Abstract
Schizophrenia is a major mental illness with a median lifetime prevalence, across studies, of 0.5%. Across definitions of treatment resistance, about 37% of schizophrenia patients do not respond to treatment, and about 24% are treatment resistant from the first-episode, itself. Treatment resistance is addressed by trialing different antipsychotics and with antipsychotic augmentation strategies; what augmenting agent is used depends on what the target symptoms are. A landmark study in 1988 demonstrated the efficacy of clozapine in treatment resistant schizophrenia (TRS). Confirmatory studies and meta-analyses followed, establishing clozapine as the drug of choice for TRS in schizophrenia treatment guidelines across the world. Between 2016 and 2025, 2 network meta analyses (NMAs) and 1 individual participant data meta-analysis (IPD-MA) examined randomized controlled trials (RCTs) of clozapine vs other antipsychotics in TRS. The NMAs found that clozapine was superior to first-generation antipsychotics; however, clozapine did not head rankings for overall symptoms, positive symptoms, or negative symptoms, and, in pairwise analyses, there was little difference between clozapine and olanzapine and clozapine and risperidone for overall symptoms, positive symptoms, and negative symptoms. The IPD-MA found that clozapine was no better than comparator second-generation antipsychotics, considered singly or together, for overall symptoms, positive symptoms, and negative symptoms, in the short term, intermediate term, and long term. These findings fly in the face of clinical experience and treatment guideline recommendations. Among possible explanations, notable was that clozapine was significantly superior to comparator drugs when disregarding RCTs sponsored by the manufacturer of olanzapine. Clozapine is associated with many inconveniencing, distressing, and serious adverse effects that may be rare or common. Given the findings that olanzapine and risperidone may be as good as clozapine in TRS, it may be worth trialing these drugs before clozapine in patients with TRS. These and related issues, including nuances, are discussed.
J Clin Psychiatry 2025;86(3):25f16038
Author affiliations are listed at the end of this article.
Schizophrenia is a major mental illness the lifetime prevalence of which is commonly stated to be about 1%. However, findings in individual studies vary widely depending on the prevalence of risk factors in the population, the method of sampling, the method of case ascertainment, the definition of what constitutes a case, the definition of how long subjects should be followed to constitute a lifetime, statistical methods employed to generalize findings from the sample to the population, and other factors. A systematic review of 29 studies obtained a median value of 0.48% for lifetime prevalence (interquartile range, 0.34% to 0.85%)1; however, this review did not include a meta-analysis. Many studies have obtained higher than 1% values. For example, lifetime prevalences of schizophrenia and related psychoses have been pegged at 1.25% in China,2 1.44% in Finland,3 and 2.3% in Singapore.4
As a side note, the median value of 0.48% cited above should be taken with a grain of salt. The authors of the review1 merely presented the median of the prevalences reported in individual studies. Such a median does not represent a real population.
Treatment-Resistant Schizophrenia: Definitions
Schizophrenia is treated with antipsychotic and other drugs,5 but not all patients respond well; some patients are considered to be treatment-resistant. Definitions of treatment-resistant schizophrenia (TRS) vary widely across studies and can be quite complex, incorporating details about the number of antipsychotics trialed, what constitutes an adequate dose for each antipsychotic, what constitutes an adequate duration of treatment in each trial, whether or not nonresponsiveness is prospectively confirmed, whether or not medication compliance is factored in, whether or not a trial with a long-acting injection is required, whether or not treatment discontinuation due to the experience of unacceptable adverse effects constitutes an adequate trial, and how positive, negative, cognitive, and other symptoms, as well as functional impairment, are weighed in duration and severity toward the operationalization of nonresponse. Consensus guidelines on diagnosis and terminology have been proposed.6–8
For the practical clinician, a working definition of TRS is the failure to respond to at least 2 trials of antipsychotic medication, prescribed in adequate doses for an adequate duration, with failure to respond operationalized as the persistence of at least one distressing or impairing positive symptom. An adequate dose is usually the highest tolerated dose in the approved dosing range, and an adequate duration is usually 6–8 weeks of treatment at the highest tolerated dose. In all definitions, medication adherence is implicit; nonresponse cannot be declared in patients who do not take their medication.
Treatment-Resistant Schizophrenia: How Common Is It?
Decades ago, textbooks used the familiar “third, third, and a third” to describe the response of schizophrenia to antipsychotic drugs. That is, a third of treated patients respond well, a third respond partially to treatment, and a third show poor to no treatment response. Surprisingly, despite the advent of newer generations of antipsychotic drugs, and clozapine, not much has changed. In a recent systematic review and meta-analysis,9 the prevalence of TRS was found to be 36.7% (50 studies; pooled N=29,390) with treatment resistance from the first-episode, itself, observed in 23.6% (3 studies; pooled N=694). In a systematic review and meta-analysis specifically of first-episode psychosis (12 studies; pooled N=11,958), the pooled rate of treatment resistance was 22.8% overall, and 24.4% in first-episode schizophrenia cohorts. Male sex emerged as the only clear predictor of TRS (rate ratio, 1.57; 95% confidence interval [CI], 1.11–2.21).10
Readers may note that the prevalence of treatment resistance is lower in first-episode cohorts than in mixed cohorts. This underscores the clinical observation that treatment-responsive patients may transit into treatment resistance later during the course of the illness.
