Patient Preferences for Treatment of Major Depressive Disorder and the Impact on Health Outcomes: A Systematic Review
Objective: To summarize the peer-reviewed literature on patient preferences for depression treatments and the impact of these preferences on the outcomes of treatment.
Data Sources: Studies were identified via a systematic search conducted simultaneously in PsycINFO and MEDLINE using EBSCOhost and EMBASE. Publications were retrieved in March 2010.
Study Selection: Search terms included depression OR MDD OR major depressive disorder, patient preference, treatment preference, intervention preference, and pharmacotherapy preference. There were no restrictions on years of publication. The search was restricted to research articles written in English.
Data Extraction: Fifteen articles contained unique information on patient preferences for depression treatments and their impact on depression-related outcomes.
Results: The patient preference literature includes a limited number of studies examining the impact of patient preferences on outcomes such as depression severity, treatment initiation, persistence and adherence, treatment engagement, the development of the therapeutic alliance, and health-related quality of life. The majority of the preference research has focused on comparisons of psychotherapy versus pharmacotherapy, with some limited information regarding comparisons of psychotherapies. Results from the research to date suggest that the impact of patient treatment preferences is mixed. The results also indicate that patient preferences have minimal impact on depression severity outcomes within the context of controlled clinical trials but may be more strongly associated with other outcomes such as entry into treatment and development of the therapeutic alliance. However, it is important to note that the literature is limited in that the impact of patient preference has been examined only through secondary analyses, and there have been few studies designed explicitly to examine the impact of patient preferences, particularly outside the context of controlled clinical trials.
Conclusions: Consideration of patient preferences for depression treatments may lead to increased treatment initiation and improved therapeutic alliance. However, despite treatment guidelines and suggestions in the literature, the value of and appropriate procedures for considering patient preferences in real-world treatment decisions deserves more careful study. Further research is needed, and future studies should be conducted in more naturalistic treatment settings that examine patient preferences for other specific approaches to depression treatments including preferences related to comparisons of individual pharmacotherapies and second-step treatments.
Prim Care Companion CNS Disord 2011;13(5):doi:10.4088/PCC.11r01161
© Copyright 2011 Physicians Postgraduate Press, Inc.
Submitted: February 8, 2011; accepted May 3, 2011.
Published online: October 13, 2011.
Corresponding author: Heather L. Gelhorn, PhD, United BioSource Corporation, 7101 Wisconsin Ave, Ste 600, Bethesda, MD 20814 ([email protected]).
Patients with major depressive disorder (MDD) often have well-defined attitudes and preferences associated with their depression treatment.1 Therefore, it is important that clinicians are aware of the relevance and potential impacts of patient preferences when they make decisions about depression treatment.2-4 Incorporation of patient preferences into treatment planning has been advocated in the peer-reviewed literature,5-9 as well as in guidelines set forth by regulatory and clinical organizations.3,10 For example, in the United Kingdom, the National Institute for Health and Clinical Excellence guidelines state that “given the current limited knowledge about what factors are associated with better antidepressant or psychotherapy response, most decisions will rely upon clinical judgment and patient preference until we have further research evidence.”3(p20) Similarly, the American Psychiatric Association’s treatment guidelines suggest that “selection of an initial treatment modality should be influenced by both clinical (eg, severity of symptoms) and other factors (eg, patient preference).”10(p10) Awareness of and consideration for depressed patients’ preferences are also important because these patients are more likely to want to participate in medical decision making compared with patients affected by other chronic conditions such as hypertension, diabetes, or heart disease.11
Clinical Points
- Studies evaluating the impact of patient preferences for depression treatments are limited and have primarily used clinical trial data; studies specifically designed to examine the impact of patient preferences on health and outcomes and conducted in more naturalistic settings are needed.
- Research shows that clinician attention to patients’ preferences may improve the likelihood of treatment initiation and positively impact the development of the therapeutic alliance, while having minimal impact on depression severity outcomes.
Consistent with the strong emphasis on patients’ depression treatment preferences, considerable research has been devoted to this general topic. Patient preferences have been evaluated through a variety of empirical research approaches, including self-report, economic or willingness-to-pay methodology, qualitative studies, and behavioral/observational data collection. Previous research studies have concluded that patient preferences are related to a variety of factors. Patients with more severe depression are more likely to opt for treatment,12 and personal experiences and knowledge about depression tend to impact patient preferences as well.13-16 Many demographic factors have been associated with patient depression treatment preferences, including ethnicity,13,15,17-19 gender,15,19 and age.19-24 In addition, some data suggest that medication costs and insurance copayments affect patients’ treatment decisions.25,26
In contrast to the many studies that have identified clinical, demographic, and economic factors associated with patient preferences for depression treatment, research examining the impact of these preferences on the outcomes of treatment is more limited. A variety of outcomes may be impacted by patient preferences for depression treatment—the most prominent of which is depression severity. Additional outcomes that have been studied include treatment initiation, persistence and adherence, engagement in treatment, and the development of the therapeutic alliance. Treatment persistence refers to continuing on a treatment for the prescribed length of time as recommended by a health care provider. In studies examining patient preferences for depression treatment, this can be measured using outcomes such as attrition from clinical trials, study dropout, or treatment discontinuation. Treatment adherence refers to participating in a treatment plan as recommended by a health care provider with respect to the timing, dosage, and frequency of medication or therapy. The therapeutic alliance refers to the nature and quality of the relationship between the health care professional and his/her patient.
Research on the impact of patient preferences for depression treatment on outcomes has important implications for clinical practice and research study design. For practicing clinicians, information regarding the impact of patient treatment preferences on health outcomes is important for at least 2 reasons. First, clinicians are interested in obtaining the most favorable outcomes for the patients they are treating, and it is important to understand how patient preferences may impact these outcomes. Second, in order to evaluate the appropriateness of various depression treatment options, knowledge about the impact of patient treatment preferences on the outcomes is critical.
