ABSTRACT
Objective: To analyze the psychopathology and pattern of remission in cannabis-induced psychotic disorder with treatment.
Methods: This was a prospective cohort study of a group of patients admitted with new-onset psychosis, cannabis use, and no evidence of other drug abuse from January 1 to June 31, 2019, to the psychiatry inpatient department of a multispecialty tertiary care hospital in Kerala, India. Patients were evaluated at admission and after 1 week in the hospital and 1 month after discharge using the Structured Clinical Interview for the Positive and Negative Syndrome Scale and the Clinical Global Impressions–Severity of illness scale.
Results: Fifty-six male subjects were recruited for the study. The mean age of the subjects was 22.2 years, and the majority were active smokers of nicotine and cannabis. Total duration of abuse and family history of substance use in first-degree relatives correlated with severity of psychosis. Hostility, excitement, and grandiosity were the predominant positive symptoms, and these symptoms showed a steady reduction toward the end of the study. The most frequent negative symptoms were emotional withdrawal, passive or apathetic social withdrawal, and difficulty in abstract thinking, and these symptoms also showed significant improvement (P < .001 for all). For symptoms such as somatic concern and guilt feelings, significant treatment response was noted only in the initial week (P < .001).
Conclusions: Cannabis-induced psychosis in the Indian setting presents with predominant positive symptoms and minimal affective symptoms. The steady improvement noted with complete cessation of cannabis indicates a possible contributory role for cannabis in precipitating psychosis.
Prim Care Companion CNS Disord 2023;25(2):22m03350
To cite: Kumar PNS, Menon V, Suresh R, et al. Psychopathology and pattern of remission of cannabis-induced psychotic disorder. Prim Care Companion CNS Disord. 2023;25(2):22m03350.
To share: https://doi.org/10.4088/PCC.22m03350
© 2023 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry, IQRAA International Hospital and Research Centre, Calicut, Kerala, India
bDepartment of Psychiatry, Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), Puducherry, India
cChethana–Center for Neuropsychiatry, Calicut, Kerala, India
*Corresponding author: P. N. Suresh Kumar, MD, MRCPsych, PhD, Department of Psychiatry, IQRAA International Hospital and Research Centre, Calicut, Kerala, India ([email protected]).
Cannabis is the most widely used illicit psychoactive substance worldwide, but there is a paucity of research about its impact on mental health.1 A variety of psychiatric conditions have been reported with cannabis use, especially psychotic disorders.2 This association, which could present as either an independent diagnosis or a comorbidity, has important clinical implications with respect to psychopathology, diagnosis, treatment outcome, course of illness, and service utilization. To date, there are only limited studies focusing on phenomenology and pattern of remission in cannabis-associated psychiatric disorders.3 Hence, it is important to study co-occurring cannabis use and psychiatric disorders.
Some studies have reported cannabis-related psychosis as a predominantly affective illness or as nonaffective psychosis, while others have not delineated this condition to any specific entity.4–6 Hence there is a need to define this condition based on the psychopathology at presentation and pattern of remission. Many of the previous studies had several methodological limitations such as single-point observational or retrospective chart review designs or no standardized assessment.5 Moreover, only a few have reported the effects of abstinence from cannabis abuse.7 To address this knowledge gap, the objective of the present study was to analyze the psychopathology at index presentation as well as the pattern of remission with treatment in cannabis-induced psychosis.
METHODS
Setting and Sample
This was a prospective cohort study of a group of patients admitted with new-onset psychosis, cannabis use, and no evidence of other drug abuse from January 1 to June 31, 2019, to the psychiatry inpatient department of a multispecialty tertiary care hospital in Kerala, India. The patients were evaluated at admission and after 1 week in the hospital and as outpatients 1 month after discharge. The study was conducted after approval was obtained from the institutional ethics committee. The work was performed in accordance with the principles of the 1983 Declaration of Helsinki.
Inclusion Criteria
Inclusion criteria were as follows:
- Subjects over aged 16 years who had psychosis and used cannabis at least 3 times a week for at least 1 month before the onset of psychosis.
