ABSTRACT
Objective: To describe persistence with and adherence to paliperidone palmitate once-monthly injection (PP1M) compared to oral second-generation antipsychotics (SGAs) in patients with schizophrenia in real-world settings in China and Japan.
Methods: Patients with a schizophrenia diagnosis (ICD-10: F20.x) who received oral or injectable antipsychotics from study start (China: January 1, 2012; Japan: January 1, 2014) until December 31, 2017, were enrolled in this retrospective cohort study. The first PP1M or oral SGA prescription date during the study period was defined as the index date. Eligible patients were followed up for up to 1 year after the index date. Persistence was measured from the index date until discontinuation or reaching 1 year. Adherence was assessed by calculating the proportion of days covered (PDC). Multivariable regression models were used to adjust for potential confounders.
Results: The study cohorts comprised 44,266 patients from Japan and 7,564 and 5,189 patients, respectively, from 2 hospitals in China. The PP1M group showed consistently lower risk of discontinuation; adjusted hazard ratios and 95% CIs were 0.75 (0.72–0.90) (Japan), and 0.76 (0.68–0.84) and 0.65 (0.56–0.76) (China) compared to oral SGAs. The PP1M group also showed better adherence; adjusted odds ratios and 95% CIs were 1.61 (1.22–2.11) (Japan), and 1.92 (1.53–2.41) and 2.25 (1.58–3.23) (China).
Conclusions: Persistence and adherence were significantly higher in PP1M users than in oral SGAs users across 3 databases comprising patients in 2 countries in Asia.
J Clin Psychiatry 2022;83(1):20m13850
To cite: Wang H, Zhang Y, Liu J, et al. Persistence with and adherence to paliperidone palmitate once-monthly injection for schizophrenia treatment in China and Japan. J Clin Psychiatry. 2022;83(1):20m13850.
To share: https://doi.org/10.4088/JCP.20m13850
© Copyright 2021 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry, Xijing Hospital, The Fourth Military Medical University, Xi’an, China
bGlobal Epidemiology, Janssen Research & Development, Shanghai, China
cPeking University Sixth Hospital & Peking University Institute of Mental Health, NHC Key laboratory of Mental Health (Peking University), National Clinical Research Center for Mental Disorders (Peking University Sixth Hospital), Beijing, China
dMedical Affairs, Xi’an Janssen Pharmaceutical Ltd, Beijing, China
eGlobal Epidemiology, Janssen Research and Development, Titusville, New Jersey
‡These authors contributed equally to this article.
*Corresponding author: Tianmei Si, Peking University Institute of Mental Health, No. 51 Huayuanbeilu, Haidian District, Bejing, China 86-10-62723748 ([email protected]).
Schizophrenia ranks among the top 20 leading causes of disability worldwide.1,2 Pharmacologic treatment remains the cornerstone of management, and adherence to antipsychotic medications is essential to optimize treatment effectiveness. However, adherence is often poor due to a complex interplay of factors including patients’ lack of illness awareness/insight, fear of specific side effects, stigma about schizophrenia, attitudes to medication and past medication experience, influence of the caregiver, and cost factors.3,4 Consequently, nonadherence represents one of the most challenging aspects of schizophrenia treatment. Long-acting injectable (LAI) antipsychotics were developed, in part, to improve medication adherence by extending dosing intervals while providing a sustained therapeutic effect.5,6 Paliperidone palmitate is an atypical LAI antipsychotic administered once a month, and real-world database studies in the United States have shown that paliperidone palmitate once-monthly injection (PP1M) was associated with higher adherence and persistence, less health care resource utilization, and lower medical costs attributable to reduced hospitalizations and long-term care admissions compared to oral second-generation antipsychotics (SGAs).7–13 PP1M use in Asian populations has been associated with reduced hospitalizations, lower health care utilization and associated costs, and improved satisfaction in patients with schizophrenia compared to other treatment alternatives.14–19 Poor adherence to antipsychotic treatment is a significant risk factor for relapse in Chinese patients.20 However, to date, these data were obtained in clinical trial participants, and to our knowledge, no real-world database studies have compared treatment patterns between PP1M and oral SGAs among Chinese or Japanese patients with schizophrenia. Therefore, we conducted a large, retrospective real-world cohort study in patients with schizophrenia in China and Japan to compare persistence and adherence between PP1M and oral SGAs.