Treatment Strategies for Treatment-Resistant Schizophrenia
Treatment resistance is addressed by trialing different antipsychotics and through antipsychotic augmentation strategies; what is done depends on what the target symptoms are. In patients incompletely responsive to their current antipsychotic drug, augmentation strategies are commonly employed. As examples, antidepressants may be added to the primary antipsychotic to treat depressive symptoms, lithium or valproate for other mood symptoms, antidepressants or anticholinergics for negative symptoms, antidementia drugs for cognitive impairment, and a second antipsychotic or an anticonvulsant drug for positive symptoms. This list is far from exhaustive; in fact, so many augmentation strategies have been trialed that it would seem that much of the pharmacopoeia has been searched, usually with unconvincing success (if at all), in the hope of finding novel interventions that might make schizophrenia patients better.11,12
Clozapine for Treatment-Resistant Schizophrenia
In a historically important, industry-sponsored, multicenter, randomized controlled trial (RCT),13 268 schizophrenia patients who had previously failed trials with at least 3 different neuroleptic drugs, and who failed a 6-week prospective trial with haloperidol, were randomized to receive clozapine or chlorpromazine for 6 weeks. At the study endpoint, response, defined as at least 20% improvement in clinical ratings, was observed in 30% vs 4% of clozapine vs chlorpromazine patients, respectively. Clozapine was superior to chlorpromazine for improvement in positive as well as negative symptoms. The findings of this study led to the approval of clozapine for patients with TRS.14
By 1999, the efficacy of clozapine in TRS was established in meta-analysis15: clozapine was superior to neuroleptic drugs for eliciting clinical improvement in short-term studies (4 RCTs; pooled N, 370; odds ratio [OR], 0.2; 95% confidence interval [CI], 0.1–0.3; number needed to treat [NNT], 4) as well as in long-term studies (2 RCTs; pooled N, 648; OR, 0.5; 95% CI, 0.3–0.7; NNT, 7). The effect size in the short-term studies was medium to large (5 studies; pooled N, 429; standardized mean difference [SMD], 0.7; 95% CI, 0.5–0.9).
With specific reference to olanzapine, in a meta-analysis of 7 RCTs (pooled N = 648) conducted in patients with TRS,16 at treatment endpoint, clozapine was superior to olanzapine for positive (3 RCTs; SMD, 0.51; 95% CI, 0.17–0.86) and negative (3 RCTs; SMD, 0.50; 95% CI, 0.16–0.85) subscale scores on the Positive and Negative Syndrome Scale (PANSS); effect sizes were medium in magnitude. At treatment endpoint, clozapine was superior to olanzapine for PANSS total scores (4 RCTs; SMD, 0.21; 95% CI, −0.04 to 0.46) and PANSS change scores (3 RCTs; SMD, 0.08; 95% CI, −0.10 to 0.27), as well, but the effect sizes were small to very small and missed statistical significance. The clozapine and olanzapine groups did not differ significantly in dropout rates (7 RCTs; relative risk [RR], 0.93; 95% CI, 0.77–1.12).
As a side note, the values for PANSS changes scores in this meta-analysis16 were taken from the forest plot; the values were wrongly stated in both abstract and text.
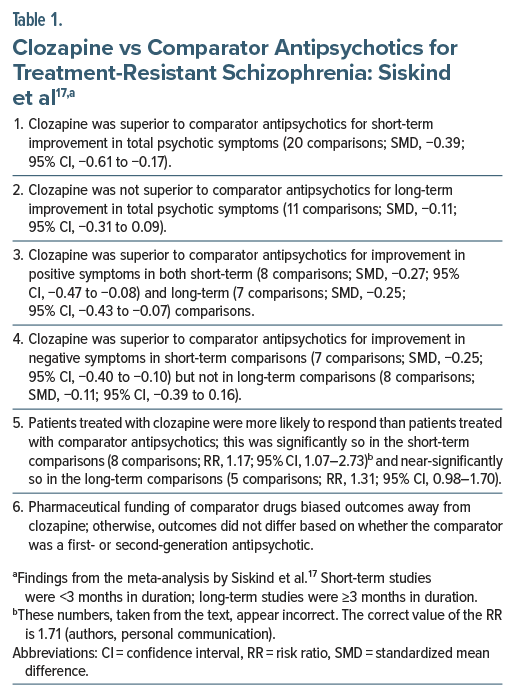
Siskind et al17 described a systematic review and meta-analysis of 21 RCTs (pooled N = 2,364) that included 25 comparisons of typical and atypical antipsychotics with clozapine in patients with TRS. The control arms were olanzapine (7 RCTs), risperidone (5 RCTs), haloperidol (4 RCTs), chlorpromazine (2 RCTs), ziprasidone (1 RCT), and assorted antipsychotics (2 RCTs). Important findings from the meta-analysis are presented in Table 1. In summary, for improvement in total ratings as well as for improvement in negative symptoms, clozapine was superior to comparator antipsychotics in short-term studies but not in long-term studies. For improvement in positive symptoms, clozapine was superior to comparator antipsychotics in both short- and long-term studies. Patients were more likely to respond to clozapine than to comparator drugs.
