk of PP1M in this high-risk population.
METHODS
Study Design
This was a post hoc analysis of a subpopulation of Black/African American patients diagnosed with schizophrenia and who had a history of contact with the criminal justice system from the PRIDE study—a randomized, prospective, open-label, event-monitoring board-blinded, parallel-group, multicenter study conducted in the United States (NCT01157351).
The PRIDE study design has been previously described.20,24 In brief, a screening/washout period of up to 2 weeks and a 15-month open-label treatment period were included (Supplementary Figure 1). Before randomization, the physician and each patient reviewed 7 OAPs available in the study (aripiprazole, haloperidol, olanzapine, paliperidone, perphenazine, quetiapine, and risperidone) to determine acceptability based on prior experience. Up to 6 of the 7 antipsychotic medications could be deselected by the patient or physician. Patients were randomly assigned in a 1:1 ratio to receive flexibly dosed monthly PP1M (78–234 mg) or flexibly dosed daily OAP within each randomization stratum, defined by the set of suitable OAPs (Supplementary Figure 1). Patients who discontinued their assigned study treatment or experienced a treatment failure were encouraged to continue in the study to the 15-month end point.
Study Population
In this analysis, eligible patients were Black/African American adults, aged 18–65 years who accepted the use of a once-monthly LAI antipsychotic medication. Other inclusion criteria entailed a current diagnosis of schizophrenia according to criteria given in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) and confirmed by the Mini-International Neuropsychiatric Interview (MINI; Version 6.0)25 administered by study investigators. In addition, eligible patients had had contact with the criminal justice system ≥ 2 times during the previous 2 years, with ≥ 1 of these events leading to incarceration, and had been released from the most recent custody within 90 days of the screening visit. Patients were ineligible if they had used clozapine within 3 months of screening or had received an injectable antipsychotic within 2 injection cycles of screening. Patients actively abusing intravenous drugs within the past 3 months and those with an opiate dependence disorder were excluded. Patients with an initial positive drug test result during screening could be retested and admitted to the study if the test result was negative and completed within 90 days of the patient’s release from most recent custody. Patients with positive drug screens during the study treatment period were eligible to continue in the study at the investigator’s discretion. The study was approved by each site’s institutional review board and was conducted in accordance with the Declaration of Helsinki. All subjects provided written informed consent, and the study was registered at ClinicalTrials.gov (identifier: NCT01157351).
Objectives
The same prespecified analyses used in the prospective PRIDE study were applied to the subpopulation in this post hoc analysis. The primary objective of this study was to compare the time to first treatment failure in PP1M-treated patients with that in OAP-treated patients. Treatment failure was defined as any one of the following: arrest/incarceration, psychiatric hospitalization, discontinuation of antipsychotic treatment because of safety or tolerability, treatment supplementation with another antipsychotic because of inadequate efficacy, need for an increase in the level of psychiatric services to prevent an imminent psychiatric hospitalization, discontinuation of antipsychotic because of inadequate efficacy, or suicide.24 Time to first institutionalization (psychiatric hospitalization or arrest/incarceration) and the mean cumulative number of treatment failures due to any event or due to institutionalization over a 15-month period were also compared between treatment groups.20 Safety was assessed by monitoring treatment-emergent adverse events (TEAEs).
Statistical Analysis
The PRIDE study was conducted from May 2010 to December 2013. The intention-to-treat (ITT) analysis set included all patients who received at least 1 dose of their randomly assigned study medication. Because the PRIDE study design combined both explanatory (randomized, controlled trial) and pragmatic (“real world” end points, treatment flexibility) elements, 2 ITT analysis sets were defined that comprised the same ITT patients but used different cutoff dates for the analyzed data.20,24 The explanatory ITT (eITT) analysis set was used for the primary efficacy and time to first institutionalization analyses; it included all data from randomization until the end of randomized treatment (28 days after the last injection of PP1M or 1 day after the last dose of OAP). The pragmatic ITT (pITT) analysis set included all data from randomization until month 15 and was used for the recurrent event efficacy analysis based on the cumulative mean function (CMF) and all safety analyses.
Time to first treatment failure and time to first psychiatric institutionalization, as determined by a blinded event monitoring board, were analyzed using the Kaplan-Meier method. Treatment differences were compared using a log-rank test, and the hazard ratio and 95% confidence intervals were estimated using a Cox proportional-hazards model, with treatment as a fixed factor. Statistical significance was based on a 2-sided α of 0.05.
Multiple treatment failures in the same patient were analyzed as recurrent events and were used to estimate the CMF,26 defined as the mean number of events per patient in a given time interval since randomization. The CMFs for the number of treatment failures due to any event or due to institutionalization were compared between the 2 treatment groups using a proportional intensity model that included the following baseline covariates: treatment, multiple prior incarcerations, being randomly assigned to receive to an antipsychotic medication that was used by the patient prior to the study, and a history of substance abuse. CMF is a useful measure to estimate event (treatment failure) rate in the setting of recurrent events in the same participant (multiple treatment failures). The CMF of treatment failure is a function of time, defined as the expected number of treatment failures per subject in a given time interval since randomization. A proportional means model including a term for treatment was used to compare mean event rates between 2 groups over time using the participant’s CMF. This is somewhat analogous to the Cox proportional hazards model, in which a participant’s hazard as a function of time is modeled to assess the hazard ratio between 2 treatment groups. Thus, in place of hazard at a given time, a participant’s CMF is modeled to compare recurrent event rates between 2 groups.
