ABSTRACT
Objective: To evaluate the effectiveness and tolerability of vortioxetine at supratherapeutic dosages in patients with treatment-resistant depression.
Methods: A retrospective observational naturalistic study was conducted in 56 depressed patients resistant to standard care treatment from September 2020 to April 2021. Effectiveness of the vortioxetine treatments was evaluated through Clinical Global Impressions (CGI) score, comparing CGI values at the beginning (T0) of the vortioxetine treatment with CGI values at the earliest of these 2 time points (T1): (1) 8 weeks of treatment with supratherapeutic dosages and (2) day of vortioxetine discontinuation or daily dosage reduction to ≤ 20 mg due to side effects. The tolerability and safe of vortioxetine were also monitored.
Results: Fifty-six patients (32 females and 24 males, mean ± SD age of 51.1 ± 9.3 years) were included in the study. Thirty-seven patients received vortioxetine 30 mg/d, while 19 patients were treated with a 40-mg/d dosage. CGI scores significantly decreased (P < .001) in patients treated with 30 mg/d and 40 mg/d, respectively. No severe side effects were reported. Weight gain and nausea were the most common reported side effects. Nausea and limited efficacy were recorded as the most frequent reasons for vortioxetine dose reduction. None of the patients required vortioxetine discontinuation.
Conclusions: Supratherapeutic doses of vortioxetine were relatively well-tolerated and effective in patients with treatment-resistant depression.
Prim Care Companion CNS Disord 2022;24(3):21m03078
To cite: Cuomo A, Santucci A, Chioccioli M, et al. Effectiveness and tolerability of supratherapeutic dosing of vortioxetine in patients with treatment-resistant depression. Prim Care Companion CNS Disord. 2022;24(3):21m03078.
To share: https://doi.org/10.4088/PCC.21m03078
© 2022 Physicians Postgraduate Press, Inc.
aDipartimento di Medicina Molecolare e dello Sviluppo, Dipartimento di Psichiatria, University of Siena, Siena, Italy
*Corresponding author: Alessandro Cuomo, MD, University of Siena Medical Center, Division of Psychiatry, Department of Mental Health and Sensory Organs, Viale Bracci, 16, Siena, 53100 Italy ([email protected]).
A relatively high number of patients do not respond to multiple courses of antidepressant treatments.1–3 In the STAR*D trials, one-third of patients never remitted, even after 4 consecutive treatments.4,5
Optimization of current medication dose, switching to another antidepressant, add-on treatments (eg, lithium, atypical antipsychotics, esketamine, or thyroid hormones), and brain stimulation techniques (eg, electroconvulsive therapy, repetitive transcranial magnetic stimulation, vagal nerve stimulation, or deep brain stimulation) are among the most used strategies to treat resistant depression.6–8
Increasing the antidepressant dose up to the standard tolerated maximal dose is considered an efficiency strategy.9,10 Patients showing good tolerability and partial response or patients who have previously required unusually high medication doses may benefit from dose optimization up to supratherapeutic dosage (eg, sertraline 250 mg to 350 mg).8
Vortioxetine is a multimodal antidepressant approved in 2013 by the US Food and Drug Administration and the European Medicines Agency for the treatment of major depressive disorder (MDD) in adults at an oral dosage of 5–20 mg/d. In Italy, it has been available since May 2016.11
Vortioxetine has a unique and multimodal mechanism of action that combines inhibition of the serotonin transporter with modulation of serotonin (5-hydroxytryptamine [5-HT]) receptor activity.12 Furthermore, it acts as a 5-HT1B receptor partial agonist and 5-HT3, 5-HT7, and 5-HT1D receptor antagonist and modulates neurotransmission in multiple systems.13 It has been hypothesized that vortioxetine also has procognitive actions.14 Contrary to selective serotonin reuptake inhibitors, it blocks self-regulating systems (5-HT1B, 5-HT1D, 5-HT7).14
Vortioxetine pharmacokinetics are linear and not affected by food intake. Plasma concentration increases proportionally with a single dose between 5 and 20 mg/d, reaching the peak within 7 to 11 hours (Tmax).15 Its absolute bioavailability is 75% after oral administration with a large volume of distribution (approximately 2,600 L), which results in a long half-life of approximately 66 hours.