There are 2 major concerns associated with patient preferences in depression treatment that are relevant to the design and interpretation of clinical trial data.27 The first is that patients participating in the clinical trials may be randomized to a treatment that is incongruent with their preference, which could adversely affect outcomes such as depression severity at follow-up, attrition from the study, adherence to study medication, or engagement in therapy. The second major concern is that patients with strong preferences may be unwilling to participate in clinical trials that require randomization, thereby resulting in a sample of patients that may not be representative of the population of patients encountered by clinicians in general practice. A greater understanding of how patient preferences impact clinical trial participation and other treatment outcomes is necessary to interpret the results of previous studies and to inform future interventions and research designs.
Despite the notable body of research on patient preference in depression, there have been few efforts to synthesize the work to date. The purpose of this systematic literature review was to summarize the peer-reviewed research on patient preferences for depression treatment and the impact of these preferences on outcomes.
Method
Data Sources
Studies were identified via a systematic search conducted simultaneously in PsycINFO and MEDLINE using EBSCOhost and EMBASE. Publications were retrieved in March 2010. Search terms included depression OR MDD OR major depressive disorder, patient preference, treatment preference, intervention preference, and pharmacotherapy preference. There were no restrictions on years of publication. The search was restricted to research articles written in English. Letters, books, editorials, dissertations, and notes were excluded. References were imported into a database,28 and duplicates were deleted.
Review Methods
The results of the literature search were evaluated by title and/or abstract in order to select empirical studies and theoretical/review articles that specifically addressed the impact of patient preference on depression treatment outcomes. The concept of depression treatment outcomes was broadly defined, and the results of the search included studies measuring self-report and clinical measures of depression severity levels, initiation of treatment, treatment persistence and adherence, development of the therapeutic alliance, and economic data. Relevant articles were limited to those that included depressed patients as the primary sample and those studies that measured patient preferences directly, either through verbal (eg, stated preference) or behavioral (eg, would only participate in a trial if assigned to their preferred treatment) responses by the participants. Full manuscripts of potentially relevant articles were obtained and assessed for inclusion.
RESULTS
Literature Search Results
After removal of duplicates, the Medline/PsycINFO search produced a total of 186 articles for potential inclusion, and the EMBASE search resulted in a total of 163 articles. A total of 73 of the 186 articles from Medline/PsycINFO and 22 of the 163 articles from EMBASE were initially accepted on the basis of a review of the titles and abstracts. After reviewing the full text of these 95 initially accepted articles, 15 met our inclusion criteria, were relevant to our review, and were found to contain unique information. These 15 articles are reviewed in-depth in this article.
Summary of Findings
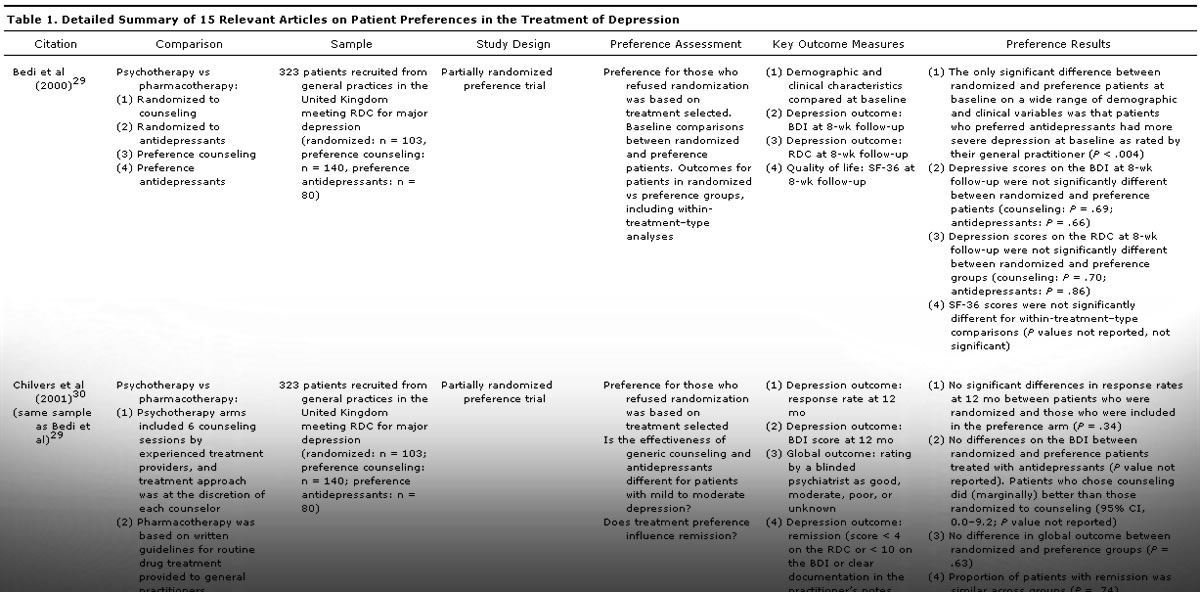
Each of the primary studies reviewed is presented in <!––>Table 1 with details including the comparison groups, sample description, study design, method of preference assessment, key outcome measures, and results relating specifically to patient preference. There were several broad categories of comparisons within the patient preference studies. Most commonly, studies examined patient preferences between pharmacotherapy and psychotherapy.29-37 Two studies examined patient preferences for different formulations or dosing schedules of antidepressant pharmacotherapies,38,39 and 2 studies examined only preferences related to psychotherapy.40,41 Four studies examined patient preferences for usual care versus alternative mental health care programs.31,36,42,43 The studies are discussed by comparison topic in the sections that follow. Two of the articles31,36 were relevant to more than 1 topic area.
