Prim Care Companion CNS Disord 2022;24(2):21cr03007
To cite: Uljon S. An adulterated urine buprenorphine specimen. Prim Care Companion CNS Disord. 2022;24(2):21cr03007.
To share: https://doi.org/10.4088/PCC.21cr03007
© Copyright 2022 Physicians Postgraduate Press, Inc.
aCore Laboratory, Pathology Department, Massachusetts General Hospital, Boston, Massachusetts
*Corresponding author: Sacha Uljon, MD, PhD, Core Laboratory, Pathology Department, Massachusetts General Hospital, 55 Fruit St, GRB 554, Boston, MA 02114-2696 ([email protected]).
Primary care providers are often responsible for monitoring adherence to buprenorphine treatment. This case report illustrates the pitfalls of urine buprenorphine screening and provides suggestions for recognizing adulterated or substituted urine specimens.
Case Report
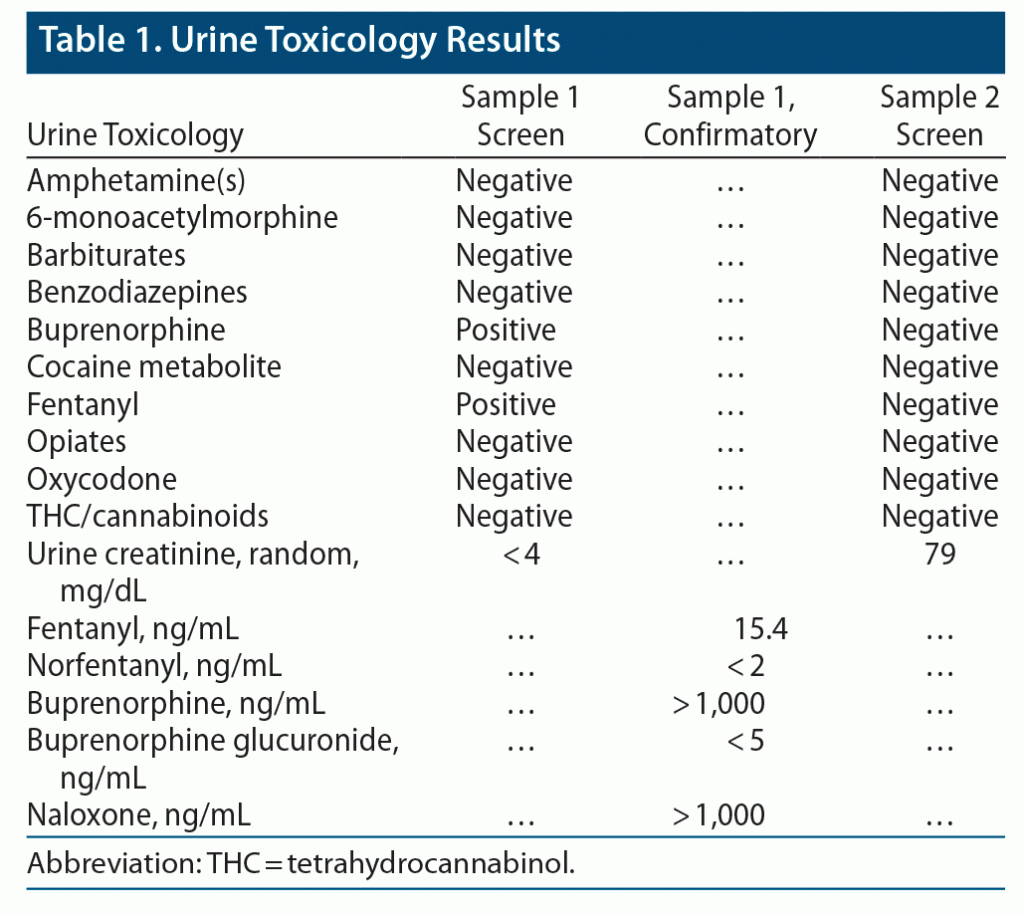
Our laboratory was consulted by a primary care provider regarding an unexpected urine toxicology result (sample 1). The urine sample was from a 45-year-old man with a history of substance use disorder, who was currently in recovery and on suboxone 8-2 twice/d. The sample was provided as part of compliance monitoring while the patient was petitioning for custody of his child.