Adherence was assessed via medication possession ratio (MPR) > 80% using injection logs for PP1M and prescriptions or refill records for OAP.
RESULTS
In the PRIDE study, a total of 444 patients were included in the ITT population, of whom 443 had race data available. Of these, 275 Black/African American patients (PP1M, n = 145; OAP, n = 130) were randomized and treated, and 118 (PP1M, n = 60; OAP, n = 58) completed the study. Consistent with the primary study, the main reasons for discontinuation were lost to follow-up (PP1M, n = 38; OAP, n = 30) and withdrawal of consent (PP1M, n = 20; OAP, n = 13).
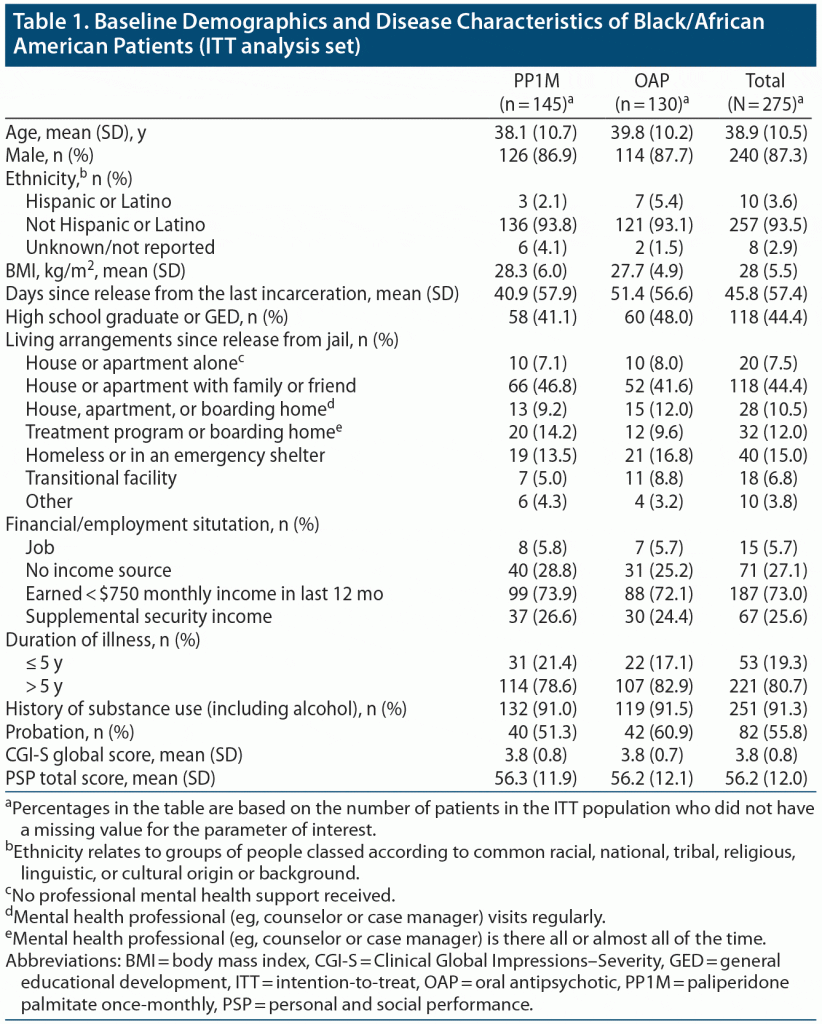
Baseline demographics and disease characteristics were similar between treatment groups (Table 1). A majority of patients were men (87.3%, n = 240), with a mean (standard deviation) age of 38.9 (10.5) years, and 80.7% (n = 221) of patients had schizophrenia for > 5 years. High school graduation or receiving a general education diploma was the highest level of education for 44.4% (n = 118) of patients. Fewer than half (44.4%, n = 118) of the patients lived with a relative or friend, and 15.0% (n = 40) were homeless or in an emergency shelter for the homeless. The highest monthly income in the prior 12 months was less than USD $750 in the majority (73.0%, n = 187) of patients, and 27.1% (n = 71) of patients had no income source since being released from jail.
Pre-randomization arrests resulted from felony (PP1M, 50.3%; OAP, 58.5%), infraction (PP1M, 29.0%; OAP, 33.8%), or misdemeanor (PP1M, 37.2%; OAP, 30.0%). The most common reasons for felony were drug charges (PP1M, 17.2%; OAP, 20.0%), assault on a private citizen (PP1M, 13.1%; OAP, 13.8%), and shoplifting/vandalism (PP1M, 9.7%; OAP, 8.5%). No arrests for homicide were reported.