The largest group of studies included comparisons of patient preferences for pharmacotherapy versus psychotherapy and is discussed first. The findings are summarized according to the outcomes of interest including findings related to depression severity, treatment initiation, persistence and adherence, development of the therapeutic alliance and treatment engagement, and health-related quality of life outcomes. Subsequently, the more limited research including comparisons within pharmacotherapies, comparisons within psychotherapies, and comparisons across different models of care is reviewed.
Studies Comparing Pharmacotherapy to Psychotherapy
Pharmacotherapy versus psychotherapy: depression severity. For practicing physicians, depression severity outcomes as measured by clinician ratings, self-reports, or other means may be the most important outcome of interest in patient preference studies of depression treatment. Many of the studies reviewed included commonly used clinician or self-reported depression severity outcomes including the Beck Depression Inventory (BDI),45 the Research Diagnostic Criteria (RDC),46 and the Hamilton Depression Rating Scale (HDRS) or 24-item HDRS (HDRS-24).47 The specific results from each of these studies are discussed below.
In a short-term study examining BDI scores at 4-week follow-up in a sample of 82 patients who were randomly assigned to treatment with cognitive-behavioral therapy, interpersonal psychotherapy, or pharmacotherapy with imipramine, there were no significant differences in BDI scores at 4-week follow-up for those who received treatment congruent with their preference compared to those who did not.32 Similarly, Bedi and colleagues29 studied 323 patients recruited from general practices in the United Kingdom who met RDC criteria for major depression. The trial design was a partially randomized preference trial, and patients who refused to be randomized to antidepressants, cognitive therapy, or placebo were included in the study and given their treatment of choice. There were no differences in either the BDI or RDC scores at 8-week follow-up between patients who were randomized and those who received their treatment of choice. The same sample was followed up 12 months later,30 and similar comparisons yielded no significant differences in depression severity outcomes (BDI scores), clinician global ratings, or relapse or remission rates.
In a randomized, placebo-controlled, clinical trial of drug versus talking treatment with 240 adult participants, Leykin and colleagues35 found no significant differences between those receiving their preferred treatment and those who did not in HDRS or BDI scores after 16 weeks. Another randomized, controlled trial by Raue et al37 that recruited 60 patients from primary care settings and compared interpersonal psychotherapy with escitalopram treatment drew similar conclusions; there were no significant differences in HDRS ratings or remission rates between the preference-congruent and preference-incongruent groups at either 12- or 24-week follow-up visits.
In contrast to these findings, a very large study (n = 429) by Kocsis et al34 measured HDRS-24 overall scores and used HDRS-24 ratings to assess remission and partial response in patients treated with either nefazodone or the Cognitive Behavioral Analysis System of Psychotherapy. They found that patients who received treatments congruent with their preferences had lower overall depression scores following treatment and were more likely to achieve remission or partial response over the course of the 12-week trial.34 This study differed from others mainly in that patient preferences were assessed not just for psychotherapy compared with pharmacotherapy, rather, patients were also given the additional preference options of “combination therapy” (ie, pharmacotherapy and psychotherapy/counseling) and/or “no preference.”34 In addition, 1 of the randomized treatment groups in the study included a “combined” treatment wherein patients received both psychotherapy and pharmacotherapy treatment concurrently.34
A similarly designed study by Lin et al36 compared patients (n = 335) who had received treatments that were either congruent or incongruent with their stated treatment preferences. Similar to the preference choices offered in the study by Kocsis and colleagues,34 patients indicated their preference among the following options: medication, counseling, both, neither, or “I don’ t know.” The results of the study at 3-month follow-up were that patients receiving preference-congruent treatment had significantly larger decreases in the 20-item Hopkins Symptom Checklist (SCL-20) depression scale compared with those receiving preference-incongruent treatment.36 One limitation of this finding is that the preference groups differed on SCL-20 depression scores at baseline, with the treatment-congruent group having higher scores and thus a greater opportunity to show improvement. In addition, by 9-month follow-up, there were no differences in SCL-20 scores between the 2 groups.36
In sum, the majority of the studies that compared psychotherapy versus pharmacotherapy and evaluated depression severity outcomes for patients found that receipt of preferred depression treatment, be it psychotherapy or pharmacotherapy, does not significantly improve posttreatment depression severity. In contrast to these findings, 2 studies34,36 did find significant differences in depression severity at 1 or more follow-up assessments for those patients receiving preference-congruent treatment. As discussed above, there were key methodological differences in both of these studies, and it is not clear how these differences may have affected the findings. The 2 studies that concluded there was an effect of patient preference on depression outcomes had very large sample sizes, suggesting perhaps that there is a small effect detectable only with sufficient power.
Pharmacotherapy versus psychotherapy: treatment initiation, persistence, and adherence. At least 2 studies have examined the impact of patient treatment preferences for pharmacotherapy versus psychotherapy on entry into treatment. Raue and colleagues37 reported that significantly more primary care patients initiated treatment when assigned to their preferred treatment. Similarly, a study of a quality improvement intervention designed to address primary care patient preferences in depression treatment (n = 742) by Dwight-Johnson et al31 found that patients who were not in treatment at baseline, and who preferred pharmacotherapy over psychotherapy, were significantly more likely to enter treatment if they were randomized to the medication-specific quality intervention program that was congruent with their preference rather than to the psychotherapy-specific quality intervention program or usual care.31
For patients who enter into depression treatment, persistence and adherence to the intervention to which they have been prescribed has also been an outcome of interest in the patient preference literature. Three randomized, controlled trials comparing pharmacotherapy and psychotherapy examined the relationship between persistence and patient treatment preferences. A smaller study by Elkin et al (n = 82)32 found that patients randomized to receive treatments incongruent with their preferences were more likely to drop out of the study (odds ratio = 4.76), while 2 larger studies by Kocsis et al34 and Leykin et al35 found no such effect (N = 429 and N = 240, respectively). The study of treatment adherence by Raue et al37 found that treatment preference congruence was not associated with adherence rates for either pharmacotherapy or psychotherapy at 12 weeks. Surprisingly, this same study also found that the strength of the patient preferences as rated on a 5-point Likert scale was significantly negatively associated with adherence,37 and patients who indicated that they strongly agreed that they needed the specific treatment to which they were assigned tended to have lower levels of adherence. This study was the only one to examine the strength of the patient preferences; future research to replicate this result is needed.
