ABSTRACT
Objective: To review the mechanism of selective serotonin reuptake inhibitor (SSRI)-mediated serotonergic neurotransmission, focusing on serotonin 1A (5-HT1A) autoreceptors, which are proposed to be involved in delaying therapeutic efficacy. Vilazodone was specifically designed to function both as an SSRI and a partial agonist at 5-HT1A receptors. This combined mechanism is proposed to decrease time to efficacy, minimize sexual side effects, and provide concomitant anxiolytic properties.
Data Sources: A PubMed search of all English-language articles from January 1990 to January 2013 was conducted using the search terms depression and 5-HT1A, depression and buspirone, depression and pindolol, and vilazodone.
Study Selection: We found 47 articles and abstracts that were selected for inclusion on the basis of information about the pharmacology of 5-HT1A receptors and the clinical data on pindolol, buspirone, and vilazodone in depression.
Data Extraction: This review summarizes current literature involving antidepressant activity, the role of 5-HT1A autoreceptors, and clinical trials involving serotonin reuptake inhibition in conjunction with 5-HT1A agonists and partial agonists, with a focus on vilazodone.
Results: Vilazodone has demonstrated efficacy in 2 large, randomized, double-blind, placebo-controlled trials in major depressive disorder. Results suggest that vilazodone has a low incidence of sexual side effects and is effective in patients with high levels of anxiety. A pooled analysis shows evidence of significant symptom reduction after only 1 week of therapy.
Conclusions: If future studies corroborate the clinical benefits attributed to its mechanism of action, vilazodone may show potential advantages in terms of onset of action, sexual side effects, and anxiolytic activity in patients with major depressive disorder.
Prim Care Companion CNS Disord 2014;16(1):doi:10.4088/PCC.13r01554
© Copyright 2014 Physicians Postgraduate Press, Inc.
Submitted: July 10, 2013; accepted October 1, 2013.
Published online: January 9, 2014.
Corresponding author: Kerri A. Pierz, PhD, c/o John Edwards, 185 Hudson St, Plaza 5 28th Floor, Jersey City, NJ 07311 ([email protected]).
Major depressive disorder (MDD) is a debilitating condition with lifetime prevalence in the United States of 19.2%.1 The World Health Organization ranks MDD as the leading cause of disease burden in high- and middle-income countries2 and the second-leading cause of years lived with disability worldwide.3 Data from the 1995-1996 National Ambulatory Medical Care Survey demonstrated that 8% of all primary care office visits were depression related,4 and the role of primary care in treating MDD is increasing due to changes in health care coverage, improved depression screening tools, and the development of newer-generation antidepressants with improved safety profiles.4-6 Since a growing share of the responsibility for MDD diagnosis and treatment falls on primary care physicians, an understanding of the history, neurobiological mechanisms, and pharmacologic treatment of MDD may enhance patient care.
In the 1950s, the discovery of the therapeutic effects of medications now classified as tricyclic antidepressants (TCAs) and monoamine oxidase inhibitors (MAOIs) led to more widespread treatment of depression.7 Although the antidepressant actions of the TCAs and MAOIs are likely to be initiated by different mechanisms, they ultimately have a similar effect of increasing neurotransmitter availability in the synaptic cleft. The TCAs inhibit reuptake of certain neurotransmitters, particularly norepinephrine and, for some TCAs, serotonin. The MAOIs, in contrast, inhibit the metabolism of serotonin, dopamine, and norepinephrine.7 One major limiting factor in the use of these 2 drug classes is their side effect profiles. For example, the TCAs can produce adverse cholinergic and adrenergic effects, and, in excessive doses or overdoses, can cause seizures and potentially lethal arrhythmias.7,8 The MAOIs can produce orthostatic hypotension and edema.9,10 Moreover, because levels of the A form of the enzyme MAO are high in the gut and liver, inhibition of MAO requires dietary restrictions to reduce the risk of a serious and potentially fatal adverse