Vortioxetine is extensively distributed into the extravascular compartment and is metabolized extensively by the liver through oxidation via several cytochrome P450 isoenzymes and subsequent glucuronic conjugation via uridine diphosphate-glucuronosyltransferase.13,15 The major metabolite Lu AA3443 is pharmacologically inactive and mainly excreted through the kidneys.16 The minor pharmacologically active metabolite is not expected to cross the blood-brain barrier.13
This study aimed to evaluate the efficacy and tolerability of supratherapeutic doses of vortioxetine in patients with treatment-resistant depression.
METHODS
This retrospective observational naturalistic study aimed to assess the efficacy and tolerability of supratherapeutic doses of vortioxetine in patients with MDD who experienced an episode of treatment-resistant depression. The study was approved by the University of Siena and Area Vasta–South-East Institutional Review Board Ethics Committee (protocol no. 17617, approval date June 15, 2020). The study was conducted from September 2020 to April 2021.
Patients were enrolled using the following criteria: (1) diagnosis of MDD according to the DSM-5; (2) diagnosis of treatment-resistant depression, defined as failure to respond to 2 or more different oral antidepressants given at an adequate dose and for a sufficiently long period; (3) a Montgomery-Asberg Depression Rating Scale17 score ≥ 20; and (4) treated with vortioxetine 30 mg/d or 40 mg/d because of treatment-resistant depression. Patients with concurrent medications/treatments and patients with bipolar traits (ie, subthreshold bipolar disorder) were also included.
All patients gave informed consent to be treated with supratherapeutic doses of vortioxetine. Patients were treated either at the Psychiatry Department of the University of Siena Medical Center or the Crotone Mental Health Center outpatient unit in Italy. Slow titration of vortioxetine was applied for all patients.
The Clinical Global Impressions (CGI)18 score was measured at baseline ([T0]; the first day in which the dose of vortioxetine was increased above the maximum recommended dose of 20 mg/d) and after 8 weeks of treatment with supratherapeutic doses (T1). If a dose reduction due to side effects was decided before the end of the 8 weeks of observation, the CGI-Severity (CGI-S) score endpoint (T1) was the CGI of the day when vortioxetine was discontinued or reduced to a daily dose of ≤ 20 mg.
Descriptive statistical analyses were presented as mean ± SD for quantitative variables and frequencies and percentages for qualitative variables. The effectiveness of treatment, ie, changes in CGI-S scores after the treatment with vortioxetine supratherapeutic doses, was assessed through Wilcoxon matched-pairs signed rank test. Statistical significance was set at 5% (P < .05).
RESULTS
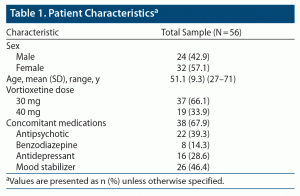
Fifty-six patients (32 females and 24 males) were included in the study. The mean ± SD age was 51.1 ± 9.3 years. Thirty-seven patients received vortioxetine 30 mg/d, and the remaining 19 patients were treated with vortioxetine 40 mg/d. Thirty-eight patients were receiving concomitant medications when vortioxetine was administered. Patient characteristics and concomitant medications are reported in Table 1.
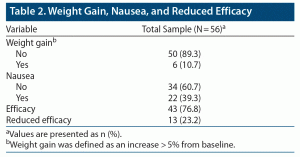
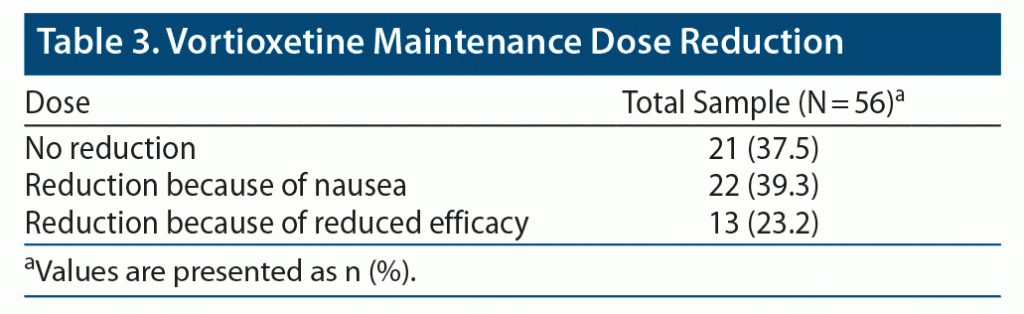
Overall, CGI scores significantly improved from T0 to T1 (CGI T0: 4.45 ± 0.74, CGI T1: 2.63 ± 1.05, P < .001) (Figure 1). No serious adverse events emerged during the observation period. Weight gain (defined as weight increased by more than 5% from baseline) and nausea were observed in 6 (10.7%) and 22 (39.3%) of the patients treated with vortioxetine 30 and 40 mg, respectively (Table 2). No symptoms or signs of serotonin syndrome were observed or recorded. A dose reduction to a daily dose of ≤ 20 mg was made before 8 weeks of treatment for 35 patients because of nausea (22 patients) or lack of improvement (13 patients) (Table 3).