Efficacy Outcomes
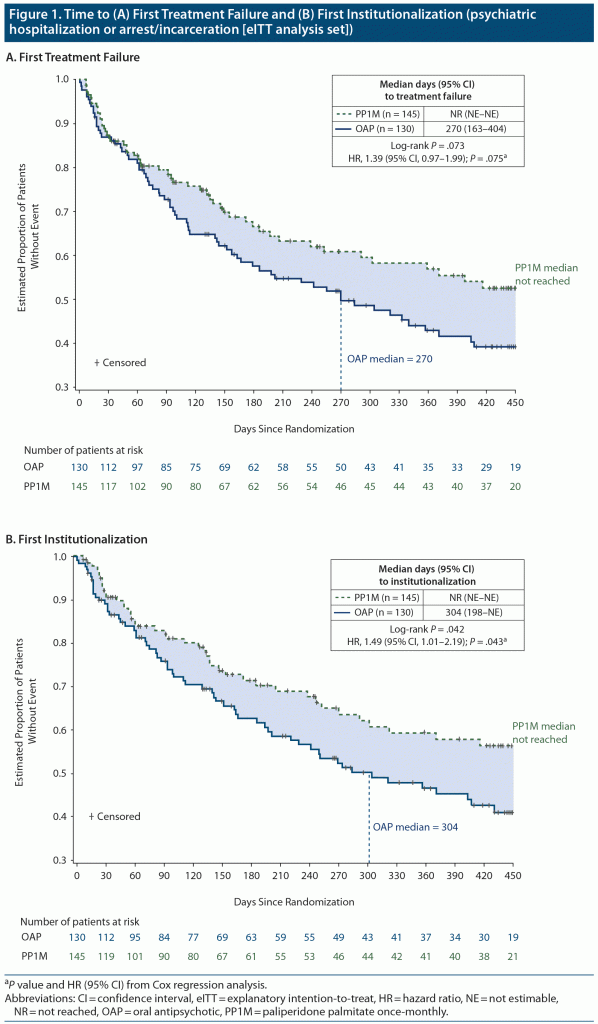
Overall, 35.9% (n = 52) of patients in the PP1M group and 52.3% (n = 68) in the OAP group had a treatment failure due to any event. The risk of first treatment failure was numerically higher in the OAP group compared with the PP1M group, although the difference was not statistically significant (hazard ratio [HR], 1.39; 95% CI, 0.97−1.99; P = .075) (Figure 1A). The median time to first treatment failure was not reached in the PP1M group (> 450 days) and was 270 days in the OAP group. The most common reason for first treatment failure was institutionalization (psychiatric hospitalization or arrest/incarceration), which occurred in 30.3% (n = 44) of patients in the PP1M group and 47.7% (n = 62) in the OAP group (Figure 1B). PP1M significantly delayed time to first institutionalization compared with OAP therapy (HR, 1.49; 95% CI, 1.01–2.19; P = .043) (Figure 1B). Median time to first institutionalization was not reached in the PP1M group (> 450 days) and was 304 days in the OAP group.
The most common first treatment failure events were arrest/incarceration followed by psychiatric hospitalization, with lower rates reported for each with the use of PP1M compared with OAP (Supplementary Figure 2).
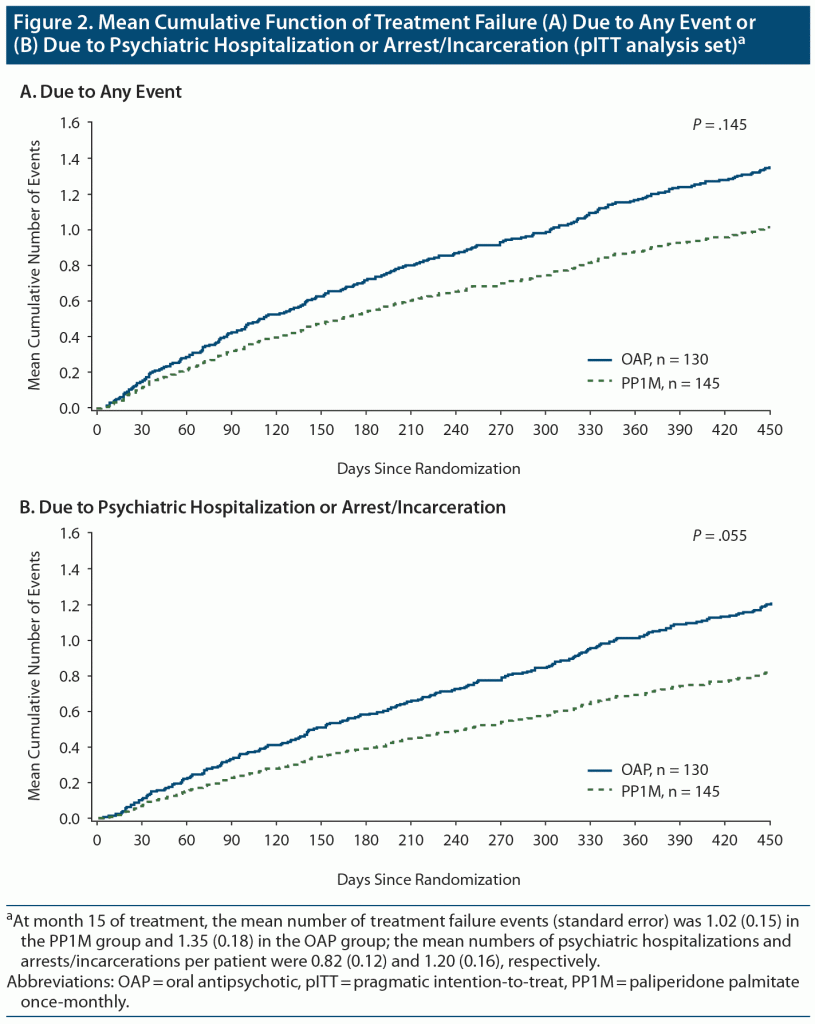
The mean cumulative number of treatment failures due to any event (Figure 2A) or due to institutionalization (Figure 2B) over the 15-month period was lower in the PP1M group versus the OAP group; the between-group differences were not statistically significant. At month 15 of treatment, the mean number of treatment failure events (standard error) was 1.02 (0.15) in the PP1M group and 1.35 (0.18) in the OAP group; the mean numbers of psychiatric hospitalizations or arrests/incarcerations per patient were 0.82 (0.12) and 1.20 (0.16), respectively.