event, hypertensive crisis.11 There was thus a clear motivation to develop newer, more selective antidepressant medications, and, by the late 1980s, research led to the introduction of a new class of antidepressants, the selective serotonin reuptake inhibitors (SSRIs). As compared with the first-generation antidepressants, SSRIs were found to be generally similar in efficacy for the treatment of depressed outpatients, with a better tolerability profile.8 Moreover, the SSRIs were profoundly safer in overdose than the TCAs. As a result of these differences, the SSRIs rapidly supplanted the TCAs and MAOIs as the antidepressant class of first choice for both psychiatrists and primary care providers. Indeed, by the end of the first decade of the “SSRI first” era, primary care physicians were prescribing more antidepressants than psychiatrists.12
Shortly after the introduction of the SSRIs, another class of antidepressants known as the serotonin-norepinephrine reuptake inhibitors (SNRIs) was introduced. As suggested by the name, these medications inhibit the reuptake of norepinephrine in addition to serotonin and, as such, directly affect both serotonergic and noradrenergic neurotransmission. However, it should be noted that most of these compounds show much greater inhibition of serotonin reuptake than norepinephrine reuptake (10-fold for duloxetine and 30-fold for venlafaxine), such that at normal therapeutic doses, most of their activity very likely results from their serotonergic effects.13,14 Although not as widely prescribed as the SSRIs, several SNRIs are also now considered to be first-line treatment options for MDD. It was proposed that the property of dual reuptake inhibition might convey an efficacy advantage for the SNRIs over SSRIs14; however, few studies have found large, statistically significant differences between the 2 drug classes, and meta-analyses generally document relatively small average differences that fall below proposed margins of clinical relevance.15-18 Moreover, no evidence of a difference in efficacy has been found in comparisons of venlafaxine and/or duloxetine versus the most selective SSRI, escitalopram.15,16,19 For these reasons, in light of the pharmacologic profile of the novel antidepressant vilazodone,20 this article focuses on alternate serotonergic mechanisms that may be drawn upon to try to address the unmet needs of antidepressant therapy.

- Vilazodone is a novel antidepressant medication that may offer clinical benefits with regard to onset of action and side effect profile.
- Serotonin 1A (5-HT1A) receptors have an important role in modulating the serotonergic system.
- Drugs that target 5-HT1A receptors, particularly in combination with inhibition of serotonin transporters, may be of value in the treatment of mood disorders.
Method
A PubMed search of all English-language articles from January 1990 to January 2013 was conducted using the search terms depression and 5-HT1A, depression and buspirone, depression and pindolol, and vilazodone. We found 47 articles and abstracts that were selected for inclusion on the basis of information about the pharmacology of serotonin (5-hydroxytryptamine) 1A (5-HT1A) receptors and the clinical data on pindolol, buspirone, and vilazodone in depression.
This review summarizes current literature involving antidepressant activity, the role of 5-HT1A autoreceptors, and clinical trials involving serotonin reuptake inhibition in conjunction with 5-HT1A agonists and partial agonists, with a focus on vilazodone.
THE SEROTONERGIC SYSTEM AND MAJOR DEPRESSIVE DISORDER
Although the discovery of the first antidepressants was serendipitous, development of the SSRI drug class resulted from the deliberate and concerted efforts of scientists working in drug discovery. As early as the 1960s, hypotheses began to circulate about the role of serotonin in the pathophysiology of depression and as a target for antidepressant drugs.21-23 Research on the SSRIs began in the 1970s, with zimelidine being the first such drug to reach clinical trials and fluoxetine becoming the first to be approved by the US Food and Drug Administration (FDA) in 1987.2,24 The other SSRIs were subsequently introduced: paroxetine, sertraline, fluvoxamine, citalopram, and escitalopram.