METHODS
Study Design and Cohort Selection
This retrospective cohort study used patient data from electronic medical record (EMR) databases from 2 university-affiliated tertiary hospitals in China and from the Japan Medical Data Center (JMDC) health insurance claims database in Japan. Study start was January 1, 2012, in the Chinese databases and January 1, 2014, in the JMDC database, reflecting the PP1M launch date in each country (2012 in China and late 2013 in Japan). Patients in the databases with at least one medical record of a schizophrenia diagnosis (International Classification of Diseases, Tenth Revision [ICD-10] code F20.x) and who received oral or injectable antipsychotics between the study start date and December 31, 2017, were enrolled. The first PP1M or oral SGA prescription date during the study period was defined as the index date and the drug prescribed termed the index drug. When the first PP1M and oral SGA were prescribed on the same day, the patients were classified into the PP1M group because short-term concomitant use of oral risperidone or paliperidone is recommended prior to PP1M initiation. Therefore, no specific restriction was applied based on antipsychotic treatment prior to the index date. The baseline period was defined as 365 days prior to the index date, and all patients were followed up for the evaluation of persistence and adherence for 1 year, or the last record of clinical activity before December 31, 2018, whichever came first. The main analysis cohort was the continuous care cohort, defined as patients with at least 3 consecutive visits during the study period with a gap of 7 to 45 days between each visit. Each unique outpatient visit, hospitalization, and hospital pharmacy prescription was considered as a visit. Patients in the continuous care cohort were classified in the PP1M group if they had received at least one PP1M prescription. Patients whose first prescription was other than PP1M or oral SGA were excluded from the study. Further, patients in the oral SGA–only group were excluded if they received any other injectable antipsychotics during the study period, because it is likely that oral SGAs may not have been intended for long-term maintenance treatment in these patients. Patients aged < 18 years at the index date were excluded because PP1M was approved for use only in adults during the study period.
Data Sources
In China, data were obtained from a tertiary mental health center in Beijing (Peking University No. 6 Hospital [PKU]) and a tertiary general hospital in Xi’an (Xijing hospital [XJH]) that both provide inpatient and outpatient care, pharmacy, and procedural and laboratory services. The Hospital Information System (HIS) at each center holds at least 10 years’ worth of health care data. As previously described,21 data held in the HIS were transformed into integrated structured EMR research databases with data verification to ensure consistency with the original HIS databases. Briefly, the research databases contained unique patient codes and information including demographic details, outpatient and inpatient dates with diagnoses as ICD-10 codes, admission and discharge information for inpatients, and prescription details including date, drug, quantity, and days of supply. Patient details were independently deidentified by the hospital Information Technology department. The study protocol was approved by the PKU and the XJH Ethical Committees.
The JDMC database holds health insurance claims information for approximately 3.78 million non-government employees aged 18–65 years and 3.33 million dependents that include children and adults up to the age of 75 years. The database captures claims information including demographic details, outpatient and inpatient services with diagnoses as ICD-10 codes, and pharmaceutical services including drug Anatomic Therapeutic Chemical code, brand name, and dispensing date. All data were anonymized and deidentified. Should persons become unemployed or not fit for work, they are withdrawn from the insurance plan and data collection ceases. Epidemiology research on anonymized data is exempted from the Ethical Guidelines from Epidemiology Research in Japan (Ministry of Education, Culture, Sports, Science and Technology and Ministry of Health, Labor and Welfare, 2002).
Study Outcomes
For eligible patients in the PP1M and oral SGA cohorts, all available records during the baseline period were retrieved. For the PP1M cohort, drug utilization information, including number of prescriptions per person, gap between prescriptions, average dose, dose at first and last prescription, and number of dose changes during the 1-year follow-up period, were summarized. For the oral SGA cohort, all drugs were treated as one class, and SGA utilization was not explored further.
Persistence with and adherence to the index drug for 1 year after the index date were assessed and compared between the PP1M and oral SGA cohorts. Persistence until day 183 and day 365 was measured using the number of days from index date until discontinuation, defined as a treatment gap (no same prescription) of at least 45 days.
Adherence was assessed by calculating the proportion of days covered (PDC), defined as the number of non-overlapping days covered by the index drug divided by the total number of days during the fixed time window of 1 year after index date (maximum 1-year follow-up period or last record, whichever came first). Good treatment adherence was defined as a PDC ≥ 80%.22–24
Statistical Methods
Statistical analyses were performed in each database separately. Descriptive analyses were used for demographics, clinical characteristics, and PP1M utilization. The persistence of PP1M and oral SGA use over the 1-year follow-up period was evaluated using Kaplan-Meier analyses and compared using log rank tests. A multivariate Cox regression model adjusted for potential confounders, including age group, sex, schizophrenia diagnosis during the baseline period, number of hospital visits during the baseline period, mental health and other psychiatric/neurologic comorbidities, and comedications within 90 days prior to the index date, was developed to evaluate the risk of discontinuation during the 1-year follow-up period. A logistic regression model with adjustment using the same set of potential confounders as in the Cox regression model was used to evaluate the likelihood of good adherence during the 1-year follow-up period.