A meta-review of 112 meta-analyses of clozapine in psychotic disorders endorsed the superiority of clozapine for positive, negative, and overall symptoms of schizophrenia, as well as for protection against relapse, in TRS and in non TRS samples.18 Clozapine is now widely considered to be the treatment of choice for TRS, with recommendations for its primacy incorporated into treatment guidelines in countries such as the US,7 Canada,19 UK,20 Australia and New Zealand,21 and elsewhere.22
Challenging the Primacy of Clozapine for Treatment-Resistant Schizophrenia
During the past decade, accumulating evidence has challenged the primacy of clozapine in TRS. In a systematic review and network meta-analysis of 40 RCTs conducted in patients (pooled N = 5,172) with TRS,23 there was little evidence for a clear advantage for any drug. Important findings from this meta-analysis are presented in Table 2. In summary, in the network meta analysis, among the 9 antipsychotic drugs for which data were available, clozapine placed third for reduction in overall symptoms of schizophrenia, second for reduction in positive symptoms, and fifth for reduction in negative symptoms. In pairwise meta-analyses, there was negligible difference between clozapine and olanzapine and between clozapine and risperidone.
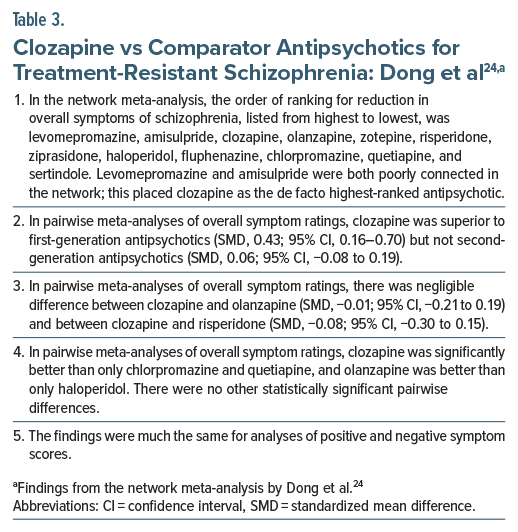
In a more recent systematic review and network meta-analysis of 60 RCTs conducted in patients (pooled N = 6,838) with TRS, including broadly defined TRS,24 there was again little evidence for a clear advantage for clozapine (Table 3). In summary, among the 12 antipsychotic drugs for which data were available, after evaluating network connectivity, clozapine could reasonably be considered as the highest-ranked antipsychotic for reduction in overall symptoms of schizophrenia. However, in pairwise meta-analyses, clozapine was significantly better than only chlorpromazine and quetiapine; there was negligible difference between clozapine and olanzapine and between clozapine and risperidone. A potentially serious limitation of this meta-analysis is that, in pairwise comparisons, the authors appear to have combined change scores with endpoint scores in generating SMDs. This is discouraged practice.25,26
Individual Participant Data Meta-Analysis
A further challenge to the primacy of clozapine in TRS arose from the systematic review and individual participant data meta-analysis (IPD-MA) by Schneider-Thoma et al.27 Readers who are unfamiliar with IPD-MA may wish to read the primer on IPD-MA which appeared as the previous article in this column.28
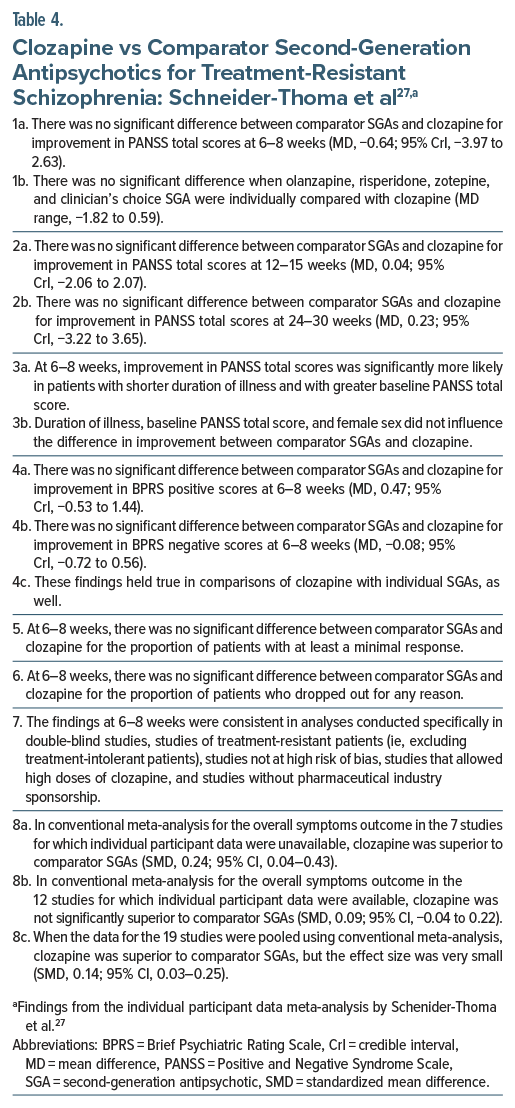
In this IPD-MA, conducted in patients with TRS, the authors27 identified 19 relevant RCTs that compared clozapine specifically with second-generation antipsychotics (SGAs). Individual participant data could be obtained for only 12 RCTs (pooled N = 1,052). These 12 RCTs compared clozapine with olanzapine (5 RCTs), risperidone (3 RCTs), assorted antipsychotics (2 RCTs), and ziprasidone and zotepine (1 RCT, each). Seven of the 12 RCTs had been sponsored by the pharmaceutical industry. In 11 of the 12 RCTs, medication dosing was flexible.