DISCUSSION
Many studies19–21 have demonstrated the efficacy of vortioxetine in the treatment of depression with a dosage between 5 and 20 mg/d. Our study aimed to evaluate the efficacy and tolerability of vortioxetine when used at supratherapeutic doses in patients with resistant depression. After 8 weeks of treatment, patients showed a significant improvement of CGI score (P < .0001) with no severe side effects reported. Consistent with what has been observed with therapeutic doses of 5–20 mg/d,22 no worsening of suicidal ideation or suicidal behaviors was recorded in our study.
Contrary to previous studies with vortioxetine at doses of 10 or 20 mg,19,23–25 weight gain was observed more frequently. However, the higher prevalence of weight gain could also be due to the concomitant medications, which included mood stabilizers (46% of study subjects) and antipsychotics (39% of study subjects). In previous reports, no major side effects were reported for accidental or intentional intake of vortioxetine dosages ranging from 40 mg to 250 mg daily.26
The main limitations of our study include the small sample size, the retrospective design, the lack of randomization, the lack of a placebo arm, and a Berkson bias due to the likelihood that the study selected patients with more severe illness than the average patient with treatment-resistant depression. Hence, our results should be considered very preliminary. However, we hope that this study will prompt more research to test the efficacy and tolerability of vortioxetine at supratherapeutic doses. This study suggests the safety and efficacy of supratherapeutic doses of vortioxetine to treat resistant depression.
Submitted: July 20, 2021; accepted September 28, 2021.
Published online: June 9, 2022.
Relevant financial relationships: Dr Cuomo has served as a consultant or speaker for Angelini, GlaxoSmithKline, Lundbeck, Janssen, Otsuka, Pfizer, and Recordati. Dr Fagiolini has served as a consultant or speaker and has received research grants from Angelini, Apsen, Boheringer Ingelheim, Daiichi Sankyo, Doc Generici, GlaxoSmithKline, Italfarmaco, Lundbeck, Janssen, Mylan, Neuraxpharm, Otsuka, Pfizer, Recordati, Sanofi Aventis, and Sunovion. Drs Santucci, Chioccioli, Goracci, and Bolognesi report no conflicts of interest related to the subject of this article.
Funding/support: None.
Clinical Points
- Only a few treatments are approved for treatment-resistant depression.
- When the approved treatments for treatment-resistant depression are not available or feasible, vortioxetine at supratherapeutic doses may be an option.
- In patients with treatment-resistant depression, vortioxetine at supratherapeutic (20–40 mg) doses has shown a favorable balance between risks and benefits.