The ordering provider and the patient were concerned about a potential false-positive fentanyl result in the urine. While the result was being confirmed by the laboratory, the patient requested the chance to provide a second urine sample (sample 2). He was adamant that he did not use fentanyl.
The laboratory confirmed the fentanyl and norfentanyl findings by re-running sample 1 on our in-house liquid chromatography-tandem mass spectrometry (LC-MS/MS). Sample 1 was also sent to an outside laboratory for buprenorphine confirmation by LC-MS/MS (Table 1). The laboratory contacted the physician with the following interpretation:
(1) Sample 1 is an adulterated or substituted sample.
(2) There is no evidence that the patient is taking fentanyl or buprenorphine.
When confronted, the patient eventually admitted that he had been adulterating his urine for months. Review of the past 3 urine samples revealed creatinine levels below 4 mg/dL. It was not known to the laboratory whether the subsequent sample (sample 2) was his urine or “clean” urine obtained elsewhere.
Urine can be diluted or substituted to avoid detection of illegal substances or can be adulterated by the direct addition of prescription drugs to mimic compliance. Oral fluid is an alternative matrix and is now accepted by the Substance Abuse and Mental Health Services Administration. However, urine has superior sensitivity for buprenorphine1 and is the most widely used matrix. This example illustrates several “red flags” that primary care providers should watch for in cases of suspected specimen urine tampering.
First, check the creatinine. Normal human urine from an adult has a creatinine level > 20 mg/dL. Creatinine values < 20 mg/dL are red flags for dilution, adulteration, or substitution. Creatinine may not appear with the urine drug results, so this may require looking into the electronic medical record.
Second, look for metabolites. In both dilute and nondilute samples, the ratio of parent drug to metabolite will be different if the drug is added directly to the sample. Because immunoassays typically detect both the parent drug and metabolites, an immunoassay cannot confirm adulteration. However, LC-MS/MS can discriminate between buprenorphine and buprenorphine metabolites (such as buprenorphine glucuronides).
The presence of the parent drug at high levels without metabolite and the presence of naloxone in high levels are both suggestive of adulteration. Some authors2,3 have even suggested using the ratio of parent drug to metabolite as a more subtle marker of adulteration. Likewise, the presence of fentanyl without the metabolite norfentanyl suggests that the sample was also contaminated with fentanyl powder. (This is likely to have been accidental.)
Finally, consult the laboratory with questions or unexpected results. At a minimum, the laboratory can provide the package insert for an immunoassay, which will list potential interferences and cross-reacting substances. The laboratory can also send the urine to a reference laboratory for LC-MS/MS, the gold standard for specificity and sensitivity.
Published online: February 24, 2022.
Potential conflicts of interest: None.
Funding/support: None.
Additional information: Information has been de-identified to protect patient anonymity.
References (3)

- Ransohoff JR, Petrides AK, Piscitello GJ, et al. Urine is superior to oral fluid for detecting buprenorphine compliance in patients undergoing treatment for opioid addiction. Drug Alcohol Depend. 2019;203:8–12. PubMed CrossRef
- Donroe JH, Holt SR, O’Connor PG, et al. Interpreting quantitative urine buprenorphine and norbuprenorphine levels in office-based clinical practice. Drug Alcohol Depend. 2017;180:46–51. PubMed CrossRef
- Donroe JH, Holt SR, O’Connor PG, et al. Interpreting quantitative urine buprenorphine and norbuprenorphine levels in office-based clinical practice. Drug Alcohol Depend. 2017;180:46–51. PubMed CrossRef
Enjoy this premium PDF as part of your membership benefits!