- Positive urine cannabis screen.
- Subjects had only a temporal relationship with cannabis and psychosis.
The study participants were identified as part of the routine clinical admission process. The diagnosis was made according to DSM-5 criteria, and urine cannabis screening was conducted immediately after admission as a routine clinical process.
Exclusion Criteria
Exclusion criteria were as follows:
- History of mental illness prior to onset of cannabis use.
- Subjects with comorbid dependence on another substance in the month preceding examination, except for nicotine.
- Major life-threatening illness like malignancy, cardiac failure, or renal failure.
Study Procedure
Subjects satisfying DSM-5 diagnostic criteria and inclusion and exclusion criteria were recruited after providing informed consent.8 The exclusion history items were obtained during patient interview, and those found to be positive on urine screening for cannabis were recruited for the study after receipt of their laboratory report. During the study period, the subjects were admitted to the hospital as inpatients in a protected, closed environment to ensure further abstinence from drug use. Acute behavioral disturbances were managed using antipsychotics of choice and lorazepam by the primary psychiatrist. The study incorporated a minimum of 10 days of inpatient treatment. Each subject was assessed with a clinical interview, physical examination, baseline laboratory tests, and urine screening for cannabis, amphetamines, barbiturates, benzodiazepines, cocaine, opiates, and phencyclidine. Only those who screened positive for cannabis were included in the study. Information was collected from both the patients and key informants. Patients were discharged after improvements with oral antipsychotics.
Subjects were assessed with a sociodemographic data form, the Structured Clinical Interview for the Positive and Negative Syndrome Scale (PANSS),9 and the Clinical Global Impressions–Severity of illness scale (CGI-S)10 on the day of admission, day 7 of admission, and 1 month after discharge. Blood testing was conducted at a laboratory associated with the hospital. Improvement was assessed as change in the PANSS subscale compared to baseline during the routine clinical visit.
Statistical Analysis
Descriptive data were expressed using mean (with standard deviation) or median (with interquartile range) for continuous variables, while frequencies and percentages were used for discrete variables. Normality of data was assessed by the Shapiro-Wilk test. One-way repeated measures analysis of variance with Greenhouse-Geisser correction was used to determine if mean scores for clinical outcome measures were statistically significantly different between timepoints. We selected 3 items with the highest mean scores on day 1 from the PANSS positive and negative subscales as well as 4 items pertaining to the affective/anxiety dimension on the general psychopathology subscale to analyze the patterns of change over time. If the overall test was significant, pairwise post hoc comparisons using Bonferroni correction were examined to determine the timepoints when the differences occurred.
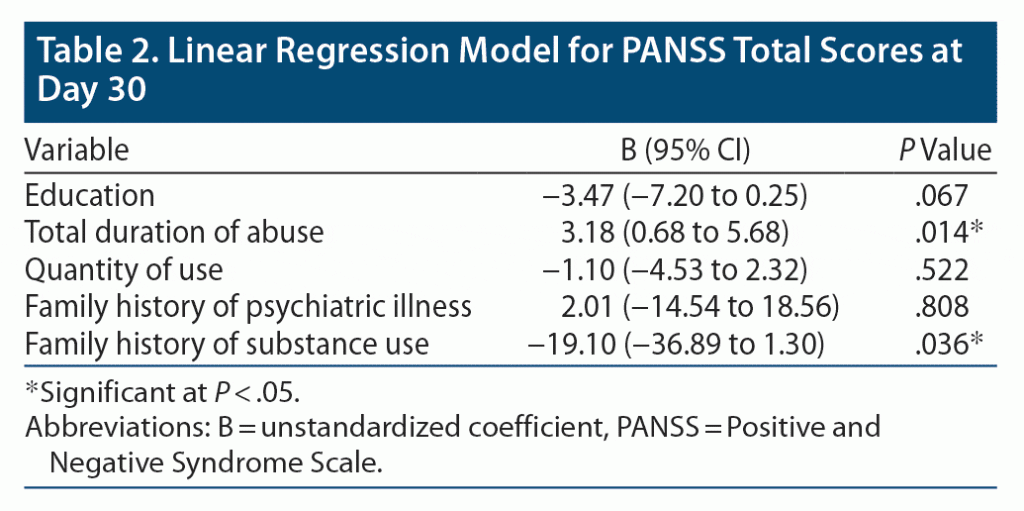
Multivariable linear regression analysis was done to determine independent predictors of endpoint (day 30) PANSS total and CGI-S scores. The covariates for all models were defined a priori and were number of years of education, total duration of abuse, quantity of use, family history of psychiatric illness, and family history of substance use.