Important findings from the IPD-MA27 are presented in Table 4. In summary, in this IPD-MA, clozapine was no better than comparator SGAs, considered singly or together, for short-, intermediate-, or long-term outcomes, whether these outcomes were total symptom scores, positive symptom scores, or negative symptom scores. However, when total symptom data at study endpoint were pooled for all 19 studies in a conventional meta-analysis, clozapine was superior to SGAs, but the effect size was very small. This last finding is iffy because it was based on different kinds of scores at different study endpoints, and on a combination of change scores and endpoint scores, which is discouraged when the SMD is the summary estimate.25,26
A worthy observation in this IPD-MA27 was that duration of illness and baseline severity of illness both predicted improvement in TRS regardless of treatment arm. It seems reasonable to infer that longer duration of illness in TRS may be a trait marker for poorer outcomes and that more severe illness at baseline may be a state marker for regression to the mean and other pathways toward improvement in clinical trials.29
Another interesting observation in this IPD-MA27 was that female sex, duration of illness, and baseline severity of illness did not predict response to clozapine. Thus, if there is a subtype of patients who respond to clozapine, these variables are not part of the subtype.
General Summary
Meta-analyses have shown, with reasonable consistency, that clozapine is superior to first-generation antipsychotic (FGA) drugs in patients with TRS15,23,24; this assertion applies primarily to clozapine vs chlorpromazine and clozapine vs haloperidol, the commonest comparisons for which data are available. The data are admittedly sparse, but this is perhaps of little consequence because FGAs are associated with extrapyramidal syndromes, secondary negative symptoms, secondary cognitive impairment, raised serum prolactin, and other untoward effects and are, consequently, currently infrequently prescribed.
An initial meta-analysis found that clozapine was superior to olanzapine in TRS; however, the advantage was evident for positive and negative symptoms but not for overall symptoms.16 Two subsequent network meta analyses of clozapine vs SGAs found that clozapine did not head rankings for overall symptoms, positive symptoms, or negative symptoms and that, in pairwise analyses, there was little difference between clozapine and olanzapine and between clozapine and risperidone for overall symptoms, positive symptoms, and negative symptoms.23,24 The most recent study, an IPD-MA27 also found that clozapine was no better than comparator SGAs, considered singly or together, for overall symptoms, positive symptoms, and negative symptoms, in the short term, intermediate term, and long term.
Olanzapine and risperidone are the mostly commonly prescribed among oral antipsychotics, and these were the antipsychotics for which the largest number of RCTs were available. So, the clozapine olanzapine and the clozapine-risperidone comparisons are of especial interest. In this context, the consistency in findings for these comparisons across the recent meta-analyses23,24,27 was not due to each meta-analysis examining the same studies; while there was certainly much overlap across meta-analyses, the overlap was not substantial.
Appraisal of the Recent Meta-Analyses
The evidence from meta-analyses during the past 10 years, presented in this article, flies in the face of clinical experience and treatment guideline recommendations. Clozapine was the revolution in the treatment of schizophrenia; so, why don’t the meta-analyses support its primacy for TRS, the indication that brought the drug back into mainstream psychopharmacology? This section considers possible explanations in what is admittedly an effort in apologetics.