Pharmacotherapy versus psychotherapy: therapeutic alliance and treatment engagement. Patient preferences for pharmacotherapy versus psychotherapy have also been examined in terms of both treatment engagement and development of the therapeutic alliance. Elkin and colleagues32 found that patients receiving treatment congruent with their preference for either pharmacotherapy or psychotherapy were more engaged in treatment and had higher ratings of their contributions to the development of the therapeutic alliance.
Another study by Iacoviello et al33 reported that among patients preferring psychotherapy, therapeutic alliance scores increased significantly more over time for those receiving congruent treatment (psychotherapy), did not change for those receiving incongruent treatment (pharmacotherapy), and decreased significantly among those receiving inactive treatment (placebo pill). In this same study, there were no differences found in the development of the therapeutic alliance for those preferring pharmacotherapy regardless of the treatment to which they were randomized.33 These results suggest that the relationship between patient preferences and development of the therapeutic alliance is particularly germane to those preferring psychotherapy, but should still be considered for patients preferring pharmacotherapy. In addition, the results suggest that treatment efficacy may be a significant mediating factor as evidenced by the fact that patients in the placebo group experienced decreases in the therapeutic alliance, while those who were in an active but preference-incongruent treatment group experienced no such decrease.33
Pharmacotherapy versus psychotherapy: health-related quality of life. Data on the health-related quality of life impact of patient preferences in the context of pharmacotherapy versus psychotherapy were extremely limited. Only a single study that met our search criteria included any measure designed to evaluate health-related quality of life.29 This study by Bedi et al29 found that patient scores on the Medical Outcomes Study 36-item Short-Form Health Survey, a broad quality of life measure, were not significantly associated with receiving preference-congruent pharmacotherapy or psychotherapy treatment.
Studies Comparing Pharmacotherapies
Two studies focused on comparisons of patient preferences within pharmacotherapies. It is likely that such studies are rare because few patients are highly knowledgeable about the specific characteristics of the various pharmacologic agents that are available to treat depression. This limited knowledge is not surprising as patients who are aware of multiple agents are likely to have gained this knowledge through negative (ie, side effects) or unsatisfactory (ie, nonresponse) experiences with a specific pharmacotherapeutic agent and subsequent treatment with another.
The first study by Delini-Stula et al,38 which examined patient preferences for different forms of pharmacotherapy, was focused on comparing different formulations of mirtazapine. The study was designed as an Internet survey of 8,811 participants and compared a fast-dissolving formulation of mirtazapine with a conventional formula. Patients tended to prefer the fast-dissolving formulation as measured by stated preferences and by increased self-reported adherence with the fast-dissolving formulation.38 In addition, a second study by Granger et al39 among patients taking bupropion sustained-release used a Web survey to assess adherence and patient preferences. Patients with an increased number of daily doses were less compliant, and the majority of patients (77% of twice-daily, 94% of thrice-daily users) expressed an interest in a once-daily formula.39 Patients cited scheduling convenience, fewer pills, and fewer missed doses as reasons for this preference.39 It is important to note the survey nature of these studies and their inherent limitations, particularly that they did not involve a controlled research design. There were no studies identified that compared patient preferences for different classes of pharmacotherapeutic medications and the impact on outcomes.
Studies Comparing Psychotherapies
Two studies that compared patient preferences for psychotherapy were identified in this review. The first study by Ward et al41 compared 464 patients who participated in either nondirective counseling or cognitive-behavioral therapy. Patients were either randomized or included in the study under a patient preference arm. There were no significant differences in the depression severity as measured by the BDI between the randomized and preference groups at either 4- or 12-month follow-up visits.41 The second study by Van et al40 compared 119 patients who were randomized to short-term psychodynamic supportive therapy versus those who were included in the same therapy under a preference arm. There were many depression-related outcome measures including depression severity as measured by response rate of > 50% reduction in symptoms on the HDRS, the depression subscale of the 90-item SCL, and Clinical Global Impressions-Severity of Illness (CGI-S) and CGI-Improvement (CGI-I) scales. In addition, persistence was measured by dropout during the first 8 weeks of treatment. There were no significant differences between the preference and randomization groups on any of the measures at 8 or 24 weeks, with the only exception being the CGI-S at 8-week follow-up for which the preference group showed a more favorable response. Both studies concluded that generally there were not significant differences between patients who were randomized versus those who insisted on a specific preference-congruent psychotherapy in terms of depression severity outcomes.40,41
Studies Comparing Different Models of Care
The literature search identified several studies that were focused on comparing alternative models of care, such as treatment received under a collaborative care model involving additional aspects of depression management including depression care managers, specialty mental health care, and medication management, to typical management in a primary care setting.36,42,43 Table 1 outlines additional details specific to each collaborative care model and the results of each of these studies that are discussed in greater detail below.
