Prim Care Companion CNS Disord 2022;24(5):22cr03296
To cite: Plasencia-García BO, García-Ligero E, Menéndez IE, et al. Attempted suicide, dexamethasone, and COVID-19: clinical case on COVID-19 treatments and the implications for mental health. Prim Care Companion CNS Disord. 2022;24(5):22cr03296
To share: https://doi.org/10.4088/PCC.22cr03296
© 2022 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry, University Hospital Virgen del Rocío, Seville, Spain
bSeville Biomedical Research Center (IBIS), Seville, Spain
cNetwork for Research in Mental Health (CIBERSAM), Seville, Spain
*Corresponding author: Benedicto Crespo-Facorro, MD, PhD, University Hospital Virgen del Rocío, Av Manuel Siurot, S/n 41013, Seville, Spain ([email protected]).
The World Health Organization (WHO) declared the outbreak of coronavirus 2019 (COVID-19) a public health emergency on January 30, 2020.1 Corticosteroids (not used in previous epidemics of severe respiratory syndromes due to risk of worsening lung lesions) have played an important role in the treatment of COVID-19, mainly because they were found to reduce mortality in hospitalized patients with severe COVID-19 by 20%.2 However, recent studies3 warn of inconsistent results and the need to reevaluate the role of corticosteroids in the treatment of COVID-19, and they are advised only for patients who are critical.
The neuropsychiatric effects derived from corticosteroid treatment are widely described in the literature. Their incidence varies from 3% to 72%4 and spans a broad spectrum of symptoms, from subtle changes in mood to severe affective syndromes and psychosis,5 as well as cognitive failure. The risk of neuropsychiatric symptoms in patients who begin treatment with corticosteroids varies with the individual (age, sex, dose, prior psychiatric history, and various biological markers).6 The time it takes for the symptoms to resolve after interrupting treatment is variable as well.5
We present the case of a patient with COVID-19 who was admitted to the hospital for bilateral pneumonia, at which time he was administered dexamethasone. After discharge, he attempted suicide, which led to admission in the mental health hospitalization unit.
Case Report
The patient was a married 38-year-old employed father of an 8-month-old son. He weighed 253 lb (115 kg) and had no personal or family history of interest or toxic habits.
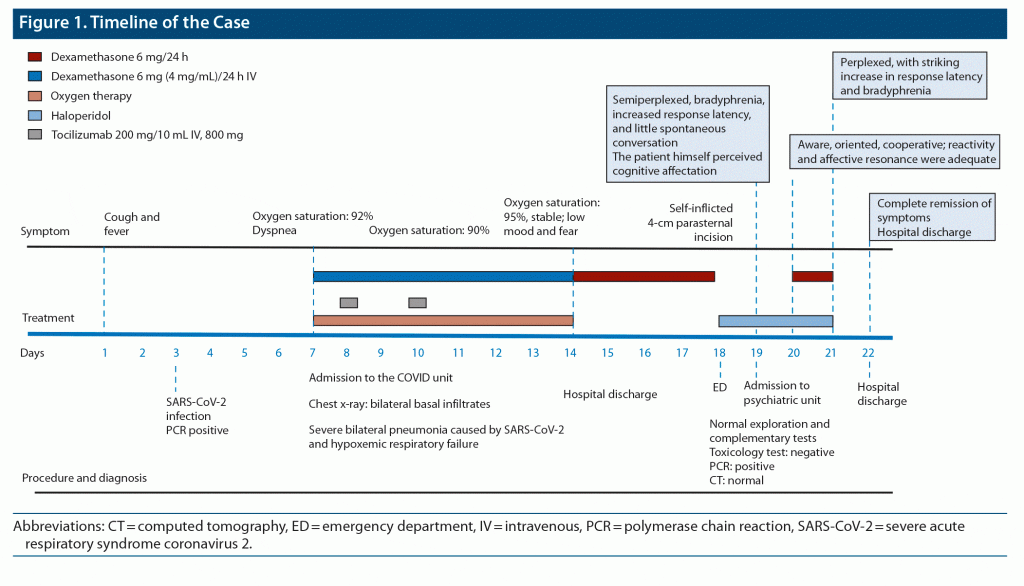
He was diagnosed with COVID-19, which started with a fever and cough, following a positive PCR (polymerase chain reaction) test result. At the beginning of his illness, he was under outpatient follow-up. On day 5 from the onset of symptoms, he presented to the emergency department (ED) with oxygen saturation of 91%–92% and was admitted to the hospital for observation. He was subsequently admitted to the COVID unit 2 days later. He was diagnosed with severe bilateral pneumonia caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and was treated with dexamethasone 6 mg 4 mg/mL every 24 hours by slow IV (intravenous), budesonide 160 mcg plus formoterol 4.5 mcg per inhaler every 12 hours, enoxaparin 80 mg once every 24 hours by subcutaneous injection (SC), dextromethorphan 15 mg every 8 hours, and losartan 25 mg (if blood pressure > 140/90 mm Hg).