RESULTS
Sample Description
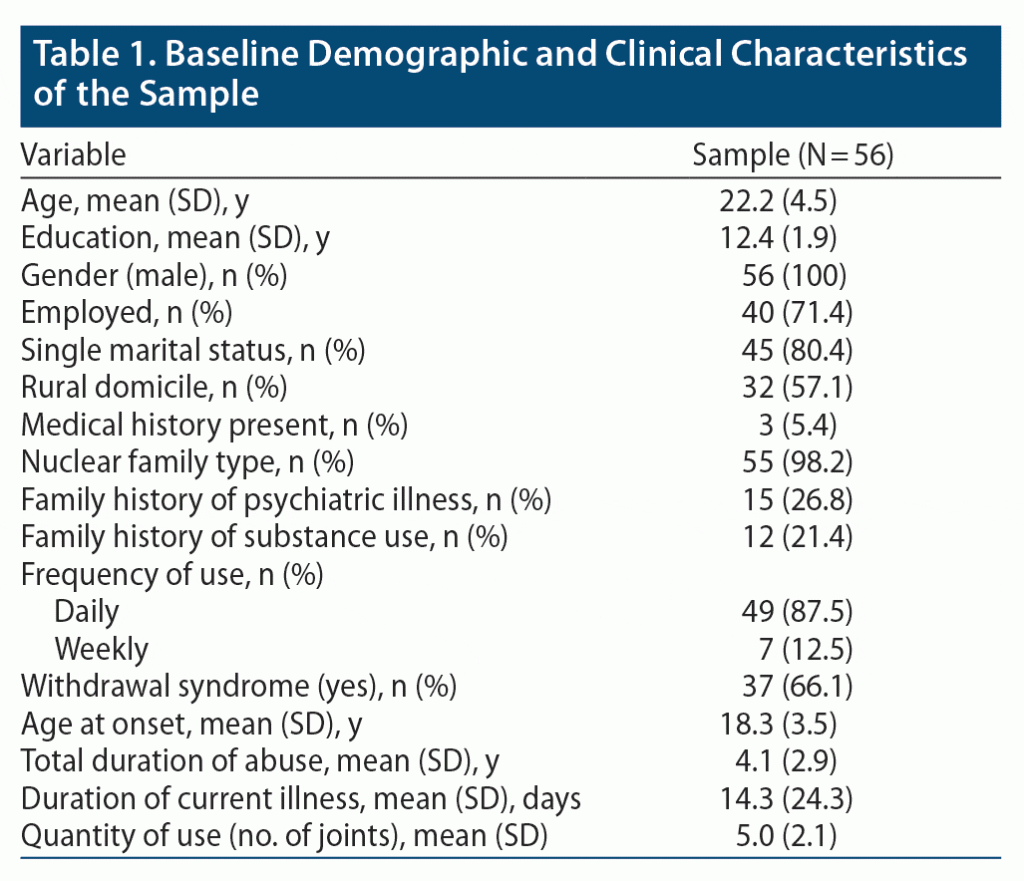
The age of the participants ranged from 17 to 34 years (mean ± SD age of 22.2 ± 4.5 years); the majority (n = 42, 75.0%) were aged ≤ 25 years. All participants actively smoked nicotine and cannabis. Apart from smoking, no other type of cannabis abuse was reported among the subjects. Other baseline characteristics are shown in Table 1.