A sensitivity analysis used a longer grace period of at least 60 days for discontinuation. Persistence and adherence were assessed in subcohorts of patients using a single antipsychotic medication at the index date. All analyses were conducted using SAS 9.2 (SAS Institute Inc; Cary, North Carolina).
RESULTS
Patient Cohort and Characteristics
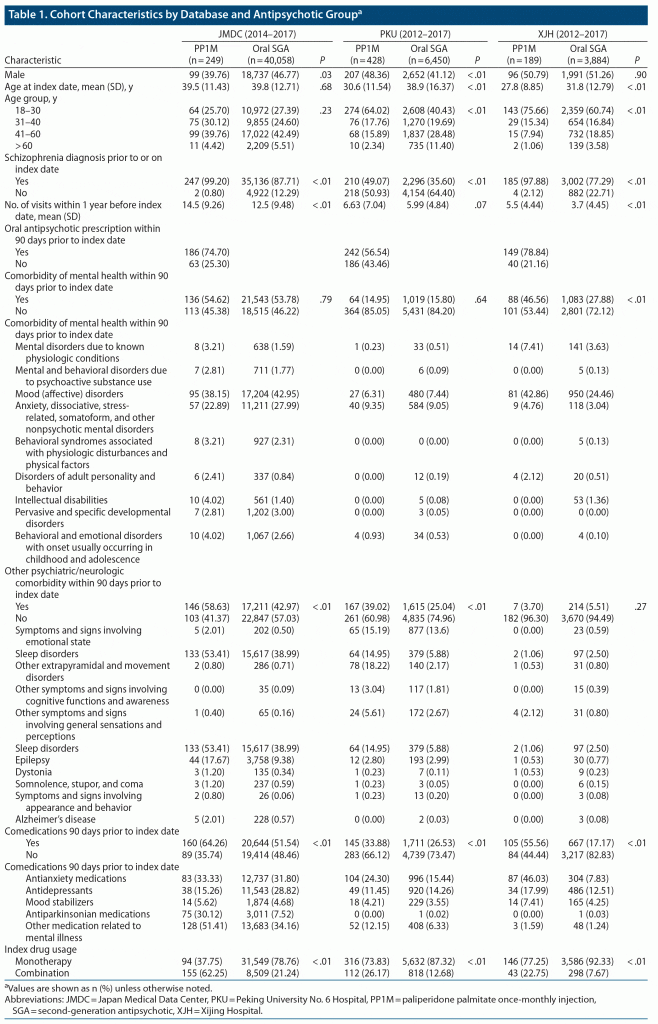
There were 44,266 patients in the JMDC database, 7,564 in the PKU database, and 5,189 in the XJH database who had continuous care, had received antipsychotics, and were ≥ 18 years of age who were included in the analysis (Supplementary Figure 1). The number of patients in the PP1M group was 249 in the JMDC database, 428 in the PKU database, and 189 in the XJH database.
In the JDMC database, the PP1M group contained proportionately fewer men than the oral SGA group (39.76% vs 46.77%) (Table 1). In the PKU database, the PP1M group contained more men than the oral SGA group (48.36% vs 41.12%), and the mean age of PP1M patients was lower (30.6 years vs 38.9 years). In the XJH database, patients in the PP1M and oral SGA groups were similar with respect to sex distribution and mean age. In the XJH database, more patients in the PP1M group than in the oral SGA group had a comorbid mental health episode within 90 days prior to the index date (46.56% vs 27.88%). Comedication use and psychiatric/neurologic comorbidities are provided in Table 1.
Overall PP1M Utilization
The median number of prescriptions per patient was similar in the PP1M and oral SGA groups in all 3 databases (Supplementary Table 1). The median number of prescriptions of PP1M and oral SGA, respectively, was 9 and 9 in the JMDC database, 6 and 7 in the PKU database, and 4 and 4 in the XJH database. The median gap between prescriptions of PP1M and oral SGA, respectively, was 28.00 and 25.29 days in the JMDC database, 31.05 and 35.32 days in the PKU database, and 31.22 and 33.33 in the XJH database. The median administered first dose and median dose over the entire course of PP1M, respectively, were 100 mg and 100 mg in the JMDC database, 150 mg and 104.55 mg in the PKU database, and 150 mg and 110 mg in the XJH database. The median number of dose changes of PP1M was 1 in all 3 databases.
