Successful Naloxone Challenge Test in a Patient With Atrial Flutter
Following opioid detoxification, naltrexone long-acting injection (LAI) (Vivitrol) is an option for maintenance medication to prevent relapse. Naltrexone is an opioid antagonist and can produce prolonged withdrawal in patients who have not fully completed detoxification from opioids. To prevent this prolonged withdrawal, it is recommended that the patient is opioid free for 7 to 10 days before injection.1 In some instances, that length of time is not feasible. One solution is the naloxone challenge test (NCT).
While useful, the NCT should still be given with caution, as naloxone carries a listed precaution for use in patients with preexisting cardiovascular disease.2,3 There have been instances of patients suffering from a wide array of cardiovascular complications, ranging from an increase in systolic blood pressure to cardiovascular arrest.2-7 Despite no particular reports of use in patients with preexisting atrial flutter, these instances have most likely influenced naloxone use in this population as well.
Case Report
A 50-year-old white man presented requesting inpatient detoxification from opiates. The patient reported injecting 1 to 2 grams of heroin daily, with his last use being the day of admission.
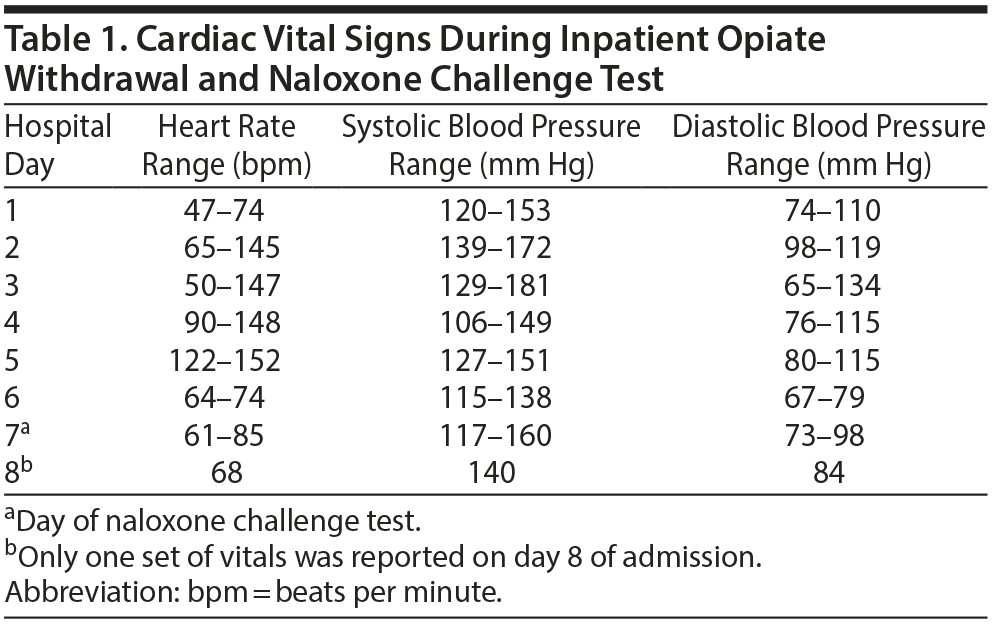
The patient’s cardiovascular history was positive for hypertension, cerebrovascular accident 4 months prior, endocarditis, atrial flutter, and atrioventricular nodal reentry tachycardia. He also had a mitral valve replacement 2 months prior and 2 ablations 4 months prior. At the time of admission, the patient was stable in regard to his cardiac history. On day 2, the patient began to experience withdrawal symptoms. Withdrawal symptoms were treated on an as-needed basis with various standard medications including clonidine 0.1 mg every 4 hours. Notably, as the admission continued, the patient’s heart rate and blood pressure began to increase (Table 1) despite otherwise improving withdrawal symptoms.