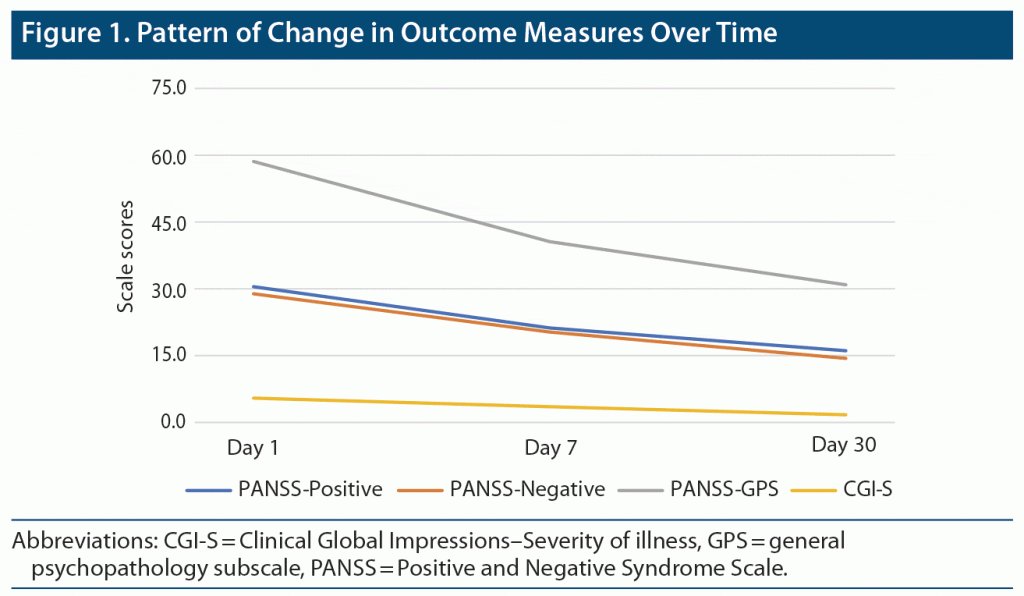
Pattern of Change in Clinical Outcome Measures
Figure 1 shows the pattern of change in outcome measures (3 subscales of the PANSS and CGI-S) over time at the 3 points of assessment. Overall, consistent improvement was noted for all outcome measures across timepoints.
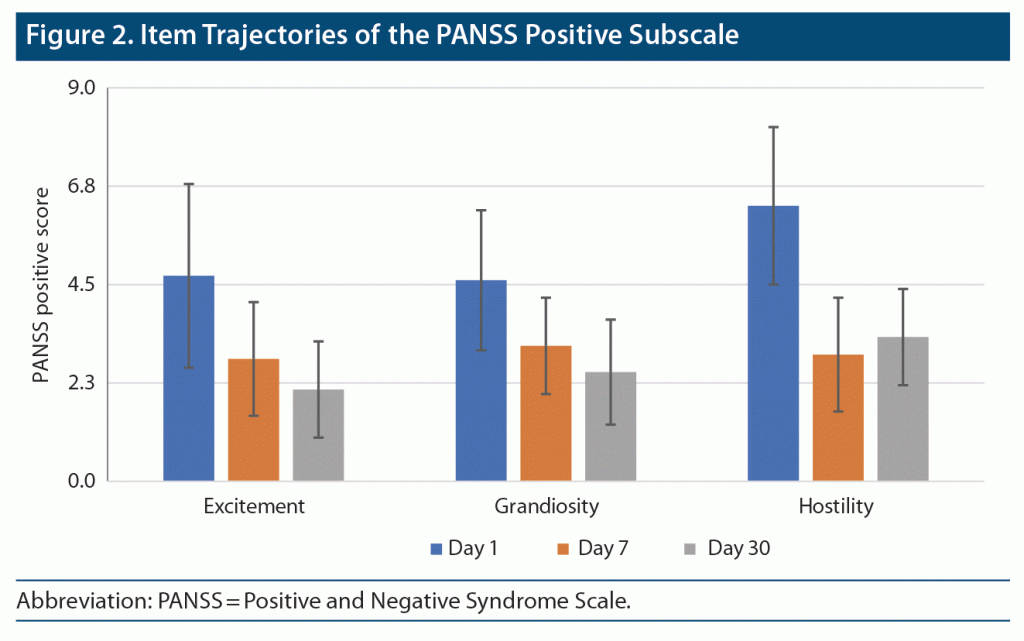
PANSS Positive Subscale Scores (items 4, 5, and 7)
Item 4 (excitement) severity scores significantly declined across the study period (F = 62.775; df = 1.33, 69.00; P < .001; partial eta square = 0.547) (Figure 2). Pairwise post hoc comparisons indicated that there were significant differences between all timepoints of observation. This finding seems to suggest a significant decrease in item 4 severity scores across 30 days. Similar findings were observed for item 5 (grandiosity) severity scores (F = 46.858; df = 1.51, 78.46; P < .001; partial eta square = 0.474). For item 7 (hostility), scores significantly declined across the study period (F = 91.845; df = 1.29, 67.24; P < .001; partial