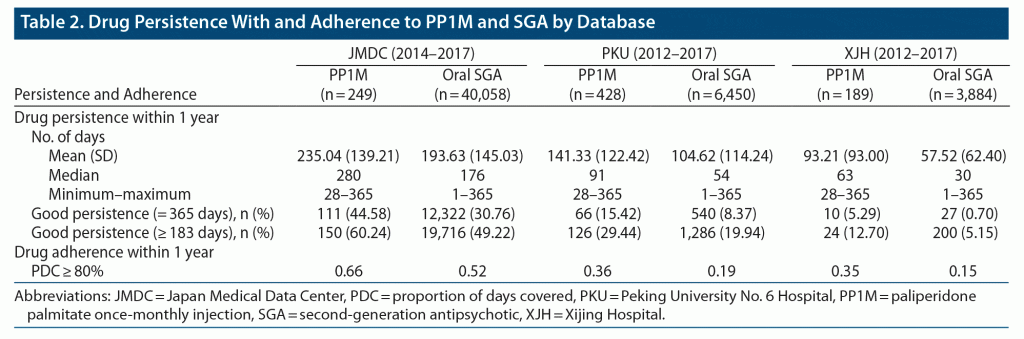
Persistence
The median persistence during the 1-year follow-up period was 280 days in the PP1M group versus 176 days in the SGA group in the JMDC database, 91 days versus 54 days in the PKU database, and 63 versus 30 days in the XJH database (Table 2). The percentage of patients who persisted on treatment for 365 days was 44.58% in the PP1M group versus 30.76% in the SGA group in the JMDC database, 15.42% versus 8.37%, respectively, in the PKU database, and 5.29% versus 0.70%, respectively, in the XJH database (Table 2).
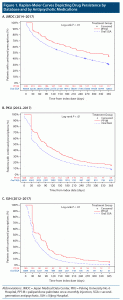
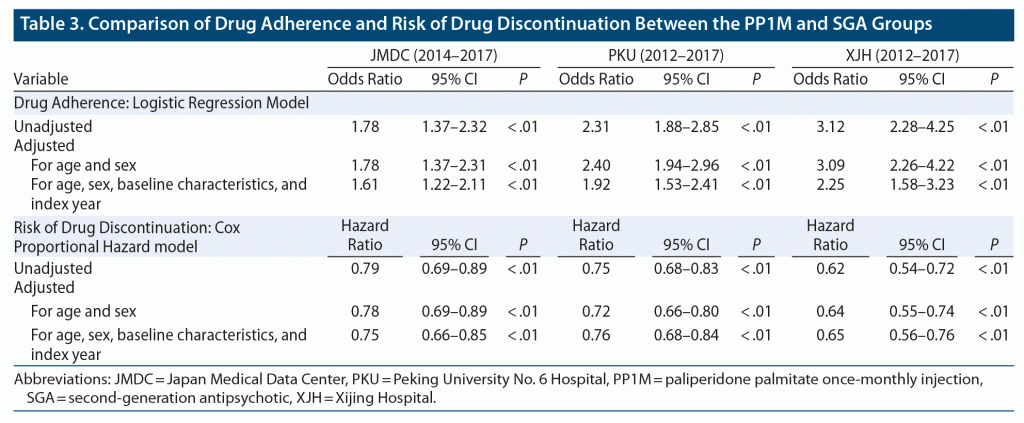
During the 1-year follow-up period, persistence was significantly higher in PP1M recipients than in recipients of oral SGAs in all 3 databases (Figure 1). The risk of drug discontinuation was 0.79-fold lower (95% CI, 0.69–0.89) in the PP1M group than the oral SGA group in the JMDC database, 0.75-fold lower (95% CI, 0.68–0.83) in the PKU database, and 0.62-fold lower (95% CI, 0.54–0.72) in the XJH database (Table 3). The risk was similar after adjustment for age and sex or for age, sex, baseline characteristics, and index year. The fully adjusted hazard ratio was 0.75 (95% CI, 0.66–0.85) in the JMDC database, 0.76 (95% CI, 0.68–0.84) in the PKU database, and 0.65 (95% CI, 0.56–0.76) in the XJH database (Table 3).