These studies concluded that alternative models of care that attempt to integrate primary care with specialty mental health care are more successful in either offering42 or providing43 patients with their preferred treatment. However, consistent with findings reported in other patient preference studies, patients receiving their preferred treatment generally did not have more favorable depression outcomes compared with those who did not receive their preferred treatment, even under these specialty models of care.36,42,43 It is interesting to note that in the study by Lin and colleagues,36 initially (ie, after 3 months) patients who had received their preferred treatment experienced significantly larger improvements in their depression scores; however, by 9-month follow-up, there were no such differences between groups. The authors suggest that this finding may reflect a more rapid treatment response for those who are matched with their preferred treatment; however, further research to confirm this conclusion is needed. In addition to the results on depression severity, patients who received their preferred treatment were no more satisfied with their treatment than those who did not.42,43
DISCUSSION
Despite clear documentation in both the literature and treatment guidelines directing clinicians to consider patient preferences when making treatment decisions for depression, this review found a limited amount of evidence supporting a significant impact of patient preference on depression-related outcomes. This finding may call into question the value of considering patients’ preferences for MDD treatments, although there are several gaps in the currently published research that should be considered. The most notable limitation is that the vast majority of studies to date have focused on the impact of preferences for psychotherapy as compared to pharmacotherapy. While clearly an important topic, this particular focus does not fully reflect the entire range of treatment options and challenges in the initial and ongoing clinical management of depression. Current trends in depression treatment increasingly involve combined regimens of pharmacotherapy and psychotherapy, and, in many cases, a sequenced approach to treatment for the significant proportion of patients who do not achieve a satisfactory outcome with initial treatment.3,10,49 In addition, research to date on the topic of patient preferences has primarily been based on secondary analyses of clinical trial data; these clinical trials were not designed explicitly to examine patient preferences and the consequent impact on the outcomes of treatment. Additional research of broader scope and with more intentional study designs is needed prior to drawing firm conclusions.
One of the more notable findings is that the majority of studies reported that patients who received treatment congruent with their preference, whether it was psychotherapy versus pharmacotherapy or among different psychotherapies, did not exhibit a greater degree of improvement in depression severity as compared to those who were randomized. One potential explanation for this surprising result is that the majority of studies have been conducted within the context of clinical trials. Treatment persistence and adherence tend to be higher in clinical trials as compared to the community practice setting. Regardless of the treatment modality, persistence and adherence represent considerable challenges in the treatment of depression.50 In a more naturalistic context, such as in the primary care setting, patient preferences and attitudes about different treatments may have a more substantial impact on depression severity outcomes through the moderators of decreased persistence and adherence. Further studies are clearly needed in more naturalistic community treatment settings. Supporting this, some evidence from the studies reviewed here suggests that patient preferences impact treatment initiation,31,37 persistence,32 treatment engagement,32 and the development of the therapeutic alliance.32,33 However, the results from the studies reviewed were mixed, with some studies failing to show positive associations between patients’ preferences and patterns of persistence34,35 or adherence.37 Further research is needed to clarify previous findings and to identify the specific preferences and impacts that warrant consideration.
Future studies could include a variety of research designs with the primary objective of assessing the impact of patient preferences on depression treatment outcomes. One study design option might be a randomized, controlled study that could include all patients seeking treatment for depression regardless of the nature or strength of their treatment preference. After an initial thorough assessment of each patient’s preference (ie, specific treatment preference and the strength of that preference), patients would be randomized to receive either preference-congruent or preference-incongruent treatment. Treatment outcomes (including all of those discussed in the current review) would be examined at follow-up to determine whether the strength and/or nature of the treatment preference had a significant impact on each outcome of interest. To more closely approximate real-world depression treatment, these studies could have participating physicians prescribe each patient’s treatment regimen (based on randomization but without knowledge of the patient’s preference) according to their usual standards of care.
A second design alternative would be a more observationally-based design that might include data gathered by examining patients’ real-world experiences with depression treatment that include restrictions on access to care based, for example, on health insurance coverage. Within this design, patients who initially seek treatment would be assessed for their treatment preferences, then provided with care as usual after consultation with their physicians and based on their health insurance coverage (and other factors). Follow-up assessments to evaluate patient outcomes could be analyzed to examine whether treatment preferences (as indicated prior to treatment) were significantly related to these outcomes. Although observational studies, such as this proposed design, would not allow for conclusions about causal relationships between patient preferences and outcomes, these designs might provide more accurate information regarding the importance and strength of the relationships among these variables in naturalistic treatment settings.
There is also a need for research that expands beyond the narrow scope of evaluating patient preferences in terms of comparisons of psychotherapy versus pharmacotherapy to more accurately reflect what is most commonly encountered in community settings. In the United States, patients are increasingly prescribed antidepressants by primary care physicians as the first-line treatment.51,52 Treatment guidelines from the American Psychiatric Association on the choice of specific pharmacologic treatment indicate that patient preferences should be considered when selecting among the available antidepressant medications.10 To our knowledge there are no studies that have formally examined patient preferences among different pharmacotherapeutic agents and their impact on outcomes, and, thus, there is limited information regarding the potential value, expected outcomes, and basis for considering patient preferences in these scenarios. The limited research to date suggests that patients may have some clear preferences related to the characteristics of antidepressants and antidepressant treatment regimens, including side effect burden, medication properties, duration of treatment, dosing schedule, symptom severity, or change in symptom severity after treatment.39,49,53,54 Not surprisingly, patients prefer pharmacotherapies that are convenient, that result in fewer side effects, and that are more effective. However, further research is needed to establish whether such preferences vary by individual, and to establish whether there are direct relationships between patients’ preferences for specific characteristics of antidepressants and other treatment outcomes, including medication persistence and adherence and depression severity.