At 15 months, the proportion of patients with arrest or incarceration was lower in the PP1M group (39.3%, n = 57) than in the OAP group (50.0%, n = 65). Arrests were classified as misdemeanor (PP1M, 43.9% [n = 25]; OAPs, 44.6% [n = 29]), felony (PP1M, 33.3% [n = 19]; OAPs, 40.0% [n = 26]), infraction (PP1M, 29.8% [n = 17]; OAPs, 46.2% [n = 30]), and unable to be classified (PP1M, 10.5% [n = 6]; OAPs, 6.2% [n = 4]).
Adherence
MPR > 80% was 96.2% for PP1M based on injection logs, and 76.7% and 25.0% for OAP based on prescriptions and refill records, respectively.
Safety
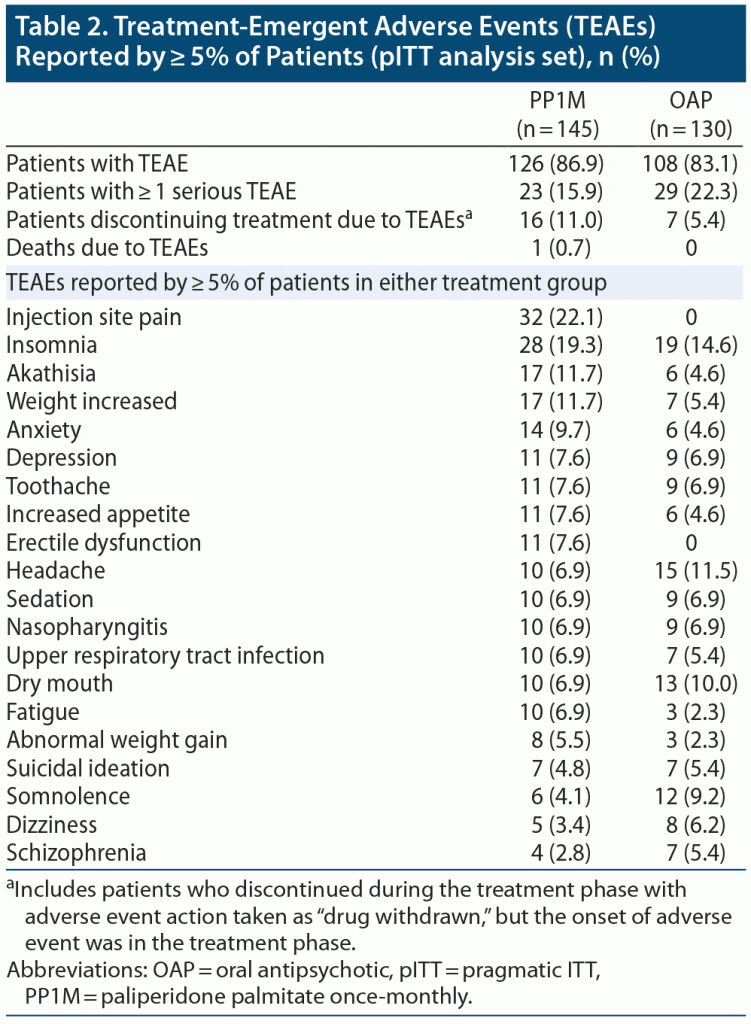
In this subpopulation, TEAEs were reported by 86.9% (126/145) of patients in the PP1M group and 83.1% (108/130) in the OAP group. Discontinuations due to TEAEs occurred in 11.0% of patients in the PP1M group and 5.4% of patients in the OAP group. Serious TEAEs occurred in 15.9% and 22.3% of patients in the PP1M and OAP groups, respectively. One death occurred in the PP1M group, but it was considered by the investigator as unlikely to be related to the study drug (Table 2). The most common TEAEs occurring in either treatment group are shown in Table 2. In the PP1M group versus the OAP group, TEAEs occurring at > 10% were injection-site pain (22.1% vs 0), insomnia (19.3% vs 14.6%), akathisia (11.7% vs 4.6%), and increased weight (11.7% vs 5.4%). No suicides were reported.