Adherence
The proportion of PDC ≥ 80% was 0.66 in the PP1M group versus 0.52 in the oral SGA group in the JMDC database, 0.36 versus 0.19 in the PKU database, and 0.35 versus 0.15 in the XJH database (Table 2).
In each database, the proportion of good adherence in the PP1M group was significantly higher than in the oral SGA group. The unadjusted odds ratio (OR) was 1.78 (95% CI, 1.37–2.32) in the JMDC database, 2.31 (95% CI, 1.88–2.85) in the PKU database, and 3.12 (95% CI, 2.28–4.25) in the XJH database (Table 3). The ORs were similar after adjustment for age and sex or for age, sex, baseline characteristics, and index year. The fully adjusted OR was 1.61 (95% CI, 1.22–2.11) in the JMDC database, 1.92 (95% CI, 1.53–2.41) in the PKU database, and 2.25 (95% CI, 1.58–3.23) in the XJH database (Table 3).
Sensitivity Analysis
Similar trends were observed in the sensitivity analysis using a treatment gap of 60 days and in the subanalysis comparing single therapy with combination therapy at the index date. Persistence and good adherence were significantly higher in the PP1M groups than in the oral SGA groups in the JMDC and XJH databases for all comparisons (Shown in Supplementary Tables 2 to 4 and Supplementary Figure 2). However, persistence using a treatment gap of 60 days was not statistically significant after full adjustment in the PKU database.
DISCUSSION
The main finding of this real-world database study on antipsychotics in 2 Asian countries is that rates of persistence and adherence were consistently significantly higher in the PP1M group than in the oral SGA group in all 3 databases studied. The results are supported by direct comparisons, adjustment for potential confounders in the statistical models, and sensitivity analyses (except for persistence in PKU when applying the 60-day criterion).
Our results in Asian populations are consistent with results of observational studies conducted in the US that have shown improved persistence and adherence using LAI antipsychotics. Several studies have been conducted that evaluated treatment patterns of PP1M compared to oral SGAs in the US. Joshi et al11 reported a significantly higher proportion of PDC ≥ 80% in 351 PP1M users compared with 4,869 users of oral SGAs after a 1-year follow-up period (28.5% vs 18.1%). The average persistence was longer in PP1M users (188 days) than in oral SGA users (148 days). Manjelievskaia et al9 achieved similar results. The average persistence was 176.8 days in PP1M users, which was significantly longer than the 148.9 days in oral SGA users. The proportion of PDC ≥ 80% was also significantly higher in PP1M users than in users of oral SGA (26.7% vs 22.3%). Lefebvre et al7 investigated the impact of PP1M and oral SGAs in Veterans Health Administration electronic health record data in the US. The PP1M users were more adherent than oral SGA users (PDC ≥ 80%: 37.0% in PP1M group vs 20.0% in oral SGA group). Conversely, Shah et al,25 who also used the Medicaid database, reported no statistically significant difference in the proportion of PDC ≥ 80% over a 1-year follow-up period between cohorts of LAI and oral antipsychotic users (36.5% in LAI antipsychotic cohort vs 34.1% in oral antipsychotic cohort, P = .096). Interestingly, the proportion of PDC ≥ 80% in the oral antipsychotic cohort was higher than in the other aforementioned studies (around 20%).
The persistence with and adherence to PP1M and oral SGAs in real-world clinical practice in Europe and Asia have not been widely reported. Sultana et al26 conducted a retrospective database study on antipsychotic utilization patterns in Italy, Spain, the UK, and the US. Persistence with antipsychotics at 1 year ranged from 30% in Italy to 45% in Spain. Kuwabara et al27 carried out a study using the JMDC database, the same claims database we used in the present study, to evaluate adherence to antipsychotics in patients with schizophrenia within 6 months after discharge from hospital. Adherence was evaluated using the Medication Possession Ratio (MPR). An MPR ≥ 80% was reported for 77.02% of patients, and the results showed that good adherence to antipsychotics reduced the risk of re-hospitalization. However, detailed persistence and adherence stratified by oral and injectable antipsychotics in each database were not shown.
Joo et al28 calculated the proportion of continuous use of LAI antipsychotics at 6 months after initiation using the Health Insurance Review Agency (HIRA) database in South Korea. LAI paliperidone had a significantly higher continuation rate (57.5%) than LAI haloperidol (36.8%) and LAI risperidone (34.5%). The continuation rate of LAI paliperidone was comparable with our results in Japan (60.24%), possibly reflecting that both of these databases are nationwide claims databases. However, adherence and oral SGA use were outside the scope of Joo and colleagues’ study.