In addition to considerations for patients’ preferences for first-line treatment of depression, many patients experience nonresponse, partial response, or response without remission to initial treatment and seek second-line treatment strategies from their health care providers.55-57 There are several options for second-line treatment for patients who have not achieved remission after the first line of therapy, and most commonly this includes switching to a different treatment or using adjunctive therapy.6 While many of these strategies are supported by efficacy data, Papakostas6 advises that treating physicians should also consider patient preference when choosing a second-line treatment strategy. Surprisingly, there is little research regarding patients’ preferences for second-step treatment strategies.49,58 Perhaps the most well-known study of second-step depression treatments is the Sequenced Treatment Alternative to Relieve Depression trial, a study wherein 1,439 participants entered second-step treatment after unsatisfactory outcomes to initial treatment with citalopram. In this study, patients considering second-line treatment options who had experienced a greater side effect burden with citalopram clearly favored switching medications, while those who had experienced a better response to citalopram in terms of depression severity outcomes favored an augmentation strategy.49 These results suggest that patients’ previous experiences with treatments, including their level of response, tolerability of the initial medication, and other factors, can impact later preferences. However, to our knowledge there is not yet published information regarding the impact that these preferences had on treatment outcomes later in the study. Further research that specifically examines patient preferences for second-step treatments and the associated outcomes, both across and within treatment modalities, is greatly needed. Additional information regarding patients’ preferences for second-line treatment strategies may be of considerable value given that patients who initially experience unsatisfactory outcomes are at increased risk for treatment discontinuation and poor long-term outcomes.
The results regarding the impact of patient preferences on depression treatment outcomes that are known to date have important clinical implications. While further research is still needed to evaluate the impact of patients’ treatment preferences on depression severity outcomes in ecologically valid settings and under varied treatment approaches, there are several reasons to conclude that attention to patients’ preferences is still of importance. The clinical importance of considering patients’ preferences is underscored by findings suggesting that entry into depression treatment is significantly more likely to occur if patients are offered treatment that is congruent with their preference, and that the development of the therapeutic alliance can be positively affected by receiving treatment congruent with preferences. Thus, clinicians who ascertain and consider patients’ treatment preferences have an opportunity to positively impact those patients in precise and potentially important ways.
Finally, researchers conducting or interpreting the results of clinical trials who are concerned about the implications of randomizing patients to treatments that are incongruent with their preferences may be comforted by the results of comparisons of depression outcomes associated with preferences for psychotherapy versus pharmacotherapy. Determining or comparing the effectiveness of pharmacotherapeutic versus psychotherapeutic depression treatments appears to be adequately ascertained by randomizing patients willing to participate in the clinical study and comparing their depression outcomes across the various treatment groups. This conclusion is based on findings that patients participating in clinical trials are unlikely to differ on key variables of interest from those unwilling to accept randomization,29,40 and assignment to a treatment incongruent with a patient’s preference is unlikely to significantly impact depression severity outcomes under controlled and carefully monitored clinical trial conditions. As suggested earlier, further research is needed to ascertain if these findings are similar in comparisons of specific pharmacotherapies. In addition, conclusions surrounding the impact of patients’ preferences on depression severity and other depression-related outcomes in real-world settings need to be evaluated using more realistic and ecologically valid study designs.
Drug names: bupropion (Wellbutrin, Aplenzin, and others), citalopram (Celexa and others), escitalopram (Lexapro and others), imipramine (Tofranil and others), mirtazapine (Remeron and others).
Author affiliations: United BioSource Corporation, Bethesda, Maryland (Drs Gelhorn and Sexton); and Eli Lilly and Company, Indianapolis, Indiana (Mr Classi).
Potential conflicts of interest: Drs Gelhorn and Sexton are employed by the United BioSource Corporation (UBC), which provides consulting and other research services to pharmaceutical, device, government, and nongovernment organizations. As UBC employees, they work with a variety of companies and organizations and are expressly prohibited from receiving any payment or honoraria directly from these organizations for services rendered. Mr Classi is an employee of and minor stock shareholder in Eli Lilly and Company.
Funding/support: Eli Lilly and Company provided funding for this research.
Acknowledgments: The authors would like to acknowledge the contributions of Tiffany Burch, MSc, United BioSource Corporation, Hammersmith, United Kingdom, for assistance with extracting data for the tables and Fritz Hamme, BA, United BioSource Corporation, Bethesda, Maryland, and Jessica Barnett, BA, United BioSource Corporation, Lexington, Massachusetts, for technical assistance with manuscript development.
REFERENCES
1. Churchill R, Khaira M, Gretton V, et al; Nottingham Counselling and Antidepressants in Primary Care (CAPC) Study Group. Treating depression in general practice: factors affecting patients’ treatment preferences. Br J Gen Pract. 2000;50(460):905-906. PubMed
2. US Dept of Health and Human Welfare. Depression in Primary Care. Vol 2. Treatment of Major Depression. Clinical Practice Guideline, No. 5. Publication No. 93-0551. Rockville, MD: Department of Health and Human Services, Agency for Health Care Policy and Research; 1993.
3. National Institute for Health and Clinical Excellence (NICE). Depression: The Treatment and Management of Depression in Adults. London, UK: National Collaborating Centre for Mental Health; 2009.
4. Richardson LP, Lewis CW, Casey-Goldstein M, et al. Pediatric primary care providers and adolescent depression: a qualitative study of barriers to treatment and the effect of the black box warning. J Adolesc Health. 2007;40(5):433-439. PubMed doi:10.1016/j.jadohealth.2006.12.006
5. Papakostas GI. Initial treatment approaches for patients with major depressive disorder. J Clin Psychiatry. 2009;70(6):e18. PubMed doi:10.4088/JCP.8001tx7c
6. Papakostas GI. Managing partial response or nonresponse: switching, augmentation, and combination strategies for major depressive disorder. J Clin Psychiatry. 2009;70(suppl 6):16-25. PubMed doi:10.4088/JCP.8133su1c.03
7. Sobczak JA. Managing high-acuity-depressed adults in primary care. J Am Acad Nurse Pract. 2009;21(7):362-370. PubMed doi:10.1111/j.1745-7599.2009.00422.x
8. Schulberg HC, Katon W, Simon GE, et al. Treating major depression in primary care practice: an update of the Agency for Health Care Policy and Research Practice Guidelines. Arch Gen Psychiatry. 1998;55(12):1121-1127. PubMed doi:10.1001/archpsyc.55.12.1121
9. APA Presidential Task Force on Evidence-Based Practice. Evidence-based practice in psychology. Am Psychol. 2006;61(4):271-285. PubMed doi:10.1037/0003-066X.61.4.271
10. American Psychiatric Association. Practice Guidelines for the Treatment of Patients with Major Depressive Disorder. 2nd ed. Washington, DC: American Psychiatric Association; 2000.