DISCUSSION
The PRIDE study was a unique, real-world clinical trial in which more than half of the study population consisted of Black/African American patients with schizophrenia and a history of criminal justice system involvement. The results of this post hoc analysis were consistent with the primary results of the PRIDE study20 with respect to magnitude of benefit of PP1M over OAP, although not achieving statistical significance in all cases owing to the reduced sample size. As in the primary study, PP1M delayed time to first treatment failure and time to first institutionalization. PP1M was significantly more effective at delaying time to first institutionalization versus daily OAP therapy. Rates of treatment failure over 15 months in Black/African American patients (PP1M, 35.9%; OAP, 52.3%) were similar to those in the overall study population (PP1M, 39.8%; OAP, 53.7%), as were the most common reasons for first treatment failure (arrest/incarceration and psychiatric hospitalization in both groups). Adherence rates and safety findings were also similar in the post hoc analysis and the primary analysis.
In the United States, approximately 20% of inmates in jails and 15% in state prisons have a serious mental illness,27 underscoring the importance of strategies that reduce recidivism in these individuals. In addition, following discharge, former inmates with schizophrenia are more likely to be hospitalized compared with people with schizophrenia who have not been incarcerated.14 In the present analysis, the rate of hospitalization or arrest/incarceration in PP1M-treated African American patients (30.3%) was similar to that reported in the primary PRIDE study (33.6%). These rates were generally lower than those reported in other studies of patients with mental illness and significant involvement with the criminal justice system (rehospitalization rates, 42%−48%; reincarceration rates, 20%−68%),28–30 supporting the benefit of PP1M in this at-risk population. The most common first treatment failure events were arrests/incarcerations and psychiatric hospitalizations, for which rates were lower in the PP1M group than in the OAP group.
The use of antipsychotic medications in patients with schizophrenia has been shown to be associated with a reduced risk of violent crime,31 arrests,32 and violent reoffending.33 Although antipsychotics may reduce the risk of violence, it should be noted that most patients with schizophrenia are nonviolent. The belief that patients with schizophrenia are dangerous or violent is a persistent stigma, despite the fact that they are more often the victims than the perpetrators of violence.16,34 Treatment adherence is a recognized factor in reducing the risk of violence in high-risk patients with schizophrenia, as suggested in a study of 11,462 patients with schizophrenia and a history of criminal justice system involvement.12 In that study, an MPR of ≥ 80% was associated with a reduction of both violent and nonviolent crime.12 In the present study, MPR > 80% was higher with PP1M (96.2% based on injection logs) than that with OAPs (76.7% and 25.0% according to prescriptions and refill records, respectively). Moreover, the overall proportion of patients with arrest or incarceration at month 15 of treatment was lower in the PP1M group than in the OAP group, suggesting a benefit for PP1M over OAPs with respect to criminal recidivism. This may be owing to the long elimination half-life of PP1M,35 which results in sustained therapeutic medication levels despite unstable post-incarceration living conditions. In contrast, OAPs are eliminated from the body after several days of missed doses. PP1M adherence may have a sustained positive impact on factors that contribute to arrest rates, such as irritability, anger, aggression, impaired judgement, and impulse control. Evidence to support the benefit of LAIs over OAPs in improving treatment adherence and thus reducing the risk of violent behavior is limited.16 In a small, open-label, prospective study in which 20 patients had ≥ 1 violent episode during the 1-year follow-up, those who received zuclopenthixol in an LAI formulation were more adherent and had significantly fewer violent episodes than those who received an oral formulation of the same drug.36
Black/African American patients treated with PP1M had fewer total treatment failures and institutionalizations than those treated with OAPs over the study period. This suggests that during treatment, patients receiving PP1M were symptomatically more stable and less likely to experience single or multiple relapses, which can be disruptive to community re-entry as patients attempt to break the cycle of frequent schizophrenia relapse, which can lead to hospitalizations and recidivism.