On day 5, it was decided to administer naltrexone LAI to the patient. However, he began to experience tachycardia and electrocardiogram (ECG) changes that revealed atrial flutter with 2:1 conduction. The Cardiology Department was consulted and recommended an electrophysiology study that would likely result in ablation. Due to the patient undergoing acute detoxification, the electrophysiology study and ablation were postponed until detoxification was complete. Until that time, anticoagulation with apixaban and rate control with diltiazem were utilized.
On day 7, the patient’s heart rate was well controlled, and he reported no cravings for heroin or symptoms of withdrawal. Given recent cardiovascular events, an NCT was given despite his being opioid free for at least 7 days. After 2 doses of naloxone 0.4 mg, the patient had no withdrawal symptoms and experienced no cardiovascular complications.
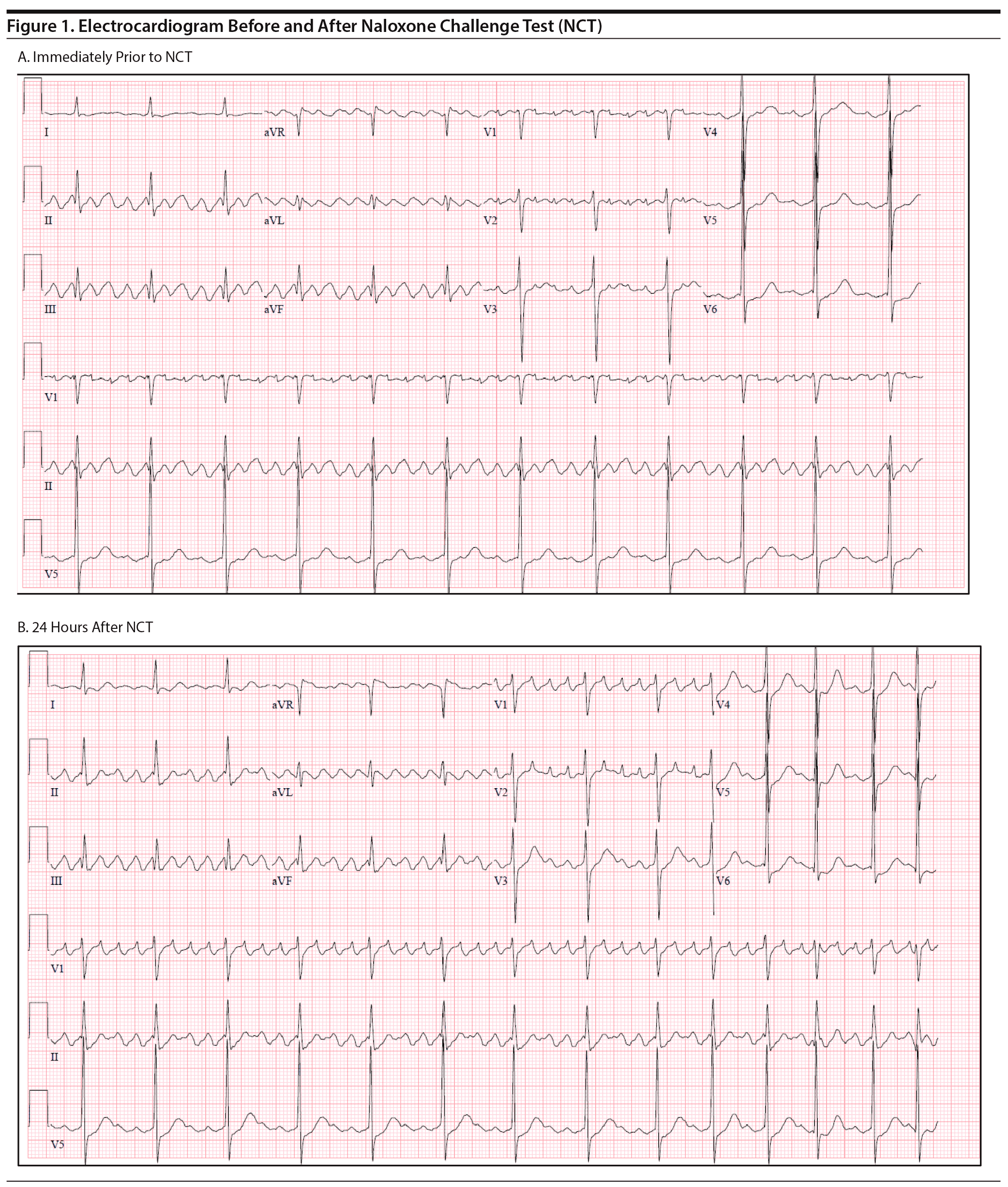
He developed no new arrhythmias or changes in heart rate or blood pressure during the NCT. The following day, the patient’s heart rate was still well controlled but remained in atrial flutter (Figure 1). He was administered naltrexone LAI and transferred for further cardiac workup and ablation. During the patient’s admission at the cardiac hospital, he successfully underwent electrophysiology study and atrial flutter ablation with no complications.