We observed that the benefit effect size of PP1M over oral SGAs was similar cross the 3 databases. PP1M significantly reduced discontinuation by approximately 25% to 35% and increased adherence by approximately 61% to 125% within the 1-year follow-up period.
Persistence and adherence in the JMDC were much better than in the 2 hospitals in China. This disparity could reflect differences in medical insurance coverage in each country. PP1M was not included in the China National Reimbursement Drug List during the study period, while it was covered by Japan medical insurance. The higher out-of-pocket costs for Chinese patients may have influenced their decision to continue with treatment. Additionally, there is no formal linked referral system for medical care in China, and patients can seek medical care in the medical facility of their choice. Difficulties in sharing electronic medical information between hospitals29 mean that medical encounters outside of the study hospital were not captured, potentially leading to underestimation of persistence and adherence. This may be particularly the case for XJH, which is a general hospital, and where patients may have occasionally visited specialized mental health centers. The JMDC captured all claims during the enrollment period, so it was less likely impacted by such underestimation.
Limitations of the Study
Drug adherence rates calculated from prescription data are not always aligned with results using clinical rating scales that may be used in clinical practice,24 such as the Medication Adherence Rating Scale. Information about disease severity was not available in all 3 databases. Although the 2 databases in China were derived from hospital medical records, in practice outpatients rarely complete disease severity scales. This means that we were unable to describe or control for clinical circumstances (eg, treatment response, adverse effects) that may have been critical factors influencing patient adherence, despite the multiple bias-controlling methods we employed. This study did not consider previous medications as potential confounders, eg, previous clozapine use, which was reported to be associated with worse outcome.30 Previous clozapine use might lead to low persistence of subsequent treatment. In addition, this study excluded the limited number of oral SGA uses prior to other injectable antipsychotics, which may potentially impact on the results as well. An additional limitation of the data is that compared to oral SGA treatment, the cost of LAI antipsychotic treatment is relatively high and, consequently, may not be selected for first-line treatment. This potential selection bias could suggest that LAI antipsychotic users might have had a better understanding of the disease, more insight, or more psychosocial supports, which selected them for better persistence and adherence.
Despite the aforementioned limitations, the observation of consistent results across 3 databases that represent 3 different clinical settings adds considerable weight to the validity of the findings. In conclusion, analysis of real-world data from Japan and China indicates that persistence and adherence were significantly higher in PP1M users than in oral SGA users. This evidence can potentially help to inform clinical practice and guide treatment decision-making for patients with schizophrenia in Asia, based on other evidence that better persistence and adherence leads to better clinical outcomes.
Submitted: December 18, 2020; accepted July 27, 2021.
Published online: December 28, 2021.
Author contributions: Huaning Wang formulated the research question, designed the study, interpreted the data, and reviewed the manuscript. Yongjing Zhang formulated the research question, designed the study, and reviewed the manuscript. Jin Liu conducted the study, analyzed the data, and reviewed the manuscript. Rui Chi conducted the study, analyzed the data, and reviewed the manuscript. Tao Wu conducted the study, analyzed the data, and reviewed the manuscript. Ting Zhang conducted the study, drafted the article, and reviewed the manuscript. Lili Zhang conducted the study, drafted the article, and reviewed the manuscript. Kun Jiang conducted the study, analyzed the data, and reviewed the manuscript. Hong Qiu interpreted the data and reviewed the manuscript. Wentian Dong interpreted the data and reviewed the manuscript. Tianmei Si formulated the research question, interpreted the data, and reviewed the manuscript.
Potential conflicts of interest: Drs Qiu and Y. Zhang, Mr Wu, and Dr T. Zhang are employees of Janssen Research & Development, LLC, and Dr L. Zhang is employee of Xi’an Janssen Pharmaceutical Ltd, which manufactures and markets risperidone and paliperidone. Drs Qiu and Y. Zhang report stock ownership in Johnson & Johnson. Drs Wang and Jiang, Ms Liu, and Drs Chi, Dong, and Si declare no conflict of interest.
Funding/support: This work was funded by Key R&D Program Projects, National Science Foundation of China (Grant No.2016YFC1306900 &2016YFC1307104); and China National Science and Technology Major Project for Investigational New Drug (Grant No. 2018ZX09201-014).
Role of the sponsor: The funding providers had no role in the conduct and publication of this study.