The transition from prison to the community can be especially difficult for people with mental illness owing to the challenges of finding a treatment provider, securing stable housing and employment, and other support services. Inmates with serious mental illness return to prison sooner than those without serious mental illness,37 and schizophrenia is a known risk factor for incarceration and reincarceration.38 Given that continuity of treatment is important for patients with schizophrenia, LAIs could help them remain adherent at this transitional time. Social stigma, unemployment, and poverty are often interrelated in people with a psychotic illness and can impede recovery.39 At baseline in many of the patients in the present study, social factors were not conducive to successful community re-entry, with fewer than half of the patients living with a relative or friend and most patients earning less than USD $750 per month. Analyses conducted in this study were not adjusted for baseline social factors, but overall, baseline sociodemographics were balanced between the treatment groups. Of note, the mean age of patients in the study population was 38.9 years, which is higher than expected given that incarcerations generally peak between the ages of 30 and 34 years. PP1M treatment was generally well tolerated in this study. The adverse event profile was consistent with that reported in previous clinical trials.40–42
The post hoc nature of the analyses in this study is a limitation, as these were not statistically powered to compare treatments in the Black/African American subpopulation. In addition, because this subanalysis represented 62.1% of the overall PRIDE population, the small sample sizes may limit the generalizability of these findings. Furthermore, patients abusing intravenous drugs and those with opiate dependence at screening were excluded from this study, which may further limit the applicability of these findings to a larger patient population. However, patients with positive drug screens during the study treatment period were eligible to continue in the study at the investigator’s discretion. This highlights a key feature of the PRIDE study design—although PRIDE was a randomized, controlled trial, the design incorporated pragmatic elements to make the study population more reflective of patients with schizophrenia encountered in real-world clinical practice.24 The proportion of patients serving county versus state sentences was not captured in this study. Because state sentences tend to be longer, and thereby reduce the opportunity to commit new crimes, it is unknown if the type of sentence (county vs state) had any impact on the outcome. Another limitation is potential inflation of MPR, which is calculated as the sum of the days’ supply for all fills of a given drug within a particular time period divided by the number of days in the time period, in the OAP group. MPR may overestimate adherence to OAPs because there is no way to verify whether a patient has taken an oral medication once they have filled their prescription. Furthermore, patients who routinely fill their prescriptions early will have an inflated MPR owing to a higher sum of days’ supply for all fills within a particular time period (numerator of the MPR ratio).
While this analysis suggests that the pharmacologic benefits of PP1M compared with OAPs in the Black/African American subpopulation are similar to the benefits observed in the overall population of the PRIDE study, the analysis does not address other pertinent factors that may affect psychiatric care in Black/African American patients, including racial disparities with regard to access to and quality of care. For example, although there is no evidence supporting a genetic basis for a higher prevalence of psychotic disorders in Black/African American patients, it is well documented that Black/African American individuals are more likely to be diagnosed with a psychotic disorder than their European American counterparts, a phenomenon thought to stem from nonclinical factors such as unconscious bias and an underdiagnosis of alternative conditions including major depressive disorder and bipolar disorder.3 Racial disparities in antipsychotic treatment have also been reported, with Black/African American Medicaid patients being more likely to receive first-generation oral antipsychotics and long-acting injectable antipsychotics and less likely to receive second-generation oral antipsychotics than White Medicaid patients.43 Future research should investigate sources of unconscious bias and evaluate potential solutions to racial disparities in psychiatric care, including ways in which psychopharmacology can address the needs of underserved patient populations.
In conclusion, in a Black/African American subpopulation of patients diagnosed with schizophrenia who had a history of criminal justice system involvement, PP1M reduced the number of treatment failures compared with daily OAPs, thereby reducing the number of psychiatric hospitalizations and/or arrests/incarcerations.
Submitted: March 23, 2020; accepted November 24, 2020.
Published online: February 24, 2021.
Potential conflicts of interest: Drs Bell Lynum and Gogate are employees and shareholders of Janssen Scientific Affairs, LLC. Dr Henderson reports receiving past research support from Roche TCRC, Reckitt Benckiser Pharmaceuticals, and the National Institute of Mental Health and honoraria from Alkermes Pharmaceuticals, Sunovion, and the National Institute of Mental Health. Dr Kim is a former employee of Janssen Scientific Affairs, LLC, and a shareholder of Janssen Scientific Affairs, LLC. Dr Wright has no conflicts to report.
Funding/support: Janssen Scientific Affairs, LLC, Titusville, New Jersey.
Role of the sponsor: The study sponsor was responsible for the design and conduct of the study; collection, management, analysis, and interpretation of data; preparation, review, and approval of the manuscript; and the decision to submit the manuscript for publication.
Previous presentation: Presented at the 2018 Psych Congress, October 25–28, 2018, Orlando, Florida • 22nd Annual Meeting of the College of Psychiatric and Neurologic Pharmacists (CPNP); April 7–10, 2019; Salt Lake City, Utah.
Acknowledgments: The authors thank Lynn Brown, PhD; Madeline Pfau, PhD; and Bettina Seri, PhD (employees of ApotheCom, LLC, Yardley, Pennsylvania), for providing writing and editorial assistance, which was funded by Janssen Scientific Affairs, LLC.
Additional information: The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.