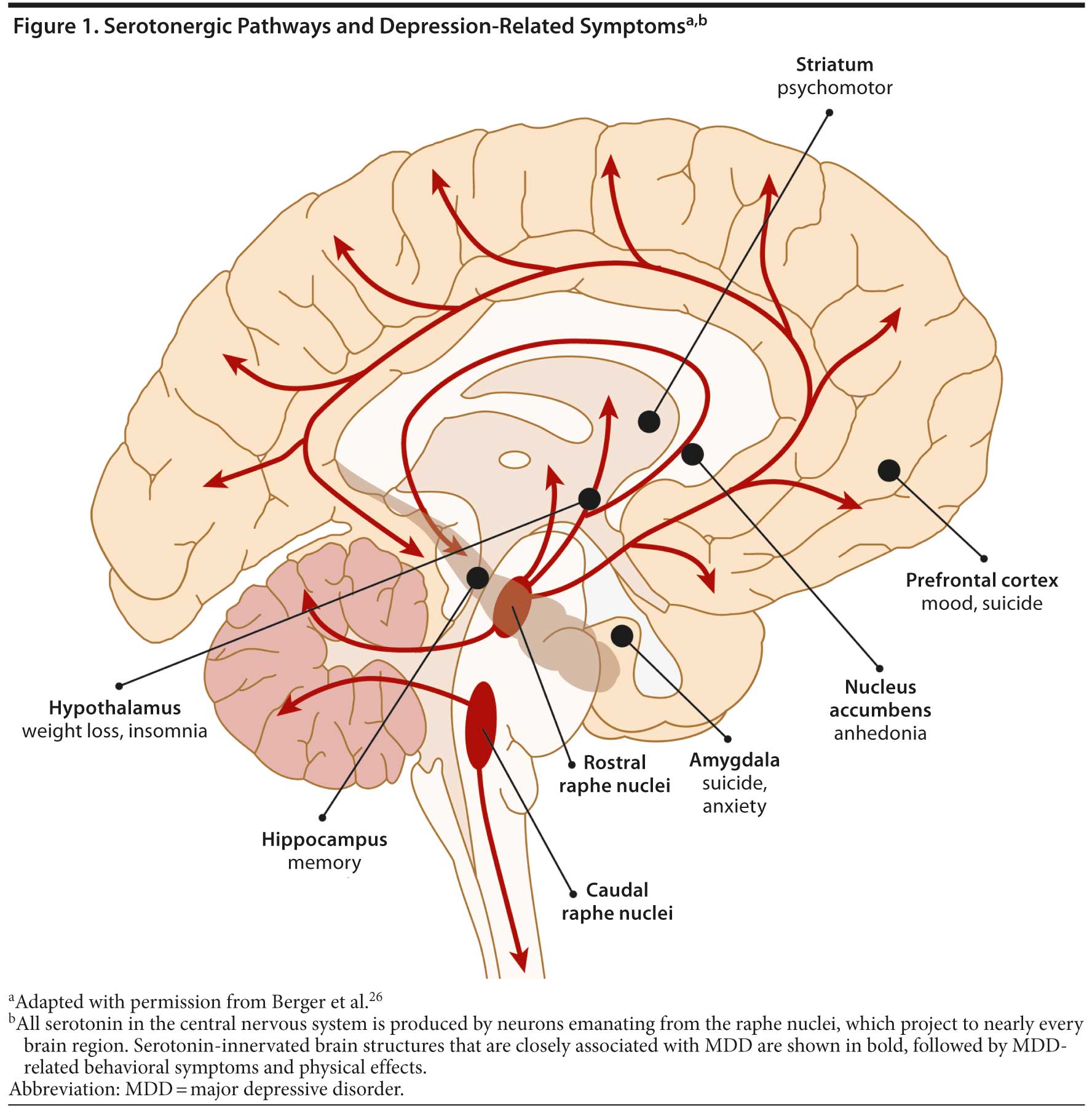
Serotonergic pathways in the brain all emanate from the raphe nuclei, branching out to nearly every region of the central nervous system (Figure 1). Serotonin plays an important role in many brain functions, including contributing to the regulation of mood, fear responses, sleep, appetite, and sexual behavior.25,26 Serotonergic neurons also project to the hippocampus, a region in which the generation of new neurons (“neurogenesis”) and synaptic plasticity have been implicated as possible factors in the development and treatment of MDD.27
SEROTONIN BIOCHEMISTRY AND PHARMACOLOGY
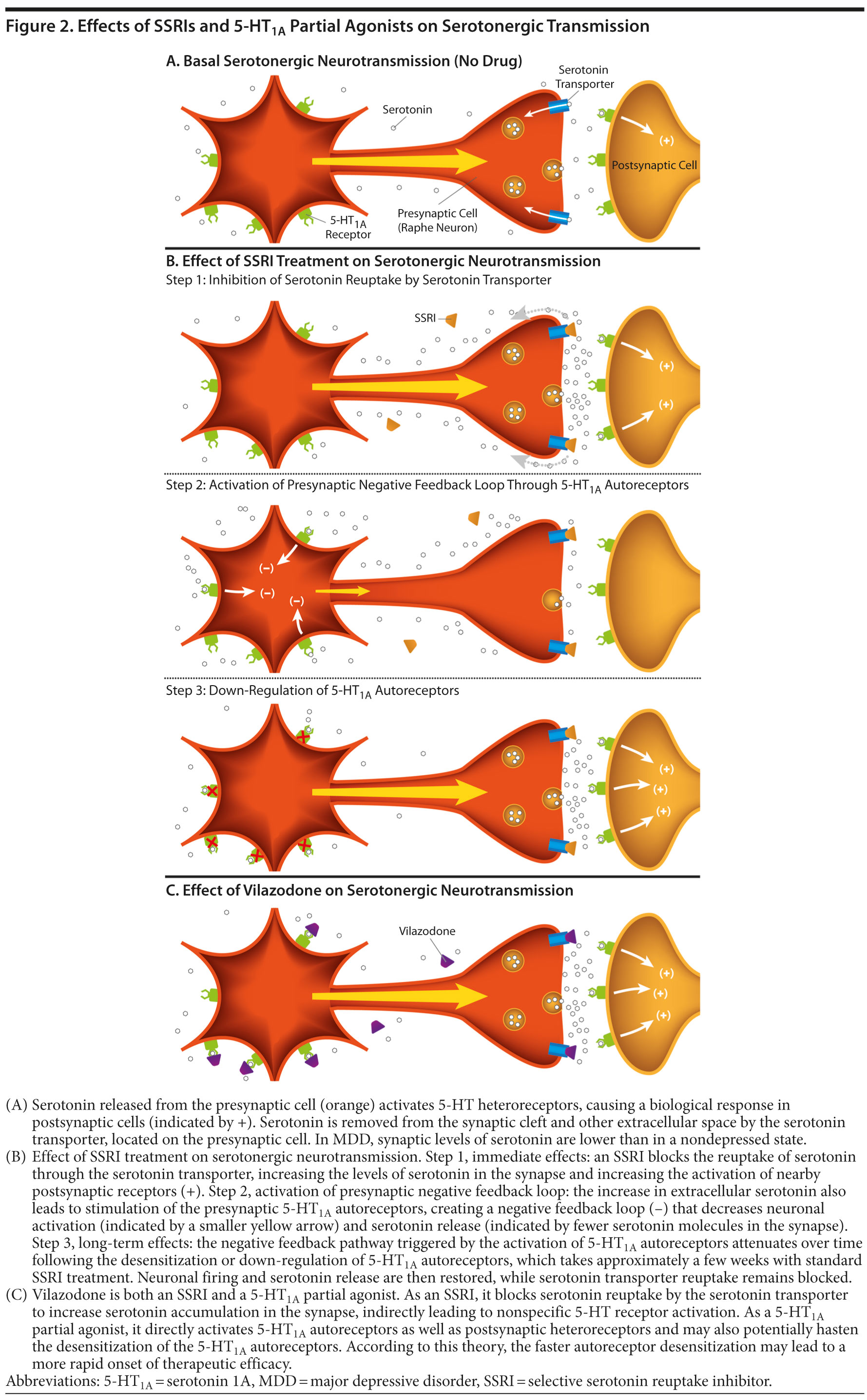
Serotonin is a monoamine neurotransmitter that is synthesized from the amino acid tryptophan. Like tryptophan and the other amino acids, serotonin contains a positive and negative charge at physiologic pH, making it hydrophilic and unable to easily cross cellular membranes or the blood-brain barrier. This property makes it unable to diffuse back into neurons after its release, thereby necessitating a specific transport mechanism for its removal from the synaptic cleft. Serotonergic uptake occurs through the serotonin transporter (SERT), an integral membrane protein located in the presynaptic neuron. Thus, serotonin is transported from the synaptic space back into the presynaptic cell from which it was released (Figure 2A), where it is either repackaged into synaptic vesicles for subsequent re-release or degraded by MAO. The therapeutic efficacy of MAOIs is believed to be initiated by inhibiting the enzymatic degradation of serotonin, while the efficacy of the SSRIs is presumed to be initiated by inhibition of the SERT (Figure 2B, step 1).