11. Arora NK, McHorney CA. Patient preferences for medical decision making: who really wants to participate? Med Care. 2000;38(3):335-341. PubMed doi:10.1097/00005650-200003000-00010
12. Berner MM, Kriston L, Sitta P, et al. Treatment of depressive symptoms and attitudes towards treatment options in a representative German general population sample. Int J Psychiatry Clin Pract. 2008;12(1):5-10. doi:10.1080/13651500701330783
13. Chandra A, Scott MM, Jaycox LH, et al. Racial/ethnic differences in teen and parent perspectives toward depression treatment. J Adolesc Health. 2009;44(6):546-553. PubMed doi:10.1016/j.jadohealth.2008.10.137
14. Johnson MD, Meredith LS, Hickey SC, et al. Influence of patient preference and primary care clinician proclivity for watchful waiting on receipt of depression treatment. Gen Hosp Psychiatry. 2006;28(5):379-386. PubMed doi:10.1016/j.genhosppsych.2006.07.006
15. Dwight-Johnson M, Sherbourne CD, Liao D, et al. Treatment preferences among depressed primary care patients. J Gen Intern Med. 2000;15(8):527-534. PubMed doi:10.1046/j.1525-1497.2000.08035.x
16. Wright A, Jorm AF, Harris MG, et al. What’s in a name? is accurate recognition and labelling of mental disorders by young people associated with better help-seeking and treatment preferences? Soc Psychiatry Psychiatr Epidemiol. 2007;42(3):244-250. PubMed doi:10.1007/s00127-006-0156-x
17. Cabassa LJ, Zayas LH. Latino immigrants’ intentions to seek depression care. Am J Orthopsychiatry. 2007;77(2):231-242. PubMed doi:10.1037/0002-9432.77.2.231
18. Givens JL, Houston TK, Van Voorhees BW, et al. Ethnicity and preferences for depression treatment. Gen Hosp Psychiatry. 2007;29(3):182-191. PubMed doi:10.1016/j.genhosppsych.2006.11.002
19. Jaycox LH, Asarnow JR, Sherbourne CD, et al. Adolescent primary care patients’ preferences for depression treatment. Adm Policy Ment Health. 2006;33(2):198-207. PubMed doi:10.1007/s10488-006-0033-7
20. Areסn PA, Hegel MT, Reynolds CF III. Treating depression in older medical patients with psychotherapy. Journal of Clinical Geropsychology. 2001;7(2):93-104. doi:10.1023/A:1009581504993
21. Bertocci D, Hirsch E, Sommer W, et al. Student mental health needs: survey results and implications for service. J Am Coll Health. 1992;41(1):3-10. PubMed doi:10.1080/07448481.1992.9936300
22. Givens JL, Datto CJ, Ruckdeschel K, et al. Older patients’ aversion to antidepressants: a qualitative study. J Gen Intern Med. 2006;21(2):146-151. PubMed
23. Hickie IB, Davenport TA, Luscombe GM, et al. The assessment of depression awareness and help-seeking behaviour: experiences with the International Depression Literacy Survey. BMC Psychiatry. 2007;7:48. doi:10.1186/1471-244X-7-48 PubMed
24. Hickie IB, Luscombe GM, Davenport TA, et al. Perspectives of young people on depression: awareness, experiences, attitudes and treatment preferences. Early Interv Psychiatry. 2007;1(4):333-339. PubMed doi:10.1111/j.1751-7893.2007.00042.x
25. Steinman MA, Sands LP, Covinsky KE. Self-restriction of medications due to cost in seniors without prescription coverage. J Gen Intern Med. 2001;16(12):793-799. PubMed doi:10.1046/j.1525-1497.2001.10412.x
26. Piette JD, Heisler M, Wagner TH. Cost-related medication underuse among chronically ill adults: the treatments people forgo, how often, and who is at risk. Am J Public Health. 2004;94(10):1782-1787. PubMed doi:10.2105/AJPH.94.10.1782
27. Brewin CR, Bradley C. Patient preferences and randomised clinical trials. BMJ. 1989;299(6694):313-315. PubMed doi:10.1136/bmj.299.6694.313