Discussion
This case report illustrates the use of the NCT in a patient who received naltrexone LAI for opioid use disorder with cardiac comorbidities. Withdrawal from opioids can lead to development of abnormal cardiac function caused by quick reversal of sympathetic suppression of long-term opioid abuse.8 However, in our patient, the NCT was successfully employed to establish safety of use of naltrexone LAI.
Appropriate medical care prior to use of naloxone may also have influenced the results in this patient. In future cases, consultation with cardiology specialists would be warranted prior to a NCT in patients with especially complex cases such as tachyarrhythmias or other forms of unstable cardiodynamics.
Published online: May 23, 2019.
Potential conflicts of interest: None.
Funding/support: None.
Patient consent: A waiver of documentation of informed consent and a full waiver of informed consent were granted by the institutional review board, as all patient information was de-identified.
REFERENCES
1. Vivitrol [package insert]. Waltham, MA: Alkermes, Inc; 2015.
2. Naloxone hydrochloride [package insert]. South El Monte, CA: Amphastar Pharmaceuticals; 2013.
3. Wermeling DP. Review of naloxone safety for opioid overdose: practical considerations for new technology and expanded public access. Ther Adv Drug Saf. 2015;6(1):20-31. PubMed CrossRef
4. Shah NG, Lathrop SL, Reichard RR, et al. Unintentional drug overdose death trends in New Mexico, USA, 1990-2005: combinations of heroin, cocaine, prescription opioids and alcohol. Addiction. 2008;103(1):126-136. PubMed CrossRef
5. Buajordet I, Naess AC, Jacobsen D, et al. Adverse events after naloxone treatment of episodes of suspected acute opioid overdose. Eur J Emerg Med. 2004;11(1):19-23. PubMed CrossRef
6. Newlin DB, Wong CJ, Cheskin LJ. Cardiovascular responses to naloxone challenge in opiate-dependent individuals. Pharmacol Biochem Behav. 1992;43(2):357-360. PubMed CrossRef
7. Cuss FM, Colaço CB, Baron JH. Cardiac arrest after reversal of effects of opiates with naloxone. Br Med J (Clin Res Ed). 1984;288(6414):363-364. PubMed CrossRef
8. Hoffman WE, McDonald T, Berkowitz R. Simultaneous increases in respiration and sympathetic function during opiate detoxification. J Neurosurg Anesthesiol. 1998;10(4):205-210. PubMed CrossRef
aDepartment of Pharmacy, Community Health Network, Indianapolis, Indiana
bDepartment of Psychiatry, Community Health Network, Indianapolis, Indiana
*Corresponding author: Taylor R. Harlow, PharmD, Pharmacy Department, Community Health Network, 7150 Clearvista Pkwy, Indianapolis, IN 46256 ([email protected]).
Prim Care Companion CNS Disord 2019;21(3):18l02385
To cite: Harlow TR, Peters JR, Anderson DL. Successful naloxone challenge test in a patient with atrial flutter. Prim Care Companion CNS Disord. 2019;21(3):18l02385.
To share: https://doi.org/10.4088/PCC.18l02385
© Copyright 2019 Physicians Postgraduate Press, Inc.
Enjoy this premium PDF as part of your membership benefits!