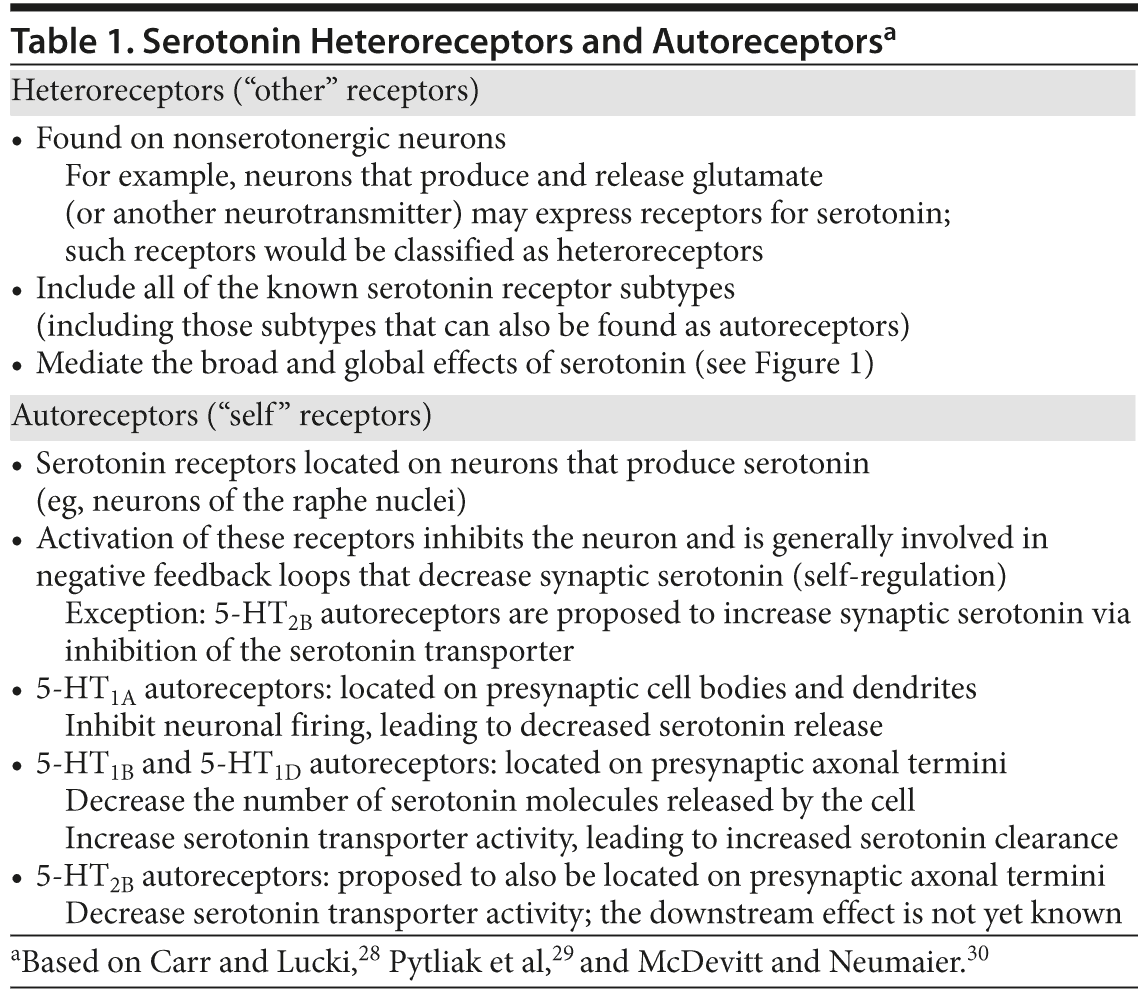
Once serotonin is released into the synapse, it can bind approximately 20 different endogenous receptor subtypes.28,29 These receptors fall into 7 distinct families based on their amino acid sequence, drug response, and cellular effects. In the serotonergic pathways that originate in the raphe nuclei, these serotonin receptor subtypes can be found on various postsynaptic, non-serotonin-containing neurons throughout the brain; these receptors are known as heteroreceptors (Table 1). A few of the receptors, notably subtypes 5-HT1A, 5-HT1B, 5-HT1D, and 5-HT2B, are also expressed on the serotonin-containing raphe neurons themselves as autoreceptors, where they regulate further serotonin release. Generally, serotonin activation of autoreceptors leads to the inhibition of neuronal firing and serotonin release through negative feedback mechanisms within the raphe neuron (Table 1). The 5-HT1A receptors are the primary 5-HT autoreceptor expressed on the cell body and dendrites (“somatodendritic”) of the raphe neurons and regulate 5-HT via modulation of action potential activity. The 5-HT1B receptors are the primary terminal autoreceptors, expressed on the axonal termini; they modulate 5-HT activity via inhibition of 5-HT synthesis and release from the raphe neurons. Finally, the 5-HT2B receptors have more recently been proposed as autoreceptors given apparent cross-talk with 5-HT1B receptors and modulation of the serotonin transporter.30 Additional investigation is needed to more fully understand the expression pattern and activity of 5-HT2B autoreceptors.