Supplementary material: Available at Psychiatrist.com
Clinical Points
- This post hoc analysis of the PRIDE study evaluated time to first treatment failure in Black/African American patients with schizophrenia and a history of criminal justice system involvement who were treated with paliperidone palmitate once-monthly versus oral antipsychotics.
- Compared with oral antipsychotic treatment, paliperidone palmitate once-monthly delayed time to first treatment failure and time to first psychiatric hospitalization or arrest/incarceration in Black/African American patients. Patients receiving paliperidone palmitate once-monthly had a lower mean number of treatment failures and institutionalization events over the 15-month study period and higher rates of medication adherence.
- The safety and tolerability profile of paliperidone palmitate once-monthly in Black/African American patients was consistent with prior studies, including the PRIDE primary study.
References (43)

- Patel KR, Cherian J, Gohil K, et al. Schizophrenia: overview and treatment options. P&T. 2014;39(9):638–645. PubMed NLM
- Schizophrenia. National Alliance on Mental Health website. https://www.nami.org/learn-more/mental-health-conditions/schizophrenia. 2020. Accessed 01/11/2020.
- Schwartz RC, Blankenship DM. Racial disparities in psychotic disorder diagnosis: a review of empirical literature. World J Psychiatry. 2014;4(4):133–140. PubMed CrossRef NLM
- McCarthy M. US jails hold 10 times more mentally ill people than state hospitals, report finds. BMJ. 2014;348:g2705. PubMed CrossRef NLM
- Baillargeon J, Binswanger IA, Penn JV, et al. Psychiatric disorders and repeat incarcerations: the revolving prison door. Am J Psychiatry. 2009;166(1):103–109. PubMed CrossRef NLM
- Ramsay CE, Goulding SM, Broussard B, et al. Prevalence and psychosocial correlates of prior incarcerations in an urban, predominantly African-American sample of hospitalized patients with first-episode psychosis. J Am Acad Psychiatry Law. 2011;39(1):57–64. PubMed NLM
- Lacro JP, Dunn LB, Dolder CR, et al. Prevalence of and risk factors for medication nonadherence in patients with schizophrenia: a comprehensive review of recent literature. J Clin Psychiatry. 2002;63(10):892–909. PubMed CrossRef NLM
- Bitter I, Fehér L, Tényi T, et al. Treatment adherence and insight in schizophrenia. Psychiatr Hung. 2015;30(1):18–26. PubMed NLM
- Dufort A, Zipursky RB. Understanding and managing treatment adherence in schizophrenia [published online ahead of print January 3, 2019]. Clin Schizophr Relat Psychoses. PubMed CrossRef NLM
- Phan SV. Medication adherence in patients with schizophrenia. Int J Psychiatry Med. 2016;51(2):211–219. PubMed CrossRef NLM
- Kane JM, Correll CU. Optimizing treatment choices to improve adherence and outcomes in schizophrenia. J Clin Psychiatry. 2019;80(5):IN18031AH3C. PubMed CrossRef NLM
- Rezansoff SN, Moniruzzaman A, Fazel S, et al. Adherence to antipsychotic medication and criminal recidivism in a Canadian provincial offender population. Schizophr Bull. 2017;43(5):1002–1010. PubMed CrossRef NLM
- Volavka J. Violence in schizophrenia and bipolar disorder. Psychiatr Danub. 2013;25(1):24–33. PubMed NLM
- Prince JD. Incarceration and hospital care. J Nerv Ment Dis. 2006;194(1):34–39. PubMed CrossRef NLM
- Spanarello S, La Ferla T. The pharmacokinetics of long-acting antipsychotic medications. Curr Clin Pharmacol. 2014;9(3):310–317. PubMed CrossRef NLM
- Mohr P, Knytl P, Voráčková V, et al. Long-acting injectable antipsychotics for prevention and management of violent behaviour in psychotic patients. Int J Clin Pract. 2017;71(9):e12997. PubMed CrossRef NLM
- Correll CU, Citrome L, Haddad PM, et al. The use of long-acting injectable antipsychotics in schizophrenia: evaluating the evidence. J Clin Psychiatry. 2016;77(suppl 3):1–24. PubMed CrossRef NLM
- Correll CU, Lauriello J. Using long-acting injectable antipsychotics to enhance the potential for recovery in schizophrenia. J Clin Psychiatry. 2020;81(4):MS19053AH5C. PubMed CrossRef NLM
- Kim B, Lee SH, Choi TK, et al. Effectiveness of risperidone long-acting injection in first-episode schizophrenia: in naturalistic setting. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(5):1231–1235. PubMed CrossRef NLM
- Alphs L, Benson C, Cheshire-Kinney K, et al. Real-world outcomes of paliperidone palmitate compared to daily oral antipsychotic therapy in schizophrenia: a randomized, open-label, review board-blinded 15-month study. J Clin Psychiatry. 2015;76(5):554–561. PubMed CrossRef NLM
- Yancy CW. Academic medicine and Black Lives Matter: time for deep listening. JAMA. 2020;324(5):435–436. PubMed CrossRef NLM
- Diversifying clinical trials. Nat Med. 2018;24(12):1779. PubMed CrossRef NLM
- Miranda J, Nakamura R, Bernal G. Including ethnic minorities in mental health intervention research: a practical approach to a long-standing problem. Cult Med Psychiatry. 2003;27(4):467–486. PubMed CrossRef NLM
- Alphs L, Mao L, Rodriguez SC, et al. Design and rationale of the Paliperidone Palmitate Research in Demonstrating Effectiveness (PRIDE) study: a novel comparative trial of once-monthly paliperidone palmitate versus daily oral antipsychotic treatment for delaying time to treatment failure in persons with schizophrenia. J Clin Psychiatry. 2014;75(12):1388–1393. PubMed CrossRef NLM
- Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22–33, quiz 34–57. PubMed NLM
- Lin DY. Non-parametric inference for cumulative incidence functions in competing risks studies. Stat Med. 1997;16(8):901–910. PubMed CrossRef NLM
- Torrey EF, Kennard AD, Eslinger D, et al. More mentally ill persons are in jails and prisons than hospitals: a survey of the states. Treatment Advocacy Center and the National Sheriff’s Association. Treatment Advocacy Center website. http://www.treatmentadvocacycenter.org/storage/documents/final_jails_v_hospitals_study.pdf. Published May 2010. Accessed January 19, 2021.
