Prim Care Companion CNS Disord 2022;24(4):21cr03119
To cite: McDermott S, Johnson BE, Balasanova AA. Phenibut and bromazolam use disorders requiring hospitalization for medically supervised withdrawal. Prim Care Companion CNS Disord. 2022;24(4):21cr03119.
To share: https://doi.org/10.4088/PCC.21cr03119
© Copyright 2022 Physicians Postgraduate Press, Inc.
aCollege of Medicine, University of Nebraska Medical Center, Omaha, Nebraska
bDepartment of Psychiatry, College of Medicine, University of Nebraska Medical Center, Omaha, Nebraska
*Corresponding author: Alëna A. Balasanova, MD, Department of Psychiatry, Poynter Hall 5th Floor, University of Nebraska Medical Center, 985578 Nebraska Medical Center, Omaha, Nebraska 68198-5578 ([email protected]).
We present the case of a patient who developed a sedative-hypnotic use disorder to the γ-aminobutyric acid (GABA) agonist phenibut and to the designer benzodiazepine bromazolam. The patient had attempted to wean himself off these substances independently but experienced severe withdrawal symptoms requiring hospitalization for medical management.
Case Report
The patient was a 30-year-old man with no known psychiatric history who self-presented to the emergency department (ED) requesting medical assistance for withdrawal from sedative-hypnotics. He reported that several years prior he began to recreationally use gabapentin obtained from friends. When he began having trouble acquiring gabapentin, he researched similar drugs and discovered phenibut, which he began ordering online and having shipped directly to his home. He reported taking phenibut consistently for the past year, initially at 500 mg/d and gradually increasing to 18 g/d due to having developed a tolerance. The patient appreciated a feeling of focus and productivity at the higher dose but also experienced insomnia, which prompted him to seek out bromazolam, a designer benzodiazepine available from the same website. The patient reported having developed a tolerance to bromazolam as well, increasing his intake to 20 mg/d.
Several months prior to presentation, the patient attempted to reduce his phenibut use on his own by titrating down to 10 g/d. However, he began experiencing severe whole-body aches, causing him to increase his dosage to 18 g/d again. These withdrawal symptoms are what led the patient to seek medical attention. On arrival to the ED, the patient explained that he sought help with the specific hope of receiving baclofen and clonazepam because he had researched that these medications could help with withdrawal.
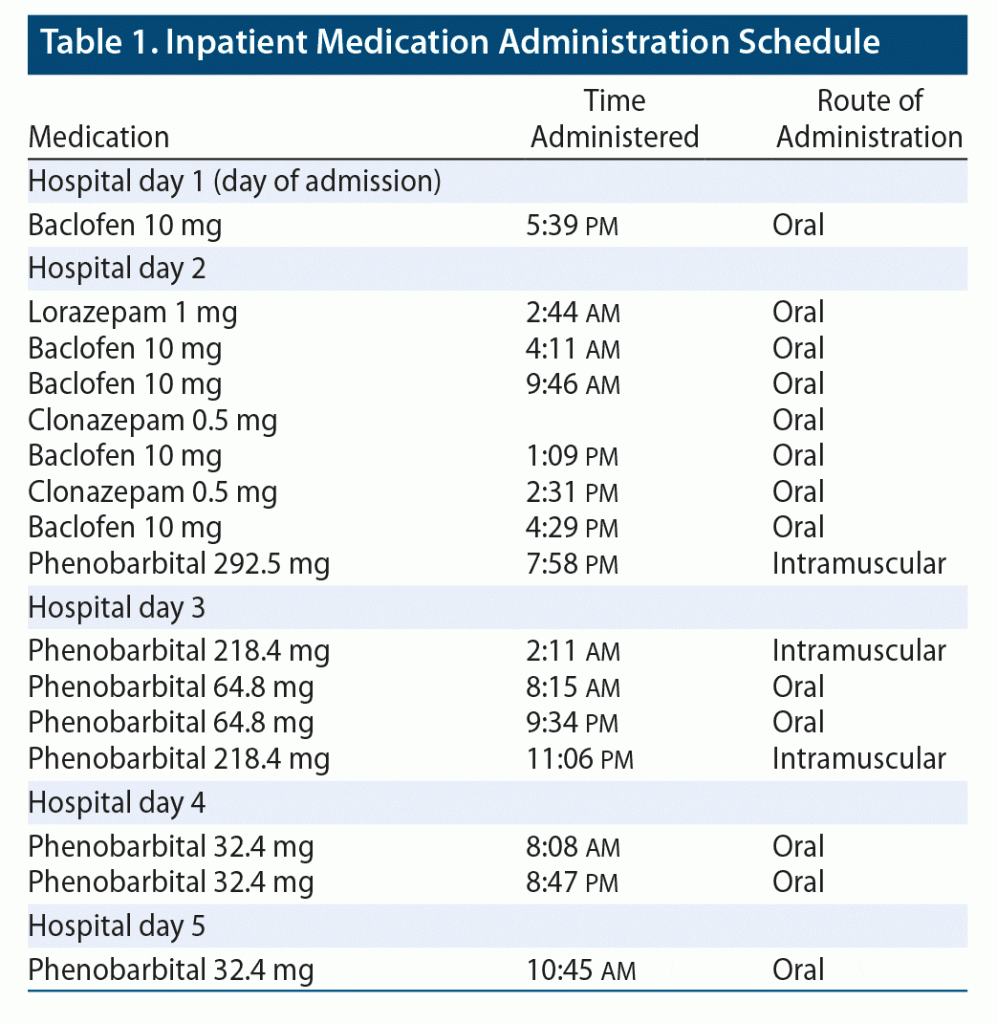
Upon admission to the medical floor, the primary team initiated baclofen as per the patient’s request and also placed him on a Clinical Institute Withdrawal Assessment of Alcohol Scale (CIWA)1 protocol with oral lorazepam. The addiction psychiatry team was consulted on the day following admission and recommended switching from baclofen and the CIWA protocol to a phenobarbital taper, which was initiated on hospital day 2 (Table 1). Over the coming days, all objective signs of withdrawal had resolved. By hospital day 3, the patient did not have tremor or diaphoresis, and his heart rate and blood pressure were within normal limits and remained so for the rest of his hospital stay. However, the patient continued to report subjective symptoms of headache, myalgias, and paresthesia, for which he repeatedly requested clonazepam and baclofen.