28. EndNote. X1 ed. Carlsbad, CA: Thomson ResearchSoft; 2007.
29. Bedi N, Chilvers C, Churchill R, et al. Assessing effectiveness of treatment of depression in primary care: partially randomised preference trial. Br J Psychiatry. 2000;177(4):312-318. PubMed doi:10.1192/bjp.177.4.312
30. Chilvers C, Dewey M, Fielding K, et al; Counselling versus Antidepressants in Primary Care Study Group. Antidepressant drugs and generic counselling for treatment of major depression in primary care: randomised trial with patient preference arms. BMJ. 2001;322(7289):772-775. PubMed doi:10.1136/bmj.322.7289.772
31. Dwight-Johnson M, Unutzer J, Sherbourne C, et al. Can quality improvement programs for depression in primary care address patient preferences for treatment? Med Care. 2001;39(9):934-944. PubMed doi:10.1097/00005650-200109000-00004
32. Elkin I, Yamaguchi J, Arnkoff D, et al. “Patient-treatment fit” and early engagement in therapy. Psychother Res. 1999;9(4):437-451. doi:10.1080/10503309912331332851
33. Iacoviello BM, McCarthy KS, Barrett MS, et al. Treatment preferences affect the therapeutic alliance: implications for randomized controlled trials. J Consult Clin Psychol. 2007;75(1):194-198. PubMed doi:10.1037/0022-006X.75.1.194
34. Kocsis JH, Leon AC, Markowitz JC, et al. Patient preference as a moderator of outcome for chronic forms of major depressive disorder treated with nefazodone, Cognitive Behavioral Analysis System of Psychotherapy, or their combination. J Clin Psychiatry. 2009;70(3):354-361. PubMed doi:10.4088/JCP.08m04371
35. Leykin Y, Derubeis RJ, Gallop R, et al. The relation of patients’ treatment preferences to outcome in a randomized clinical trial. Behav Ther. 2007;38(3):209-217. PubMed doi:10.1016/j.beth.2006.08.002
36. Lin P, Campbell DG, Chaney EF, et al. The influence of patient preference on depression treatment in primary care. Ann Behav Med. 2005;30(2):164-173. PubMed doi:10.1207/s15324796abm3002_9
37. Raue PJ, Schulberg HC, Heo M, et al. Patients’ depression treatment preferences and initiation, adherence, and outcome: a randomized primary care study. Psychiatr Serv. 2009;60(3):337-343. PubMed doi:10.1176/appi.ps.60.3.337
38. Delini-Stula A, Van Oers H, Van Willigenburg A, et al. Treating depression with different galenical drug formulations: does it make a difference? the comparison of mirtazapine fast dissolving formulation (FDT) with conventional mirtazapine tablets (CT). Int J Psychiatry Clin Pract. 2009;13(2):109-116. doi:10.1080/13651500802485262
39. Granger AL, Fehnel SE, Hogue SL, et al. An assessment of patient preference and adherence to treatment with Wellbutrin SR: a Web-based survey. J Affect Disord. 2006;90(2-3):217-221. PubMed doi:10.1016/j.jad.2005.08.018
40. Van HL, Dekker J, Koelen J, et al. Patient preference compared with random allocation in short-term psychodynamic supportive psychotherapy with indicated addition of pharmacotherapy for depression. Psychother Res. 2009;19(2):205-212. PubMed doi:10.1080/10503300802702097
41. Ward E, King M, Lloyd M, et al. Randomised controlled trial of non-directive counselling, cognitive-behaviour therapy, and usual general practitioner care for patients with depression, 1: clinical effectiveness. BMJ. 2000;321(7273):1383-1388. PubMed doi:10.1136/bmj.321.7273.1383
42. Dobscha SK, Corson K, Gerrity MS. Depression treatment preferences of VA primary care patients. Psychosomatics. 2007;48(6):482-488. PubMed doi:10.1176/appi.psy.48.6.482
43. Gum AM, Areסn PA, Hunkeler E, et al. Depression treatment preferences in older primary care patients. Gerontologist. 2006;46(1):14-22. PubMed
44. King M, Sibbald B, Ward E, et al. Randomised controlled trial of non-directive counseling, cognitive-behaviour therapy and usual general practitioner care in the management of depression as well as mixed anxiety and depression in primary care. Health Technol Assess. 2000;4(19):1-83. PubMed
45. Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561-571. PubMed
46. Spitzer RL, Endicott J, Robins E. Research diagnostic criteria: rationale and reliability. Arch Gen Psychiatry. 1978;35(6):773-782. PubMed
47. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56-62. PubMed doi:10.1136/jnnp.23.1.56
48. Ware JE Jr, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36), 1: conceptual framework and item selection. Med Care. 1992;30(6):473-483. PubMed doi:10.1097/00005650-199206000-00002
49. Wisniewski SR, Fava M, Trivedi MH, et al. Acceptability of second-step treatments to depressed outpatients: a STAR*D report. Am J Psychiatry. 2007;164(5):753-760. PubMed doi:10.1176/appi.ajp.164.5.753
50. Trivedi MH, Lin EH, Katon WJ. Consensus recommendations for improving adherence, self-management, and outcomes in patients with depression. CNS Spectr. 2007;12(suppl 13):1-27. PubMed
51. Stafford RS, Ausiello JC, Misra B, et al. National patterns of depression treatment in primary care. Prim Care Companion J Clin Psychiatry. 2000;2(6):211-216. PubMed doi:10.4088/PCC.v02n0603
52. Stafford RS, MacDonald EA, Finkelstein SN. National patterns of medication treatment for depression, 1987 to 2001. Prim Care Companion J Clin Psychiatry. 2001;3(6):232-235. PubMed doi:10.4088/PCC.v03n0611
53. Mendels J, Schless AP. Antidepressant effects of desipramine adminstered in two dosage schedules. Dis Nerv Syst. 1977;38(4):249-251. PubMed
54. Morey E, Thacher JA, Craighead WE. Patient preferences for depression treatment programs and willingness to pay for treatment. J Ment Health Policy Econ. 2007;10(2):73-85. PubMed
55. Driscoll HC, Karp JF, Dew MA, et al. Getting better, getting well: understanding and managing partial and non-response to pharmacological treatment of non-psychotic major depression in old age. Drugs Aging. 2007;24(10):801-814. PubMed doi:10.2165/00002512-200724100-00002
56. Saghafi R, Brown C, Butters MA, et al. Predicting 6-week treatment response to escitalopram pharmacotherapy in late-life major depressive disorder. Int J Geriatr Psychiatry. 2007;22(11):1141-1146. PubMed doi:10.1002/gps.1804
57. Bondolfi G, Aubry JM, Golaz J, et al. A stepwise drug treatment algorithm to obtain complete remission in depression: a Geneva study. Swiss Med Wkly. 2006;136(5-6):78-85. PubMed
58. Schatzberg AF, Rush AJ, Arnow BA, et al. Chronic depression: medication (nefazodone) or psychotherapy (CBASP) is effective when the other is not. Arch Gen Psychiatry. 2005;62(5):513-520. PubMed doi:10.1001/archpsyc.62.5.513