5-HT1A Autoreceptors in Depression
A growing body of evidence suggests that 5-HT1A receptors are involved in depression and antidepressant activity.28 In patients with MDD, 5-HT1A receptor expression and activity are altered in raphe nuclei, hippocampus, and many cortical regions.31-34 The activation of 5-HT1A autoreceptors, through the binding of serotonin (or full or partial receptor agonists), initially produces an inhibition of serotonin release by the neuron (Figure 2B, step 2).35 However, current theory predicts that sustained, long-term 5-HT1A receptor stimulation soon leads to a desensitization and/or down-regulation of the autoreceptors so that, over time, serotonin release is no longer inhibited (Figure 2B, step 3).25 The time required for this process of inhibition followed by disinhibition of the serotonergic system may partially explain why serotonergic antidepressant drugs35-38 often require several weeks or more to achieve maximal symptomatic improvement.
Initially, drugs like SSRIs and SNRIs inhibit serotonin reuptake through SERT, producing an increase in synaptic serotonin, which binds postsynaptic receptors found proximal to the synaptic cleft to increase serotonergic signaling (Figure 2B, step 1). However, the increased serotonin also begins to bind more distal 5-HT1A autoreceptors, for example, those found back on the raphe neuron cell bodies, which ultimately inhibit further neuronal firing and result in decreased serotonin release (Figure 2B, step 2). Eventually, 5-HT1A autoreceptors desensitize or are down-regulated, removing the inhibitory signal and permitting synaptic serotonin levels to rise in the presence of sustained blockade of SERT by the SSRI (Figure 2B, step 3). The net result over time is the sustained increase of serotonergic neurotransmission made possible by the removal of the 5-HT1A receptor-mediated negative feedback loop (“disinhibition”).
Ligands that bind to 5-HT1A autoreceptors and heteroreceptors, as antagonists (which block serotonin activity), full agonists (which mimic serotonin activity), or partial agonists (which mimic and compete with serotonin binding, but only produce a partial response relative to endogenous serotonin), have shown beneficial effects on depression symptoms, both clinically and in research situations.35,37 Buspirone, a drug used to treat generalized anxiety disorder, is a full agonist at presynaptic 5-HT1A autoreceptors, where it initially inhibits both serotonin synthesis and neuronal firing. Buspirone is also a partial agonist at 5-HT1A heteroreceptors in the hippocampus and frontal cortex, where it can help attenuate dysfunctional serotonergic transmission in MDD.35 Pindolol, a β-adrenergic receptor antagonist and a partial agonist of 5-HT1A receptors, shows similar effects.35,39 Both compounds have been shown to enhance the effects of SSRIs, possibly by directly binding to 5-HT1A autoreceptors and hastening their desensitization and down-regulation (Figure 2B, step 3).35,39-42
5-HT1A Agonists/Partial Agonists in Treatment of Depression
It has been hypothesized that pharmacotherapy with 5-HT1A agonists or partial agonists alone could be effective in the treatment of depression and anxiety, while avoiding the typical side effects of many SSRIs (eg, nausea, sexual dysfunction, sleep disturbances); however, the effectiveness of 5-HT1A agonists and partial agonists as monotherapy for MDD has been modest, and they are primarily used only as augmenting agents in combination with SSRIs.42,43
Preliminary studies using case reports, chart reviews, and open-label trials suggested that buspirone augmentation was effective in improving depression symptoms in patients who were refractory to SSRI treatment.44-46 Double-blind studies with buspirone augmentation of SSRIs, however, have shown mixed results. In patients with severe depression who failed to respond to fluoxetine or citalopram, buspirone augmentation compared with placebo significantly reduced Montgomery-Asberg Depression Rating Scale (MADRS) scores at endpoint; early onset of action was observed for augmentation with buspirone versus placebo in patients with less severe depression, but differences at endpoint were not significant.47 Conversely, buspirone combined with fluoxetine did not show faster onset or increased response rates relative to fluoxetine alone in patients with non-treatment-resistant depression.48