On hospital day 5, the patient requested discharge from the hospital. He was outside the window of concern for severe gabaminergic withdrawal, and his primary and consulting teams believed him to be at low risk for seizures or autonomic instability. The patient displayed decision-making capacity and was discharged at his request on hospital day 5. He reported plans to follow up on his own with an intensive outpatient treatment program in the future.
Discussion
Phenibut is a glutamate derivative that acts as an agonist predominately on GABA-B receptors, and to a lesser extent, GABA-A receptors.2 Literature regarding the pharmacokinetics and toxicology of phenibut is scarce; however, it appears to be renally cleared with a half-life in humans of approximately 5.3 hours.2 Originally developed in the Soviet Union in the 1960s, it is still used to treat anxiety and associated psychiatric disorders in Russia.2 It is characterized in Australia as a prohibited substance by the Therapeutic Goods Administration, whereas in the United States it is considered “food” by the US Food and Drug Administration (FDA).3 As such, phenibut can be purchased over the counter through a number of online venders, though its retail sale was banned by the FDA in 2019.3,4 The extent to which phenibut is used and misused in the United States is unknown; however, US poison control centers reported 1,320 phenibut exposures between 2009 and 2019.5
Prolonged use of phenibut is thought to cause down-regulation of GABA receptors, which gives rise to tolerance as well as symptoms of withdrawal upon abrupt cessation.2,6 Clinical signs of withdrawal include anxiety, altered mental status, agitation, autonomic instability, seizure, muscle rigidity, and inducible clonus, which can mimic both alcohol and benzodiazepine withdrawal, as well as serotonin syndrome.3,7
A literature search revealed differing approaches to the management of phenibut withdrawal.3,7 One strategy, performed in the outpatient setting over the course of several months, began with a baclofen cross-taper over 9 weeks with 10 mg of baclofen substituted for each gram of phenibut; this was followed by a baclofen taper over 12 additional weeks.6 Another report8 described successful withdrawal from phenibut in the acute care setting with a 9-day inpatient phenobarbital taper. Due to phenibut’s nonspecific binding to GABA-A receptors, concern for autonomic instability and seizures was prioritized in our case. In the inpatient setting, we found that a phenobarbital taper over the course of 4 days successfully provided seizure prophylaxis and maintained autonomic stability.