First, many of the RCTs in the meta-analyses were industry-sponsored. This is problematic because sponsored trial designs commonly contain characteristics that are intended to promote the sponsor’s interests, and because trial findings that do not support the sponsor’s interests may remain unpublished. In their meta-analysis, Siskind et al17 observed that pharmaceutical funding of comparator drugs appeared to disfavor clozapine. However, whereas 12 of the 19 RCTs in the IPD-MA by Schneider-Thoma et al27 were sponsored, a sensitivity analysis conducted in studies without pharmaceutical industry support did not uncover an advantage for clozapine; a limitation of this analysis is that it included only 276 subjects. In contrast, and of critical importance, in the most recent network meta-analysis,24 when studies from the manufacturer of olanzapine were excluded, clozapine was significantly better than olanzapine in the network meta-analysis (SMD, −0.36; 95% CI, −0.67 to −0.04) and near significantly better in the pairwise meta-analysis (SMD, −0.43; 95% CI, −0.88 to 0.01). The findings for clozapine changed little when studies from the manufacturer of clozapine were excluded.24
Second, there are many pathways to clinical improvement beyond the pharmacological effect and the placebo effect,29 and these pathways are magnified in clinical trials, narrowing drug vs placebo differences; these pathways, and statistical noise in multicenter trials,30 may therefore explain why it is so hard for new treatments to outperform placebo. In such circumstances, it could be even harder to find one active drug superior to other active drugs, as with clozapine vs other SGAs. However, this argument does not carry weight in meta-analysis because if a small advantage exists, it should be detectable when studies are pooled and statistical power increases. So, the consistent failure to identify an advantage for clozapine in meta-analysis suggests that the posited advantage may not exist.
Third, meta-analysis does not provide as definitive an answer to a research question as readers may expect.31 In the meta-analyses that compared clozapine with other antipsychotics, study factors and meta-analysis factors could both have influenced results. Study factors include how TRS was defined (narrow or broad criteria), blinding protocols (single-blind, double-blind, or not blinded), drug dosing protocols (fixed or flexible dosing, optimal or suboptimal dosing), how treatment response was defined, what the study duration was (short- or long-term), etc. Meta-analysis factors include how studies were chosen for inclusion, what outcomes and the measurement thereof were selected for analysis, what time point was selected for examination of the outcomes of interest, whether endpoint scores or change scores were examined, whether mean difference or SMD was examined, and other statistical methods. Some of these factors could have narrowed differences between clozapine and comparator drugs; others could have introduced heterogeneity and statistical noise. However, most of the meta-analyses included sensitivity analyses, and the sensitivity analyses broadly supported the findings of the main analyses. The only exception is that when studies from the manufacturer of olanzapine were excluded, an advantage for clozapine emerged.
Adverse Effects of Clozapine
As a reminder to readers, all treatment decisions in medicine involve risk-benefit analyses. Therefore, whether or not clozapine should be the go-to antipsychotic for TRS should be based not only on its efficacy profile but also on its adverse effect profile. Clozapine has many adverse effects. It is associated with rare but potentially serious adverse effects, such as myocarditis, seizures, ileus, and agranulocytosis, and with common and inconveniencing as well as common and problematic adverse effects, such as sialorrhea, excessive sleep, constipation, and the metabolic syndrome, and consequences and complications thereof.32 On the positive side, clozapine is not associated with extrapyramidal syndromes or with raised serum prolactin.
Verdict
At the risk of oversimplification, the issue comes down to whether to accept the consistent findings in the recent meta-analyses that clozapine is no better than other SGAs for TRS, or assert that clozapine is superior to SGAs with its superiority masked (in the meta analyses) by industry-sponsored studies. The latter choice assumes that industry-sponsored studies are automatically untrustworthy. Because there is uncertainty around such an assumption, the verdict must be that … the jury is out. Therefore, the clinician, patient, and caregivers must, in a shared decision-making process, choose between another SGA trial and going directly to clozapine. This choice should include a consideration of the adverse effect profile of the drugs.
Suggestions
Olanzapine and risperidone are among the most commonly prescribed oral SGAs, and both drugs were among the top-ranked antipsychotics in the network meta-analyses23,24; in efficacy, clozapine differed negligibly from each. Moving directly to clozapine may be justified if the patient with TRS has already had trials of olanzapine and risperidone that were adequate in dose and duration, and/or if the illness or any aspect thereof is so severe as to make clozapine desirable as a matter of immediate need.