eta square = 0.638). However, pairwise post hoc comparisons indicated that there were significant differences only between day 7 and day 30, but not day 1 and day 7. This finding suggests only a nominal decrease in PANSS positive subscale item 7 scores across the first week of admission, but significant improvements accrued thereafter.
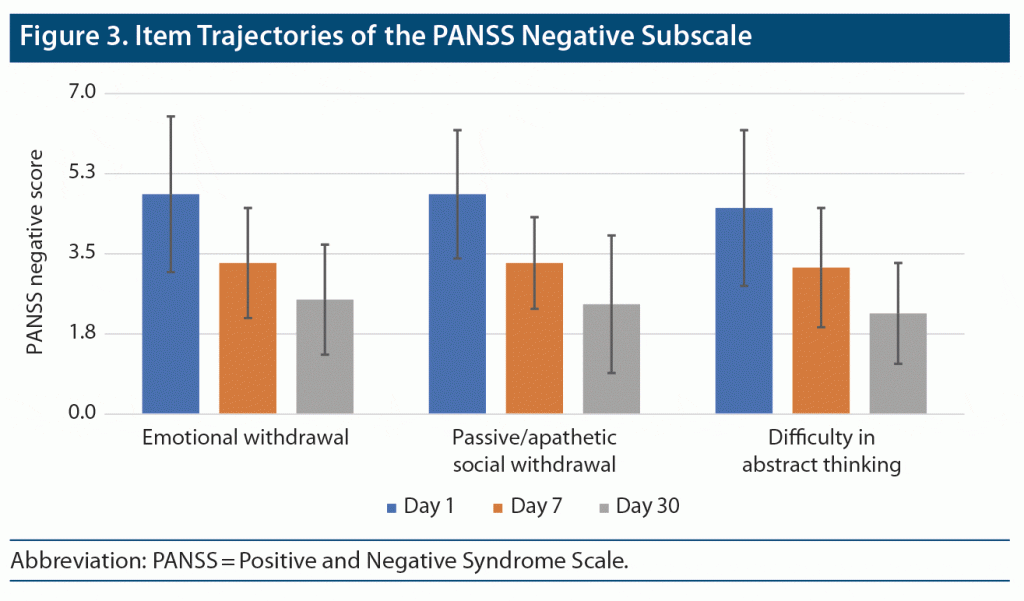
PANSS Negative Subscale Scores (items 2, 4, and 5)
Item 2 (emotional withdrawal) severity scores significantly declined across the study period (F = 65.393; df = 1.31, 67.96; P < .001; partial eta square = 0.557) (Figure 3). Pairwise post hoc comparisons indicated that there were significant differences between all timepoints of observation. This finding suggests a significant decrease in item 2 severity scores across 30 days. Similar findings were observed for item 4 social withdrawal (F = 85.694; df = 1.50, 76.52; P < .001; partial eta square = 0.627) and item 5 difficulty in abstract thinking (F = 55.548; df = 1.59, 82.72; P < .001; partial eta square = 0.516).
