Neutropenia is defined as an absolute neutrophil count (ANC) below 1,500/μL, a threshold derived from studies in predominantly white populations, which demonstrated increased infection risk below this level.1 Despite clozapine being the gold standard antipsychotic for treatment-resistant schizophrenia, its use is constrained by the risk of agranulocytosis.2 Current guidelines recommend initiating clozapine only in patients with ANC >2,000/μL and discontinuing treatment if ANC drops below 1,500/μL.3
However, individuals of African, Middle Eastern, or West Indian descent may exhibit benign ethnic neutropenia (BEN), a chronic, asymptomatic neutropenia not linked to increased infection risk.3 BEN is typically defined by ANC levels between 1,000 and 1,500/μL, with no other cytopenias, lymphadenopathy, organomegaly, or secondary causes.1,3 This condition is associated with a Duffy antigen/ chemokine receptor gene variant and the Duffy-null red blood cell phenotype.4 Although the precise mechanism remains unclear, evidence suggests that patients with BEN can be safely treated with clozapine using modified hematological thresholds.1,7 Failure to recognize BEN can lead to inappropriate discontinuation or withholding of clozapine, thus depriving patients of an effective treatment.5
Case Report
A 24-year-old woman from Angola, residing in Portugal since 2023, with no previous medical or psychiatric history, developed an insidious psychotic syndrome at age 20. Symptoms included disorganized behavior, persecutory delusions, and auditory and cenesthetic hallucinations. After 3 years of untreated psychosis, she was evaluated in January 2024 and prescribed olanzapine 5 mg, but adherence was poor.
In February 2024, clinical deterioration prompted hospitalization. On admission, her Positive and Negative Syndrome Scale (PANSS) score was 92. Imaging and laboratory results were within normal limits. Paliperidone was initiated and later switched to its long-acting injectable (LAI) form. Persistent symptoms led to the addition of olanzapine (up to 20 mg/day), but galactorrhea and hyperprolactinemia developed by day 35. Aripiprazole was introduced and later transitioned to its LAI form.
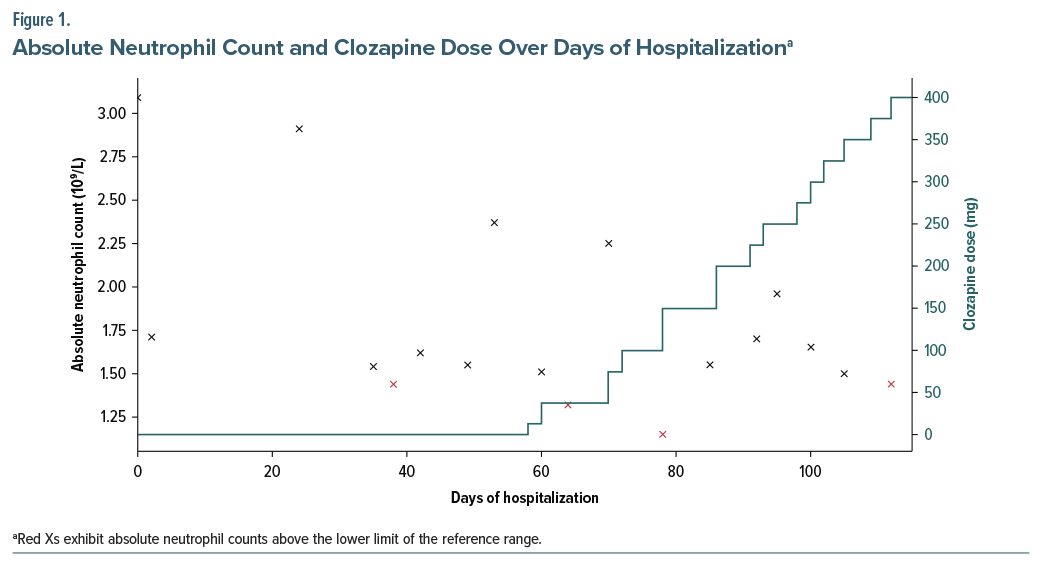
On day 38, routine bloodwork revealed neutropenia (ANC: 1,400/ μL), with further fluctuations during hospitalization, reaching a minimum of 1,150/μL (Figure 1), without infection or other symptoms. BEN versus drug-induced neutropenia was considered. Duffy antigen testing confirmed a Duffy-null phenotype, supporting a diagnosis of BEN. Since no prior ANC measurements were available, this finding was most likely indicative of a longstanding BEN. Given persistent psychosis despite 3 antipsychotics, clozapine was initiated on day 58 and titrated to 300 mg/day without complications.