If the patient has been ill for long and is willing to postpone trying clozapine, an adequate trial of olanzapine or risperidone may be recommended, whichever has not been already adequately trialed. The trial should be at least 6–8 weeks long, and perhaps even up to 3 months in duration, going by what was done in many of the RCTs populating the meta-analyses discussed in this article. Between olanzapine and risperidone, the former may be preferred, based on the advantage for olanzapine in the network meta-analyses. It could also be worth considering a long-acting injectable antipsychotic if only because these are generally associated with better outcomes than oral antipsychotics.33,34
The shared decision-making process should also consider that whatever works is likely to be continued for years and perhaps a lifetime. The long-term adverse effect profiles of different drugs merit attention during such discussions. Also worth considering is that, at least in observational studies, treatment with clozapine is associated with consistently reported advantages. For example, a meta-analysis found that, relative to other SGAs, clozapine was associated with lower risk of hospitalization (19 studies; pooled N, 49,453; RR, 0.82; 95% CI, 0.73–0.92) and all-cause discontinuation (16 studies; pooled N, 56,368; RR, 0.73; 95% CI, 0.64–0.84).35 Data from Finland (hazard ratio [HR], 0.64; 95% CI, 0.49–0.84) and Sweden (HR, 0.66; 95% CI, 0.43–0.99) showed that clozapine was the only antipsychotic associated with a reduced risk of attempted or completed suicide.36 In a meta-analysis with data from 4 studies, patients continuing on clozapine were at lower risk of mortality than those on other antipsychotics (RR, 0.56; 95% CI, 0.36–0.85).37
Parting Notes
Some authors argue for the early introduction of clozapine to improve outcomes in schizophrenia. For example, in a systematic review and network meta analysis of RCTs of 32 oral antipsychotics used to treat acute psychosis in adults with multiepisode schizophrenia, among 402 relevant studies (pooled N = 53,463), clozapine had the largest effect size for reduction in overall symptoms and negative symptoms and the second largest effect size for reduction in positive symptoms and depressive symptoms.38
Delay in initiating clozapine is common; one study found that patients were started on clozapine after a median of 6 failed trials.39 Delay in initiating clozapine, including after recognition of treatment resistance, has been associated with poorer response to clozapine.40,41 However, in at least some patients, late introduction of clozapine may merely be a marker for worse prognosis, with no assurance that earlier introduction of clozapine would have improved outcomes.
Clozapine is not a magic bullet for every patient with TRS. In a meta-analysis of 77 studies of assorted design, the median response rate was 50% (39 studies) for clozapine monotherapy; this was higher than the median response rate to all other antipsychotics, considered individually. The median response rate was 25% (20 studies) with typical antipsychotics, and 42% (36 studies) with nonclozapine atypical antipsychotics.42 Readers may note that these medians were medians of studies and not medians of pooled samples; so, the medians need to be interpreted with caution.
The response rate is expectedly lower with extreme treatment resistance. In the Kane et al13 RCT, which required at least 3 historical and 1 prospective failed antipsychotic trials, although clozapine was superior to chlorpromazine, the response rate to clozapine was a mere 30%; and this [response rate], with the threshold set at a low of 20% reduction in clinical ratings. Patients who continue to remain symptomatic after treatment with clozapine may benefit from augmentation strategies; these include psychopharmacological agents and electroconvulsive therapy, and the choice will depend on what the target symptoms are.43
Article Information
Published Online: August 25, 2025. https://doi.org/10.4088/JCP.25f16038
© 2025 Physicians Postgraduate Press, Inc.
To Cite: Andrade C. The superiority of clozapine over second-generation antipsychotics in patients with treatment-resistant schizophrenia: room for doubt.
J Clin Psychiatry 2025;86(3):25f16038.
Author Affiliations: Department of Psychiatry, Kasturba Medical College, Manipal Academy of Higher Education, Manipal, India; Department of Clinical Psychopharmacology and Neurotoxicology, National Institute of Mental Health and Neurosciences, Bangalore, India.
Corresponding Author: Chittaranjan Andrade, MD, Department of Clinical Psychopharmacology and Neurotoxicology, National Institute of Mental Health and Neurosciences, Bangalore 560029, India ([email protected]).
Relevant Financial Relationships: None.
Funding/Support: None.
 Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Department of Clinical Psychopharmacology and Neurotoxicology, National Institute of Mental Health and Neurosciences, Bangalore, India. Please contact Chittaranjan Andrade, MD, at Psychiatrist.com/contact/andrade.