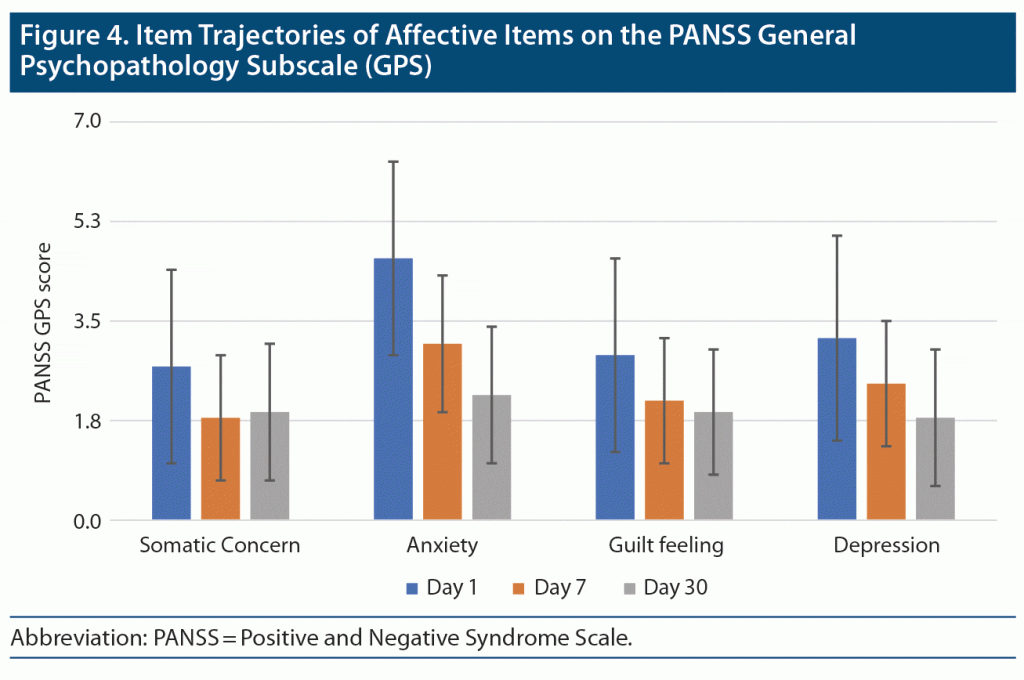
PANSS General Psychopathology Subscale Scores (items 1, 2, 3, and 6)
Item 1 (somatic concern) severity scores significantly declined during the first part of the study period (F = 16.75; df = 1.48, 77.21; P < .001; partial eta square = 0.244) (Figure 4). Pairwise post hoc comparisons indicated that there were significant differences only between day 1 and day 7, but not day 7 and day 30. This finding seems to suggest a significant decrease in item 1 of PANSS general psychopathology subscale scores across the first week of admission but nominal improvements thereafter. For item 2 anxiety (F = 52.610; df = 1.44, 75.15; P < .001; partial eta square = 0.503) and item 6 depression (F = 25.293; df = 1.57, 80.10; P < .001; partial eta square = 0.332), there was a significant decline in severity scores, and pairwise post hoc comparisons indicated significant differences between all timepoints of observation. This finding suggests a significant decrease in item 2 severity scores across 30 days. Item 3 (guilt feeling) severity scores significantly declined across the study period (F = 17.695; df = 1.48, 77.09; P < .001; partial eta square = 0.254). However, pairwise post hoc comparisons indicated significant differences between day 1 and day 7, but not day 7 and day 30. This finding suggests a significant decrease in PANSS general psychopathology subscale item 3 scores across the first week of admission but only nominal improvements thereafter.
Regression Analysis: PANSS Total Score
Total duration of abuse and family history of substance use emerged as positive predictors of PANSS total scores at study endpoint. Other variables were not significantly associated with endpoint PANSS total scores (Table 2). The adjusted R square of the model was 0.163, meaning that the covariates together explained 16.3% of the variance in PANSS scores.