References (26)

- Han K-M, De Berardis D, Fornaro M, et al. Differentiating between bipolar and unipolar depression in functional and structural MRI studies. Prog Neuropsychopharmacol Biol Psychiatry. 2019;91:20–27. PubMed CrossRef
- Kasper S, Frazer A. Editorial for treatment-resistant depression (TRD). Int J Neuropsychopharmacol. 2019;22(2):83–84. PubMed CrossRef
- Sinyor M, Schaffer A, Levitt A. The Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial: a review. Can J Psychiatry. 2010;55(3):126–135. PubMed CrossRef
- Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905–1917. PubMed CrossRef
- Gaynes BN, Warden D, Trivedi MH, et al. What did STAR*D teach us? results from a large-scale, practical, clinical trial for patients with depression. Psychiatr Serv. 2009;60(11):1439–1445. PubMed CrossRef
- Bennabi D, Charpeaud T, Yrondi A, et al. Clinical guidelines for the management of treatment-resistant depression: French recommendations from experts, the French Association for Biological Psychiatry and Neuropsychopharmacology and the fondation FondaMental. BMC Psychiatry. 2019;19(1):262. PubMed CrossRef
- Thase ME, Rush AJ. When at first you don’t succeed: sequential strategies for antidepressant nonresponders. J Clin Psychiatry. 1997;58(suppl 13):23–29. PubMed
- Ionescu DF, Rosenbaum JF, Alpert JE. Pharmacological approaches to the challenge of treatment-resistant depression. Dialogues Clin Neurosci. 2015;17(2):111–126. PubMed CrossRef
- Fava M. Diagnosis and definition of treatment-resistant depression. Biol Psychiatry. 2003;53(8):649–659. PubMed CrossRef
- Rush AJ. STAR*D: what have we learned? Am J Psychiatry. 2007;164(2):201–204. PubMed CrossRef
- Adamo D, Pecoraro G, Aria M, et al. Vortioxetine in the treatment of mood disorders associated with burning mouth syndrome: results of an open-label, flexible-dose pilot study. Pain Med. 2020;21(1):185–194. PubMed
- Sanchez C, Asin KE, Artigas F. Vortioxetine, a novel antidepressant with multimodal activity: review of preclinical and clinical data. Pharmacol Ther. 2015;145:43–57. PubMed CrossRef
- Chen G, Lee R, Højer A-M, et al. Pharmacokinetic drug interactions involving vortioxetine (Lu AA21004): a multimodal antidepressant. Clin Drug Investig. 2013;33(10):727–736. PubMed CrossRef
- Sowa-Kućma M, Pańczyszyn-Trzewik P, Misztak P, et al. Vortioxetine: a review of the pharmacology and clinical profile of the novel antidepressant. Pharmacol Rep. 2017;69(4):595–601. PubMed CrossRef
- Spina E, Santoro V. Drug interactions with vortioxetine, a new multimodal antidepressant. Riv Psichiatr. 2015;50(5):210–215. PubMed
- Salagre E, Grande I, Solé B, et al. Vortioxetine: a new alternative for the treatment of major depressive disorder. Rev Psiquiatr Salud Ment (Engl Ed). 2018;11(1):48–59. PubMed CrossRef
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134(4):382–389. PubMed CrossRef
- Guy W. Clinical Global Impressions. ECDEU Assessment Manual for Psychopharmacology. DHEW Publication No. 76-338. Rockville, MD: National Institute of Mental Health; 1976:217–222.
- Alvarez E, Perez V, Dragheim M, et al. A double-blind, randomized, placebo-controlled, active reference study of Lu AA21004 in patients with major depressive disorder. Int J Neuropsychopharmacol. 2012;15(5):589–600. PubMed CrossRef
- Thase ME, Mahableshwarkar AR, Dragheim M, et al. A meta-analysis of randomized, placebo-controlled trials of vortioxetine for the treatment of major depressive disorder in adults. Eur Neuropsychopharmacol. 2016;26(6):979–993. PubMed CrossRef
- Katona CL, Katona CP. New generation multi-modal antidepressants: focus on vortioxetine for major depressive disorder. Neuropsychiatr Dis Treat. 2014;10:349–354. PubMed CrossRef
- Mahableshwarkar AR, Affinito J, Reines EH, et al. Suicidal ideation and behavior in adults with major depressive disorder treated with vortioxetine: post hoc pooled analyses of randomized, placebo-controlled, short-term and open-label, long-term extension trials. CNS Spectr. 2020;25(3):352–362. PubMed CrossRef
- Henigsberg N, Mahableshwarkar AR, Jacobsen P, et al. A randomized, double-blind, placebo-controlled 8-week trial of the efficacy and tolerability of multiple doses of Lu AA21004 in adults with major depressive disorder. J Clin Psychiatry. 2012;73(7):953–959. PubMed CrossRef
- Citrome L. Vortioxetine for major depressive disorder: a systematic review of the efficacy and safety profile for this newly approved antidepressant–what is the number needed to treat, number needed to harm and likelihood to be helped or harmed? Int J Clin Pract. 2014;68(1):60–82. PubMed CrossRef
- Baldwin DS, Chrones L, Florea I, et al. The safety and tolerability of vortioxetine: analysis of data from randomized placebo-controlled trials and open-label extension studies. J Psychopharmacol. 2016;30(3):242–252. PubMed CrossRef
- Mazza MG, Rossetti A, Botti ER, et al. Vortioxetine overdose in a suicidal attempt: a case report. Medicine (Baltimore). 2018;97(25):e10788. PubMed CrossRef
Enjoy this premium PDF as part of your membership benefits!