A gradual encapsulation of delusional beliefs was observed. At discharge (day 103), the PANSS score was 53, and she was prescribed clozapine 400 mg/day and aripiprazole LAI. At 1-year follow-up, she remains in full remission despite ongoing ANC fluctuations unrelated to clozapine dose.
Discussion
Lack of recognition of BEN is a common reason for delaying or avoiding clozapine therapy in patients with severe psychosis.5 Importantly, BEN does not elevate the risk of agranulocytosis and is not a contraindication to clozapine.1 A UK study found the average delay in BEN diagnosis postclozapine initiation to be 2.7 years.2
Ideally, BEN status should be identified before initiating clozapine. The condition poses a clinical challenge when drugs with known neutropenic potential are needed. Notably, up to 79% of patients with previous treatment-emergent neutropenia were able to resume clozapine successfully, and up to 95% could have avoided discontinuation if BEN had been correctly identified and monitored under adjusted protocols.1,6,7 Recent data also show similar infection risk among BEN patients on clozapine compared to the general treated population.
Historically, clozapine monitoring guidelines were based on Caucasian populations, creating barriers for ethnically diverse groups. Since 2000, 7 countries have implemented BEN-specific monitoring algorithms.3 The 2015 US Food and Drug Administration revision lowered the ANC discontinuation threshold to <500/μL and removed the white blood cell monitoring requirement, allowing patients with BEN to start clozapine under adjusted criteria.1 This case highlights the critical need for evidence-based international guidelines that accommodate ethnic variations in ANC and promote equitable access to clozapine treatment.
Article Information
Published Online: January 22, 2026. https://doi.org/10.4088/PCC.25cr04053
© 2026 Physicians Postgraduate Press, Inc.
Prim Care Companion CNS Disord 2026;28(1):25cr04053
Submitted: July 30, 2025; accepted October 9, 2025.
To Cite: Ribeiro ML, Albuquerque S, Saraiva R, et al. Benign ethnic neutropenia and clozapine. Prim Care Companion CNS Disord 2026;28(1):25cr04053.
Author Affiliations: Department of Psychiatry, Unidade Local de Saúde de Santa Maria, Lisbon, Portugal (Ribeiro, Albuquerque, Saraiva, Coentre); University Clinic of Psychiatry and Medical Psychology, Faculty of Medicine, University of Lisbon, Lisbon, Portugal (Coentre).
Corresponding Author: Marta Loureiro Ribeiro, MD, Av. Prof. Egas Moniz MB, 1649-028 Lisboa, Portugal ([email protected]).
Author Contributions: Literature review, writing of the manuscript (Ribeiro, Albuquerque). Writing and critical review of the manuscript (Saraiva, Coentre).
Relevant Financial Relationships: None.
Funding/Support: None.
Previous Presentation: A different and simplified version of this clinical case was presented as an oral communication by Dr Ribeiro at the 9° Encontro Nacional Intervenção Precoce na Psicose; May 23–24, 2024; Vila Nova de Gaia, Portugal.
Patient Consent: Consent was received from the patient to publish the case report, and information, including dates, has been de-identified to protect patient anonymity.
References (7)

- Atallah-Yunes SA, Ready A, Newburger PE. Benign ethnic neutropenia. Blood Rev. 2019;37:100586. PubMed CrossRef
- Dotson S, Shtasel D, Freudenreich O. Race-based medicine, clozapine, and benign (ethnic) neutropenia: a call for nuance. Psychiatr Serv. 2021;72(2):232–233. PubMed CrossRef
- Kelly DL, Glassman M, Wonodi I, et al. Clozapine and neutrophil response in patients of African descent: a six-month, multinational, prospective, open-label clinical trial. Schizophr Res. 2024;268:312–322. PubMed CrossRef
- Merz LE, Story CM, Osei MA, et al. Absolute neutrophil count by Duffy status among healthy Black and African American adults. Blood Adv. 2023;7(3):317–320. PubMed CrossRef
- Munro J, O’Sullivan D, Andrews C, et al. Active monitoring of 12,760 clozapine recipients in the UK and Ireland: beyond pharmacovigilance. Br J Psychiatry. 1999;175:576–580. PubMed CrossRef
- Oloyede E, Casetta C, Dzahini O, et al. Life after the UK clozapine central non-rechallenge database: a national perspective. Schizophr Bull. 2021;47(4):1088–1098. PubMed CrossRef
- Wu S, Powell V, Chintoh A, et al. Safety of BEN guidelines in clozapine treatment: a Canadian perspective. Schizophr Res. 2024;264:451–456. PubMed CrossRef
Enjoy this premium PDF as part of your membership benefits!