DISCUSSION
This study evaluated the psychopathology and pattern of remission in cannabis-induced psychotic disorder longitudinally. The study found consistent improvement in all outcome measures across timepoints: PANSS positive subscale scores (excitement, grandiosity, hostility), PANSS negative subscale scores (emotional withdrawal, social withdrawal, difficulty in abstract thinking), PANSS general psychopathology subscale scores (somatic concern, anxiety, depression, guilt feeling), and CGI-S scores.
Psychopathology
Analysis of the baseline visit composite scores revealed that PANSS scoring was loaded for positive symptoms in the majority of the sample (60.7%). Previous studies have reported that cannabis produces psychosis with prominent positive symptoms.3 Thomas11 reported the phenomenology of cannabis use disorders to be vague and the symptoms short-lived.
Hostility, excitement, and grandiosity were the predominant positive symptoms at baseline, and these symptoms showed steady reduction toward the end of the study. Goel and Netto12 reported that regular cannabis users presented with hyperactivity, mood changes, and delusions. Rottanburg et al4 also noted improvement in agitation, irritability, and grandiose delusions recorded serially by the Present State Examination. These are important symptoms in the diagnosis of psychosis, which explains their high frequency.13 Among negative symptoms, the most frequently reported symptoms were emotional withdrawal, passive or apathetic social withdrawal, and difficulty in abstract thinking. These symptoms also consistently improved across the study.
Andréasson et al14 have argued that cannabis produces worsening of positive symptoms. Contrary to this argument, in our study both positive and negative symptoms improved with no significant difference. It can also be argued that faster improvement was due to remission of a toxic confusional state. However, severe withdrawal symptoms were not reported in our sample, as 64.5% had only minor symptoms.
In the PANSS subsection on general psychopathology, symptoms like somatic concern and guilt feelings showed improvement in the initial week of the study, but the improvement plateaued later. Maximum scoring was for excitement, suspiciousness, and grandiosity. Similar findings have been reported in previous Indian studies.15 Although anxiety, fearfulness, and depression have been described in cannabis psychosis,16 very few subjects in our study reported such symptoms. The reason could be that we did not use a specific scale to assess mood symptoms.
The significant improvement in most symptoms with complete cessation of cannabis indicates that cannabis may have either caused or increased the intensity of psychosis. Previous studies focused on the outcome of cannabis-related psychosis also noted similar observations.17 This finding suggests an indirect contributory role of cannabis in precipitating psychosis in the user.18
Family History
Few studies have reported family history of substance abuse and mental illnesses in patients with cannabis-related psychosis.19 In our study, substance use in first-degree relatives was a risk factor for developing cannabis-related psychosis in the regression analysis, which was similar to a previous finding.3 Family history of substance abuse as a risk factor for developing substance abuse and psychosis suggests a shared genetic vulnerability to psychotic symptoms as well as substance abuse including cannabis.20
Regarding limitations of this study, generalization of our findings is limited by the small sample size, lack of females, lack of a control group, short-term follow-up, and lack of control over the treatment, as it was decided by the primary psychiatrist. The possible role of nicotine is another important limitation, as its withdrawal symptoms can confound the clinical picture. Another limitation was that there was no laboratory assessment for cannabis or other substance use at the visit 1 month after discharge, which could have influenced the clinical picture. It could also be argued that our sample was heterogenous with high comorbidity of other substances of abuse. However, we took the utmost caution to select subjects using only cannabis. In the real world, it would be difficult to get a homogenous population using only cannabis and developing psychosis.
CONCLUSIONS
From this study, we can infer that cannabis contributed to precipitation of psychosis and that cannabis-induced psychosis in the Indian setting presents predominantly with positive and comparatively lesser affective and negative symptoms.
Submitted: June 28, 2022; accepted October 19, 2022.
Published online: April 18, 2023.
Relevant financial relationships: None.