References (43)

- Simeone JC, Ward AJ, Rotella P, et al. An evaluation of variation in published estimates of schizophrenia prevalence from 1990─2013: a systematic literature review. BMC Psychiatry. 2015;15:193. PubMed CrossRef
- Chang WC, Wong CSM, Chen EYH, et al. Lifetime prevalence and correlates of schizophrenia-spectrum, affective, and other non-affective psychotic disorders in the Chinese adult population. Schizophr Bull. 2017;43(6):1280–1290. PubMed CrossRef
- Perälä J, Suvisaari J, Saarni SI, et al. Lifetime prevalence of psychotic and bipolar I disorders in a general population. Arch Gen Psychiatry. 2007;64(1):19–28. PubMed CrossRef
- Subramaniam M, Abdin E, Vaingankar JA, et al. Lifetime prevalence and correlates of schizophrenia and other psychotic disorders in Singapore. Front Psychiatry. 2021;12:650674. PubMed CrossRef
- Kinon BJ, Leucht S, Tamminga C, et al. Rationale for adjunctive treatment targeting multiple mechanisms in schizophrenia. J Clin Psychiatry. 2024;85(3):23nr15240. PubMed CrossRef
- Howes OD, McCutcheon R, Agid O, et al. Treatment-resistant schizophrenia: Treatment Response and Resistance in Psychosis (TRRIP) Working Group consensus guidelines on diagnosis and terminology. Am J Psychiatry. 2017;174(3):216–229. PubMed CrossRef
- American Psychiatric Association. The American Psychiatric Association Practice Guideline for the Treatment of Patients With Schizophrenia, 3rd ed, American Psychiatric Association; 2020. Accessed July 02, 2025. https://psychiatryonline.org/doi/abs/10.1176/appi.books.9780890424841
- Correll CU, Howes OD. Treatment-resistant schizophrenia: definition, predictors, and therapy options. J Clin Psychiatry. 2021;82(5):MY20096AH1C. PubMed CrossRef
- Diniz E, Fonseca L, Rocha D, et al. Treatment resistance in schizophrenia: a meta analysis of prevalence and correlates. Braz J Psychiatry. 2023;45(5):448–458. PubMed CrossRef
- Siskind D, Orr S, Sinha S, et al. Rates of treatment-resistant schizophrenia from first-episode cohorts: systematic review and meta-analysis. Br J Psychiatry. 2022;220(3):115–120. PubMed CrossRef
- Correll CU, Rubio JM, Inczedy-Farkas G, et al. Efficacy of 42 pharmacologic cotreatment strategies added to antipsychotic monotherapy in schizophrenia: systematic overview and quality appraisal of the meta-analytic evidence. JAMA Psychiatry. 2017;74(7):675–684. PubMed CrossRef
- Andrade C. The use of statins for antipsychotic augmentation in schizophrenia: examination of meta-analyses with flawed methods and conclusions. J Clin Psychiatry. 2018;79(5):18f12562. PubMed CrossRef
- Kane J, Honigfeld G, Singer J, et al. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45(9):789–796. PubMed CrossRef
- The Lancet Psychiatry. Clozapine: past, present, and future. Lancet Psychiatry. 2024;11(1):1. PubMed CrossRef
- Wahlbeck K, Cheine M, Essali A, et al. Evidence of clozapine’s effectiveness in schizophrenia: a systematic review and meta-analysis of randomized trials. Am J Psychiatry. 1999;156(7):990–999. PubMed CrossRef
- Souza JS, Kayo M, Tassell I, et al. Efficacy of olanzapine in comparison with clozapine for treatment-resistant schizophrenia: evidence from a systematic review and meta-analyses. CNS Spectr. 2013;18(2):82–89. PubMed CrossRef
- Siskind D, McCartney L, Goldschlager R, et al. Clozapine v. first- and second generation antipsychotics in treatment-refractory schizophrenia: systematic review and meta-analysis. Br J Psychiatry. 2016;209(5):385–392. PubMed CrossRef
- Wagner E, Siafis S, Fernando P, et al. Efficacy and safety of clozapine in psychotic disorders-a systematic quantitative meta-review. Transl Psychiatry. 2021;11(1):487. PubMed CrossRef
- Remington G, Addington D, Honer W, et al. Guidelines for the pharmacotherapy of schizophrenia in adults. Can J Psychiatry. 2017;62(9):604–616. PubMed CrossRef
- Barnes TR, Drake R, Paton C, et al. Evidence-based guidelines for the pharmacological treatment of schizophrenia: updated recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2020;34(1):3–78. PubMed CrossRef
- Galletly C, Castle D, Dark F, et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for the management of schizophrenia and related disorders. Aust N Z J Psychiatry. 2016;50(5):410–472. PubMed CrossRef
- McCutcheon RA, Pillinger T, Varvari I, et al. INTEGRATE: international guidelines for the algorithmic treatment of schizophrenia. Lancet Psychiatry. 2025;12(5):384–394. PubMed CrossRef
- Samara MT, Dold M, Gianatsi M, et al. Efficacy, acceptability, and tolerability of antipsychotics in treatment-resistant schizophrenia: a network meta-analysis. JAMA Psychiatry. 2016;73(3):199–210. PubMed CrossRef
- Dong S, Schneider-Thoma J, Bighelli I, et al. A network meta-analysis of efficacy, acceptability, and tolerability of antipsychotics in treatment-resistant schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2024;274(4):917–928. PubMed CrossRef
- Deeks JJ, Higgins JPT, Altman DG. On Behalf of the Cochrane Statistical Methods Group. Analysing Data and Undertaking Meta-Analyses. In: Higgins JPT, Thomas J, Chandler J, et al, eds. Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed;2019:241–284.