Acknowledgements: The authors thank Yanhua Yang, BS (Xijing Hospital, the Fourth Military Medical University); Yong He, BS (Peking University Sixth Hospital); Meiqi Zhang, MS (Janssen R&D); Hsingwen Chung, MS (Janssen R&D) and Joanne Wolter, MD, PhD (Janssen R&D) for database establishment and writing assistance. Meiqi Zhang, Hsingwen Chung, and Joanne Wolter were contractors for Janssen Research & Development.
Additional information: Data availability: The original data in China were generated during routine practice at Xijing Hospital, the Fourth Military Medical University, and the Peking University Sixth Hospital. The analysis data supporting the findings of this study are available from the corresponding authors H.W. and T.S. on reasonable request. For the Japan Medical Data Center (JMDC), restrictions apply to the license agreement between the JMDC and Johnson & Johnson. Data holders had access to their data, separately.
Supplementary material: Available at Psychiatrist.com.
Clinical Points
- Paliperidone palmitate once-monthly injection (PP1M) was associated with improved drug adherence and persistence in patients with schizophrenia. However, to date, PP1M utilization in China and Japan has been limited.
- In real-world clinical practice in China and Japan, treatment persistence and adherence were significantly higher in PP1M users than in oral second-generation antipsychotic users.
Editor’s Note: We encourage authors to submit papers for consideration as a part of our Focus on Psychosis section. Please contact Ann K. Shinn, MD, MPH, at [email protected].
References (30)

- Findlow J, Borrow R, Snape MD, et al. Multicenter, open-label, randomized phase II controlled trial of an investigational recombinant Meningococcal serogroup B vaccine with and without outer membrane vesicles, administered in infancy. Clin Infect Dis. 2010;51(10):1127–1137. PubMed CrossRef
- Whiteford HA, Degenhardt L, Rehm J, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382(9904):1575–1586. PubMed CrossRef
- Velligan DI, Weiden PJ, Sajatovic M, et al; Expert Consensus Panel on Adherence Problems in Serious and Persistent Mental Illness. The expert consensus guideline series: adherence problems in patients with serious and persistent mental illness. J Clin Psychiatry. 2009;70(suppl 4):1–46, quiz 47–48. PubMed CrossRef
- Haddad PM, Brain C, Scott J. Nonadherence with antipsychotic medication in schizophrenia: challenges and management strategies. Patient Relat Outcome Meas. 2014;5:43–62. PubMed CrossRef
- Kaplan G, Casoy J, Zummo J. Impact of long-acting injectable antipsychotics on medication adherence and clinical, functional, and economic outcomes of schizophrenia. Patient Prefer Adherence. 2013;7:1171–1180. PubMed CrossRef
- Medic G, Higashi K, Littlewood KJ, et al. Dosing frequency and adherence in chronic psychiatric disease: systematic review and meta-analysis. Neuropsychiatr Dis Treat. 2013;9:119–131. PubMed CrossRef
- Lefebvre P, Muser E, Joshi K, et al. Impact of paliperidone palmitate versus oral atypical antipsychotics on health care resource use and costs in veterans with schizophrenia and comorbid substance abuse. Clin Ther. 2017;39(7):1380–1395.e4. PubMed CrossRef
- Pilon D, Muser E, Lefebvre P, et al. Adherence, healthcare resource utilization and Medicaid spending associated with once-monthly paliperidone palmitate versus oral atypical antipsychotic treatment among adults recently diagnosed with schizophrenia. BMC Psychiatry. 2017;17(1):207. PubMed CrossRef
- Manjelievskaia J, Amos TB, El Khoury AC, et al. A comparison of treatment patterns, healthcare resource utilization, and costs among young adult Medicaid beneficiaries with schizophrenia treated with paliperidone palmitate or oral atypical antipsychotics in the US. J Med Econ. 2018;21(12):1221–1229. PubMed CrossRef
- Kamstra R, Pilon D, Lefebvre P, et al. Treatment patterns and Medicaid spending in comorbid schizophrenia populations: once-monthly paliperidone palmitate versus oral atypical antipsychotics. Curr Med Res Opin. 2018;34(8):1377–1388. PubMed CrossRef
- Joshi K, Lafeuille MH, Kamstra R, et al. Real-world adherence and economic outcomes associated with paliperidone palmitate versus oral atypical antipsychotics in schizophrenia patients with substance-related disorders using Medicaid benefits. J Comp Eff Res. 2018;7(2):121–133. PubMed CrossRef