On day 8 since the onset of symptoms, partial hypoxemic respiratory failure was identified, and tocilizumab 200-mg/10-mL injection 800-mg infusion IV was added to his treatment. On day 10 from the onset of symptoms, oxygen saturation of 97% with oxygen therapy via Ventimask face mask at 50% and 15 L/minute, respiratory rate 20 breaths/minute was started; dexamethasone was increased to 7.2 mg IV every 24 hours; and the second 800-mg bolus IV of tocilizumab was administered. On day 11, oxygen saturation of 95% was achieved with the nasal cannula at 4 L/minute with good test values (PCR, ferritin, decreasing D-dimer).
On day 12 since the onset of symptoms and 6 days since the start of treatment with dexamethasone, according to the clinical history, the patient first began to speak of fear of poor evolution. Both the patient and his wife reported his low mood, fear of death from the disease, and sleep problems during the days before discharge. On day 14, which was 8 days since admission to the COVID unit, the patient was discharged and sent home with the following treatment: dexamethasone 6 mg/24 hours for 4 days and enoxaparin 40 mg SC/24 hours for 14 days.
From the time the patient arrived home, his wife said he did not seem “normal” and that he was afraid he would not recover and constantly repeated he would not “be the same again.” He was in a very low mood, his spontaneous conversation decreased, and he lacked motivation, had no appetite, and had insomnia. The patient later admitted that during those days, he had intense anxiety, unfounded fears about financial concerns, and ruminative thoughts about ideas of incapacity and desperation.
Four days after his release (day 18 since symptom onset and 12 days since starting treatment with dexamethasone), the patient was taken to the hospital ED for a self-inflicted 4-cm parasternal incision. He had attempted suicide, taking advantage of his wife’s absence from the home. He was admitted to the emergency observation ward for assessment and given analgesic treatment with no prescription of corticosteroids (not knowing that the patient had been taking the treatment at home).
The thoracic surgery team evaluated the patient and found no need for intervention related to the wound. Neither the hemogram nor the general biochemistry provided pathological findings, and a head computed tomography scan was unremarkable.
A psychiatric evaluation 24 hours after admission described the patient as perplexed, with striking increase in response latency and bradyphrenia. He said he felt sad and worried and had insomnia. His emotional response did not fit the situation. Therefore, admission to the psychiatric unit was scheduled.
The patient was reevaluated in the psychiatry unit 48 hours after withdrawal of dexamethasone. He showed remarkable recovery from the psychopathological state described at admission. He was aware, oriented, and cooperative in the interview; his discourse was adequate and coherent; and reactivity and affective resonance were sufficient. The patient said his mood had improved, and there were no psychotic symptoms. He had an appropriate critical view of the suicide attempt, for which he was very sorry and explained what had happened due to his low mood and anxiety.
He agreed to remain hospitalized for a follow-up assessment. According to the infectious medicine team, treatment with dexamethasone was restarted at 4 mg for progressive reduction, as the patient had been doing at home (4 mg administered the first day of admission to the psychiatry unit and another 4 mg administered on the second day after admission).
On the following day, the patient’s symptoms worsened again. He seemed semiperplexed, with bradypsychia, increased response latency, and little spontaneous conversation, and he perceived his cognitive impairment (“The ideas don’t come to me, I’m blocked”). He complained again of lack of energy and low mood.
As it was suspected that corticosteroids could induce neuropsychiatric symptoms, treatment was suspended. Twenty-four hours after the last dose of dexamethasone, the patient had improved remarkably, with complete remission of symptoms at 72 hours. Five months after release from the hospital, the patient was stable and asymptomatic. Figure 1 provides a timeline of the patient’s course.
Discussion
We present what is, to our knowledge, the first report of a failed suicide attempt induced by corticosteroids in the context of SARS-CoV-2 infection. Suicide is an important public health problem, with almost 700,000 deaths per year.7 The use of corticosteroids is related to a dose-dependent increase in risk of suicide (7 times higher in patients with cancer and twice as high in those treated for other diseases).8 The mechanism that produces secondary lesions in the central nervous system is not yet clear, although they seem to be related to neurotoxicity of the glutamate and high arterial pressure.9 Corticosteroids increase harm to the prefrontal cortex and hippocampus even after brief exposure to those molecules. The corticosteroids, through their regulatory function of the raphe-hippocampal serotonin system, can alter neurotransmission and thereby generate affective symptoms.10
Both neuroinflammatory mechanisms and neurotropism from SARS-CoV-2 infection, hypoxia, cerebrovascular events, and the effect of the steroid treatment itself have been proposed as possible biological mediators of neuropsychiatric alterations in COVID-19.11 Psychosocial factors may also affect the psychiatric consequences of COVID-19. Thus, for example, the quarantine implicit after infection by SARS-CoV-2 has been related to negative psychological effects including posttraumatic stress symptoms, confusion, anger,12 anxiety related to fear of spreading the disease to family, and anxiety related to fear of death.11
For a drug to be considered responsible for an adverse reaction, it must generally meet the following criteria. First, the drug must be able to initiate the symptom within a specified period. Second, removal of the drug must reduce the symptom. Third, there must not be any other confounding variable that could be causing the symptom or its improvement. Reinstitution of the drug should cause reappearance of the symptom.13
These 3 criteria were met in our case. The evident chronology found between the appearance and resolution of neuropsychiatric symptoms and the start and withdrawal of the corticosteroids leads to the conclusion of causality of corticosteroids in neuropsychiatric symptoms and suicidal behavior. Any possible confounding factors derived from infection by SARS-CoV-2 and psychosocial stress associated with the pandemic were determinants not considerably modified after resolution of the affective symptoms.