- Andrade C. Understanding the basics of meta-analysis and how to read a forest plot: as simple as it gets. J Clin Psychiatry. 2020;81(5):20f13698. PubMed CrossRef
- Schneider-Thoma J, Hamza T, Chalkou K, et al. Efficacy of clozapine versus second-generation antipsychotics in people with treatment-resistant schizophrenia: a systematic review and individual patient data meta-analysis. Lancet Psychiatry. 2025;12(4):254–265. PubMed CrossRef
- Andrade C. A primer on individual participant data meta-analysis and its strengths and limitations. J Clin Psychiatry. 2025;86(3):25f16001. PubMed CrossRef
- Andrade C. There’s more to placebo-related improvement than the placebo effect alone. J Clin Psychiatry. 2012;73(10):1322–1325. PubMed CrossRef
- Andrade C. Signal-to-noise ratio, variability, and their relevance in clinical trials. J Clin Psychiatry. 2013;74(5):479–481. PubMed CrossRef
- Streiner DL. I have the answer, now what’s the question?: why metaanalyses do not provide definitive solutions. Can J Psychiatry. 2005;50(13):829–831. PubMed CrossRef
- Siskind D, Northwood K, Pillinger T, et al; Clozapine Delphi Expert Panel. Absolute neutrophil count and adverse drug reaction monitoring during clozapine treatment: consensus guidelines from a global Delphi panel. Lancet Psychiatry. 2025;1:S2215-0366(25)00098-7.
- Kishimoto T, Hagi K, Nitta M, et al. Effectiveness of long-acting injectable vs oral antipsychotics in patients with schizophrenia: a meta-analysis of prospective and retrospective cohort studies. Schizophr Bull. 2018;44(3):603–619. PubMed CrossRef
- Lopena OJ, Alphs LD, Sajatovic M, et al. Earlier use of long-acting injectable paliperidone palmitate versus oral antipsychotics in patients with schizophrenia: an integrated patient-level post hoc analysis. J Clin Psychiatry. 2023;84(6):23m14788. PubMed CrossRef
- Masuda T, Misawa F, Takase M, et al. Association with hospitalization and all cause discontinuation among patients with schizophrenia on clozapine vs other oral second-generation antipsychotics: a systematic review and meta-analysis of cohort studies. JAMA Psychiatry. 2019;76(10):1052–1062. PubMed CrossRef
- Taipale H, Lahteenvuo M, Tanskanen A, et al. Comparative effectiveness of antipsychotics for risk of attempted or completed suicide among persons with schizophrenia. Schizophr Bull. 2020;46(suppl 1):S118–S119. CrossRef
- Vermeulen JM, van Rooijen G, van de Kerkhof MPJ, et al. Clozapine and long-term mortality risk in patients with schizophrenia: a systematic review and meta-analysis of studies lasting 1.1-12.5 years. Schizophr Bull. 2019;45(2):315–329. PubMed CrossRef
- Huhn M, Nikolakopoulou A, Schneider-Thoma J, et al Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi episode schizophrenia: a systematic review and network meta-analysis. Lancet. 2019;394(10202):939–951. Erratum in: Lancet. 2019;394(10202):918. PubMed CrossRef
- Law C, See YM, Yee JY, et al. The impact of clozapine delay on clinical outcomes in schizophrenia. J Clin Psychiatry. 2023;84(5):22m14588. PubMed CrossRef
- Griffiths K, Millgate E, Egerton A, et al. Demographic and clinical variables associated with response to clozapine in schizophrenia: a systematic review and meta-analysis. Psychol Med. 2021;51(3):376–386. PubMed CrossRef
- Hatano M, Kamei H, Takeuchi I, et al. Long-term outcomes of delayed clozapine initiation in treatment-resistant schizophrenia: a multicenter retrospective cohort study. BMC Psychiatry. 2023;23(1):673. PubMed CrossRef
- Seppälä A, Pylvänäinen J, Lehtiniemi H, et al. Predictors of response to pharmacological treatments in treatment-resistant schizophrenia - a systematic review and meta-analysis. Schizophr Res. 2021;236:123–134. PubMed CrossRef
- Wagner E, Kane JM, Correll CU, et al. Clozapine combination and augmentation strategies in patients with schizophrenia -recommendations from an international expert survey among the Treatment Response and Resistance in Psychosis (TRRIP) Working Group. Schizophr Bull. 2020;46(6):1459–1470. PubMed CrossRef
This PDF is free for all visitors!