- El Khoury AC, Pilon D, Morrison L, et al. The prospective economic impact of once monthly paliperidone palmitate versus oral atypical antipsychotics in Medicaid patients with schizophrenia. Curr Med Res Opin. 2019;35(3):395–405. PubMed CrossRef
- Joshi K, Muser E, Xu Y, et al. Adherence and economic impact of paliperidone palmitate versus oral atypical antipsychotics in a Medicare population. J Comp Eff Res. 2018;7(8):723–735. PubMed CrossRef
- Chiou CF, Wang BC, Caldwell R, et al. The cost reduction in hospitalization associated with paliperidone palmitate in the People’s Republic of China, Korea, and Malaysia. Neuropsychiatr Dis Treat. 2015;11:1989–1994. PubMed
- Kwon JS, Kim SN, Han J, et al. Satisfaction of immediate or delayed switch to paliperidone palmitate in patients unsatisfied with current oral atypical antipsychotics. Int Clin Psychopharmacol. 2015;30(6):320–328. PubMed CrossRef
- Zhang F, Si T, Chiou CF, et al. Efficacy, safety, and impact on hospitalizations of paliperidone palmitate in recent-onset schizophrenia. Neuropsychiatr Dis Treat. 2015;11:657–668. PubMed CrossRef
- Li N, Zhuo JM, Turkoz I, et al. A post hoc analysis on hospitalization risk in Asian patients with schizophrenia switching to once-monthly paliperidone palmitate from oral antipsychotics. Expert Opin Pharmacother. 2019;20(16):2033–2039. PubMed CrossRef
- Choon JWY, Wu DBC, Chong HY, et al. Real-world evidence of improved healthcare utilization in patients with schizophrenia or schizoaffective disorder after early treatment of paliperidone palmitate once-monthly treatment in Hong Kong. J Med Econ. 2019;22(3):273–279. PubMed CrossRef
- Si T, Fan J, Wang X, et al. A subgroup analysis of Chinese patients switched to paliperidone palmitate one-month injectable by prior oral antipsychotic treatment. Pharmacopsychiatry. 2016;49(1):32–41. PubMed
- Si T, Li N, Lu H, et al. Impact of paliperidone palmitate one-month formulation on relapse prevention in patients with schizophrenia: a post-hoc analysis of a one-year, open-label study stratified by medication adherence. J Psychopharmacol. 2018;32(6):691–701. PubMed CrossRef
- Qiu H, He Y, Zhang Y, et al. Antipsychotic polypharmacy in the treatment of schizophrenia in China and Japan. Aust N Z J Psychiatry. 2018;52(12):1202–1212. PubMed CrossRef
- Quality Alliance, Pharmacy Quality Alliance. Adherence – PQA adherence measures. Alexandria, VA. PQA website. https://www.pqaalliance.org/adherence-measures. Accessed November 09, 2019.
- National Committee for Quality Assurance. Adherence to antipsychotic medications for individuals with schizophrenia. Washington, DC. NCQA website. https://www.ncqa.org/hedis/measures/adherence-to-antipsychotic-medicationsfor-individuals-with-schizophrenia/. Accessed November 09, 2018.
- Forbes CA, Deshpande S, Sorio-Vilela F, et al. A systematic literature review comparing methods for the measurement of patient persistence and adherence. Curr Med Res Opin. 2018;34(9):1613–1625. PubMed CrossRef
- Shah A, Xie L, Kariburyo F, et al. Treatment patterns, healthcare resource utilization and costs among schizophrenia patients treated with long-acting injectable versus oral antipsychotics. Adv Ther. 2018;35(11):1994–2014. PubMed CrossRef
- Sultana J, Hurtado I, Bejarano-Quisoboni D, et al. Antipsychotic utilization patterns among patients with schizophrenic disorder: a cross-national analysis in four countries. 2019;75(7):1005–1015.
- Kuwabara H, Saito Y, Mahlich J. Adherence and rehospitalizations in patients with schizophrenia: evidence from Japanese claims data. Neuropsychiatr Dis Treat. 2015;11:935–940. PubMed
- Joo SW, Shon SH, Choi G, et al. Continuation of schizophrenia treatment with three long-acting injectable antipsychotics in South Korea: a nationwide population-based study. Eur Neuropsychopharmacol. 2019;29(9):1051–1060. PubMed CrossRef
- Zhou L, Nunes MB. Barriers to knowledge sharing in Chinese healthcare referral services: an emergent theoretical model. Glob Health Action. 2016;9(1):29964. PubMed CrossRef
- Deslandes PN, Dwivedi M, Sewell RD. Five-year patient outcomes with risperidone long-acting injection or oral aripiprazole. Ther Adv Psychopharmacol. 2015;5(3):151–157. PubMed CrossRef
This PDF is free for all visitors!