Conclusion
The effects on mental health of the COVID-19 pandemic, infection by SARS-CoV-2, and treatment with corticosteroids are well known, although the latter is occasionally underestimated. Their confluence may cause a “perfect storm” situation, such as observed in our case. As the risk of neuropsychiatric alterations and increase in risk of suicide with dexamethasone treatment are known, evaluation of patients with COVID-19 should include psychopathologic assessment and the risk of suicide in all cases. Participation of liaison psychiatric teams is fundamental to diagnosis and intervention.
Published online: September 1, 2022.
Relevant financial relationships: Dr Plasencia-García has received honoraria for consulting/advisory boards from Otsuka, Lundbeck, and Janssen Johnson & Johnson and lecture honoraria from Otsuka, Lundbeck, Janssen Johnson & Johnson, Angelini, and Pfizer. Dr García-Ligero has received honoraria for attending meetings and/or travel from Otsuka, Lundbeck, and Janssen Johnson & Johnson. Dr Menéndez has received honoraria for attending meetings and/or travel from Otsuka, Lundbeck, Janssen Johnson & Johnson, and Ademed. Dr Gotor has received honoraria for consulting/advisory boards from Otsuka and Lundbeck and lecture honoraria from Otsuka, Lundbeck, Adamed, and Janssen Johnson & Johnson. Dr Crespo-Facorro has received honoraria for consulting/advisory boards from Otsuka, Takeda, and Angelini and lecture honoraria from Janssen Johnson & Johnson, Lundbeck, Roche, and Otsuka. Ms González-Fuentes reports no conflicts of interest related to the subject of this report.
Funding/support: None.
Patient consent: Verbal consent was received from the patient to publish the case report, and information has been de-identified to protect anonymity.
References (13)

- WHO. Coronavirus Disease (COVID-19) - events as they happen. World Health Organization website. Accessed June 27, 2021. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen
- Horby P, Lim WS, Emberson JR, et al; RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384(8):693–704. PubMed CrossRef
- Cheng B, Ma J, Yang Y, et al. Systemic corticosteroid administration in coronavirus disease 2019 outcomes: an umbrella meta-analysis incorporating both mild and pulmonary fibrosis-manifested severe disease. Front Pharmacol. 2021;12:670170. PubMed CrossRef
- Sánchez García MD, Pecino Esquerdo B, Pérez Martínez E. Manía inducida por el tratamiento con corticoesteroides: revisión a partir de un caso clínico. Rev Asoc Esp Neuropsiq. 2015;35(126):323–340. CrossRef
- Warrington TP, Bostwick JM. Psychiatric adverse effects of corticosteroids. Mayo Clin Proc. 2006;81(10):1361–1367. PubMed CrossRef
- Judd LL, Schettler PJ, Brown ES, et al. Adverse consequences of glucocorticoid medication: psychological, cognitive, and behavioral effects. Am J Psychiatry. 2014;171(10):1045–1051. PubMed CrossRef
- World Health Organization. Suicide Worldwide in 2019: Global Health Estimates. World Health Organization website. Accessed October 24, 2021. https://apps.who.int/iris/handle/10665/341728
- Laugesen K, Farkas DK, Vestergaard M, et al. Glucocorticoid use and risk of suicide: a Danish population-based case-control study. World Psychiatry. 2021;20(1):142–143. PubMed CrossRef
- Zhang W, Egashira N, Masuda S. Recent topics on the mechanisms of immunosuppressive therapy-related neurotoxicities. Int J Mol Sci. 2019;20(13):3210. PubMed CrossRef
- Popoli M. Agomelatine: innovative pharmacological approach in depression. CNS Drugs. 2009;23(suppl 2):27–34. PubMed CrossRef
- Rogers JP, Watson CJ, Badenoch J, et al. Neurology and neuropsychiatry of COVID-19: a systematic review and meta-analysis of the early literature reveals frequent CNS manifestations and key emerging narratives. J Neurol Neurosurg Psychiatry. 2021;92(9):932–941. CrossRef
- Brooks SK, Webster RK, Smith LE, et al. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet. 2020;395(10227):912–920. PubMed CrossRef
- Jenkins CA, Bruera E. Difficulties in diagnosing neuropsychiatric complications of corticosteroids in advanced cancer patients: two case reports. J Pain Symptom Manage. 2000;19(4):309–317. PubMed CrossRef
Enjoy this premium PDF as part of your membership benefits!