- Kunz M, Yates KF, Czobor P, et al. Course of patients with histories of aggression and crime after discharge from a cognitive-behavioral program. Psychiatr Serv. 2004;55(6):654–659. PubMed CrossRef NLM
- Harris V, Koepsell TD. Criminal recidivism in mentally ill offenders: a pilot study. Bull Am Acad Psychiatry Law. 1996;24(2):177–186. PubMed NLM
- Feder L. A comparison of the community adjustment of the mentally ill offenders with those from the general prison population. Law Hum Behav. 1991;15(5):477–493. CrossRef
- Fazel S, Zetterqvist J, Larsson H, et al. Antipsychotics, mood stabilisers, and risk of violent crime. Lancet. 2014;384(9949):1206–1214. PubMed CrossRef NLM
- Van Dorn RA, Desmarais SL, Petrila J, et al. Effects of outpatient treatment on risk of arrest of adults with serious mental illness and associated costs. Psychiatr Serv. 2013;64(9):856–862. PubMed CrossRef NLM
- Chang Z, Lichtenstein P, Långström N, et al. Association between prescription of major psychotropic medications and violent reoffending after prison release. JAMA. 2016;316(17):1798–1807. PubMed CrossRef NLM
- Choe JY, Teplin LA, Abram KM. Perpetration of violence, violent victimization, and severe mental illness: balancing public health concerns. Psychiatr Serv. 2008;59(2):153–164. PubMed CrossRef NLM
- Invega Sustenna [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc; 2019.
- Arango C, Bombín I, González-Salvador T, et al. Randomised clinical trial comparing oral versus depot formulations of zuclopenthixol in patients with schizophrenia and previous violence. Eur Psychiatry. 2006;21(1):34–40. PubMed CrossRef NLM
- Cloyes KG, Wong B, Latimer S, et al. Time to prison return for offenders with serious mental illness released from prison: a survival analysis. Crim Justice Behav. 2010;37(2):175–187. CrossRef
- Hawthorne WB, Folsom DP, Sommerfeld DH, et al. Incarceration among adults who are in the public mental health system: rates, risk factors, and short-term outcomes. Psychiatr Serv. 2012;63(1):26–32. PubMed CrossRef NLM
- Sweeney S, Air T, Zannettino L, et al. Psychosis, socioeconomic disadvantage, and health service use in South Australia: findings from the Second Australian National Survey of Psychosis. Front Public Health. 2015;3:259. PubMed CrossRef NLM
- Hough D, Gopal S, Vijapurkar U, et al. Paliperidone palmitate maintenance treatment in delaying the time-to-relapse in patients with schizophrenia: a randomized, double-blind, placebo-controlled study. Schizophr Res. 2010;116(2–3):107–117. PubMed CrossRef NLM
- Gopal S, Hough DW, Xu H, et al. Efficacy and safety of paliperidone palmitate in adult patients with acutely symptomatic schizophrenia: a randomized, double-blind, placebo-controlled, dose-response study. Int Clin Psychopharmacol. 2010;25(5):247–256. PubMed CrossRef NLM
- Markowitz M, Fu DJ, Levitan B, et al. Long-acting injectable paliperidone palmitate versus oral paliperidone extended release: a comparative analysis from two placebo-controlled relapse prevention studies. Ann Gen Psychiatry. 2013;12(1):22. PubMed CrossRef NLM
- Lawson W, Johnston S, Karson C, et al. Racial differences in antipsychotic use: claims database analysis of Medicaid-insured patients with schizophrenia. Ann Clin Psychiatry. 2015;27(4):242–252. PubMed NLM
This PDF is free for all visitors!