The other novel substance used by our patient, bromazolam, is a triazolobenzodiazepine that is known as research chemical XLI-268.9 Bromazolam is structurally similar to alprazolam but is the bromo rather than the chloro analog.9,10 To date, literature on bromazolam is remarkably scarce. Much of what is known about the substance, aside from fundamental chemical properties, currently comes from crowdsourcing online forums for user experiences.11–14 These sources describe a common dose of 2 mg, an onset of 15–45 minutes, and a duration of 5–8 hours if taken orally, but peer-reviewed data on its half-life and potency are not available.12
This case describes a patient who developed a sedative-hypnotic use disorder to phenibut and bromazolam requiring hospitalization for medically supervised withdrawal. This is of particular interest because while neither of these substances are commonly used, they both have the potential to precipitate severe and potentially fatal withdrawal. As such, accurate identification of the withdrawal syndrome and swift initiation of medical management is paramount.
Conclusion
The clinical significance of this case is that an individual may develop a phenibut and/or bromazolam use disorder requiring hospitalization for medical management of withdrawal. It is therefore critically important to take a thorough substance use history including asking about novel agents purchased over the internet. Awareness of phenibut and bromazolam use disorders can help clinicians anticipate withdrawal and provide appropriate medical management including hospitalization.
Published online: July 12, 2022.
Relevant financial relationships: None.
Funding/support: None.
Patient consent: Consent was received from the patient to publish the case report, and information has been de-identified to protect anonymity.
ORCID: Alëna A. Balasanova: https://orcid.org/0000-0001-9735-2712
References (14)

- Sullivan JT, Sykora K, Schneiderman J, et al. Assessment of alcohol withdrawal: the revised Clinical Institute Withdrawal Assessment for Alcohol Scale (CIWA-Ar). Br J Addict. 1989;84(11):1353–1357.
- Lapin I. Phenibut (β-Phenyl-GABA): a tranquilizer and nootropic drug. CNS Drug Rev. 2006;7(4):471–481.
- Jouney EA. Phenibut (β-Phenyl-γ-Aminobutyric Acid): an easily obtainable “dietary supplement” with propensities for physical dependence and addiction. Curr Psychiatry Rep. 2019;21(4):23.
- Mash JE, Leo RJ. Phenibut: a novel nootropic with abuse potential. Prim Care Companion CNS Disord. 2020;22(4):19l02587.
- Graves JM. Notes from the field: phenibut exposures reported to poison centers—United States, 2009–2019. MMWR Morb Mortal Wkly Rep. 2020;69(35):1227–1228. Accessed August 27, 2021. https://www.cdc.gov/mmwr/volumes/69/wr/mm6935a5.htm
- Samokhvalov AV, Paton-Gay CL, Balchand K, Rehm J. Phenibut dependence. BMJ Case Rep. 2013:bcr2012008381.
- Hardman MI, Sprung J, Weingarten TN. Acute phenibut withdrawal: a comprehensive literature review and illustrative case report. Bosn J Basic Med Sci. 2018;19(2):125–129.
- Brunner E, Levy R. Case report of physiologic phenibut dependence treated with a phenobarbital taper in a patient being treated with buprenorphine. J Addict Med. 2017;11(3):239–240.
- PubChem. 8-Bromo-1-methyl-6-phenyl-4H-[1,2,4]triazolo[4,3-a][1,4]benzodiazepine. pubchem.ncbi.nlm.nih.gov website. Accessed August 18, 2021. https://pubchem.ncbi.nlm.nih.gov/compound/12562546
- PubChem. Alprazolam. @pubchem. PubChem website. Accessed August 18, 2021. https://pubchem.ncbi.nlm.nih.gov/compound/Alprazolam#section=Structures
- Zawilska JB, Wojcieszak J. An expanding world of new psychoactive substances—designer benzodiazepines. Neurotoxicology. 2019;73:8–16.
- TripSit Factsheets. drugs.tripsit.me website. Accessed August 18, 2021. http://drugs.tripsit.me/
- Bromazolam vad vet vi om denna? (rc benso). flashback.org website. Accessed August 18, 2021. https://www.flashback.org/t2805274
- Manchester KR, Lomas EC, Waters L, et al. The emergence of new psychoactive substance (NPS) benzodiazepines: a review. Drug Test Anal. 2017;10(1):37–53.
Enjoy this premium PDF as part of your membership benefits!