Funding/support: None.
Acknowledgments: The authors thank Jaison Joy, MSc (research coordinator); Binsha Thandam Punathil, MSc (clinical psychologists); and Thoohima Parol, MSW (clinical psychologist) of IQRAA Hospital, Kerala, India, for their valuable assistance in data collection and in preparing this manuscript. Mr Joy and Mss Punathil and Parol report no conflicts of interest related to the subject of this article.
References (20)

- Singh S, Balhara YPS. A review of Indian research on co-occurring cannabis use disorders & psychiatric disorders. Indian J Med Res. 2017;146(2):186–195. PubMed CrossRef
- Leweke FM, Koethe D. Cannabis and psychiatric disorders: it is not only addiction. Addict Biol. 2008;13(2):264–275. PubMed CrossRef
- Kulhalli V, Isaac M, Murthy P. Cannabis-related psychosis: presentation and effect of abstinence. Indian J Psychiatry. 2007;49(4):256–261. PubMed CrossRef
- Rottanburg D, Robins AH, Ben-Arie O, et al. Cannabis-associated psychosis with hypomanic features. Lancet. 1982;320(8312):1364–1366. PubMed CrossRef
- Basu D, Malhotra A, Varma VK. Cannabis related psychiatric syndromes: a selective review. Indian J Psychiatry. 1994;36(3):121–128. PubMed
- Hall W, Degenhardt L. Cannabis use and psychosis: a review of clinical and epidemiological evidence. Aust N Z J Psychiatry. 2000;34(1):26–34. PubMed CrossRef
- Carney P, Lipsedge M. Psychosis after cannabis abuse. Br Med J (Clin Res Ed). 1984;288(6427):1381. PubMed CrossRef
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fifth Edition. Arlington, VA: American Psychiatric Association; 2013.
- Kay SR, Opler LA, Fiszbein A. Positive and Negative Syndrome Scale (PANSS; 2006) Manual. Toronto, Ontario: Multi Health Systems, Inc; 2006.
- Guy W. Clinical Global Impressions (CGI) Scale, Modified. In: Rush JA; Task Force for the Handbook of Psychiatric Measures, ed. Handbook of Psychiatric Measures. 1st ed. Washington, DC: American Psychiatric Association; 2000.
- Thomas H. Psychiatric symptoms in cannabis users. Br J Psychiatry. 1993;163(2):141–149. PubMed CrossRef
- Goel D, Netto D. Cannabis: the habit and psychosis. Indian J Psychiatry. 1975;17:238–243.
- Negrete JC. What’s happened to the cannabis debate? Br J Addict. 1988;83(4):359–372. PubMed CrossRef
- Andréasson S, Allebeck P, Rydberg U. Schizophrenia in users and nonusers of cannabis: a longitudinal study in Stockholm County. Acta Psychiatr Scand. 1989;79(5):505–510. PubMed CrossRef
- Thacore VR. Bhang psychosis. Br J Psychiatry. 1973;123(573):225–229. PubMed CrossRef
- Pålsson A, Thulin SO, Tunving K. Cannabis psychoses in south Sweden. Acta Psychiatr Scand. 1982;66(4):311–321. PubMed CrossRef
- Patton GC, Coffey C, Carlin JB, et al. Cannabis use and mental health in young people: cohort study. BMJ. 2002;325(7374):1195–1198. PubMed CrossRef
- Menhiratta SS, Wig NN, Verma SK. Some psychological correlates of long-term heavy cannabis users. Br J Psychiatry. 1978;132(5):482–486. PubMed CrossRef
- Núñez LA, Gurpegui M. Cannabis-induced psychosis: a cross-sectional comparison with acute schizophrenia. Acta Psychiatr Scand. 2002;105(3):173–178. PubMed CrossRef
- Peralta V, Cuesta MJ. Influence of cannabis abuse on schizophrenic psychopathology. Acta Psychiatr Scand. 1992;85(2):127–130. PubMed CrossRef
Enjoy this premium PDF as part of your membership benefits!