In the largest depression study conducted to date, the Sequenced Treatment Alternatives to Relieve Depression (STAR*D), buspirone augmentation was one of multiple second-step treatment strategies in patients who failed to achieve remission with up to 14 weeks of citalopram treatment.49 In this study,49 augmentation with buspirone was associated with a 30% remission rate, which was similar to the rate seen with sustained-release bupropion augmentation.42
Multiple double-blind trials have shown efficacy for pindolol augmentation of SSRIs to both hasten onset of therapeutic action and/or improve symptom resolution at endpoint.50-56 The effectiveness of pindolol augmentation, however, was not supported in other studies.57-60 Discrepant results may be due to differences between studies in regard to patient selection, study design, and pindolol dose.61 Meta-analyses of pindolol augmentation studies suggest that the addition of pindolol to SSRI treatment was effective in accelerating the onset of clinical action, with significant benefits versus placebo observed in the initial weeks of treatment.62,63
VILAZODONE
Vilazodone was approved by the FDA for the treatment of MDD in January 2011. Pharmacologically, vilazodone increases serotonin levels by inhibiting SERT, with additional partial agonist properties at 5-HT1A receptors.64 It has been hypothesized that this dual mechanism of action has the potential to shorten the onset of antidepressant activity, decrease side effects attributed to serotonin reuptake inhibition (eg, sexual dysfunction), and provide enhanced benefits for symptoms of anxiety.31
As a result of the partial agonist activity at autoreceptors, the desensitization or down-regulation of 5-HT1A autoreceptors that purportedly underlies the delayed efficacy of SSRIs is hypothesized to occur with vilazodone over a shorter period of time (Figure 2C). Studies in animal models support this hypothesis, as vilazodone appears to desensitize 5-HT1A autoreceptors more rapidly than conventional SSRIs.65 Additionally, vilazodone has also been shown to increase serotonin levels in rat cortex and hippocampus to an extent greater than other SSRIs, as measured by in vivo microdialysis.66,67
Clinical Trials of Vilazodone for MDD
The clinical efficacy of vilazodone was demonstrated through the results of two 8-week, phase III, randomized, double-blind, placebo-controlled trials41,68 in adult outpatients diagnosed with MDD according to DSM-IV-TR criteria.69 In both studies, vilazodone compared with placebo demonstrated statistically significant improvement on the primary outcome measure, change from baseline to week 8 in MADRS70 total score, as well as on the 17-item Hamilton Depression Rating Scale (HDRS-17) total score.71
In the first study,68 significant improvements in MADRS and HDRS-17 total scores were observed in patients treated with vilazodone compared with placebo as early as week 1, suggesting a rapid onset of action. In the second trial,41 a significant difference in MADRS total score for vilazodone compared with placebo was observed after 6 weeks of treatment, with a trend for improvement (P = .051) noted as early as week 4.
These individual studies were not powered or designed to evaluate the clinical relevance of the effects at time points earlier than 8 weeks; however, post hoc analyses of the pooled data (436 vilazodone-treated and 433 placebo-treated patients) indicated significant treatment differences favoring vilazodone at week 1 in MADRS total score change from baseline, as well as in rates of response (defined as ≥ 50% improvement on the MADRS).72
The most commonly reported adverse events that occurred with significantly (P < .05) greater incidence in vilazodone-treated versus placebo-treated patients were diarrhea (28.0% vs 9.2%, respectively), nausea (23.4% vs 5.1%, respectively), dizziness (8.5% vs 4.6%, respectively), insomnia (6.0% vs 2.1%, respectively), vomiting (4.6% vs 1.2%, respectively), and abnormal dreams (4.1% vs 1.2%, respectively).20,73 Adherence to the recommended dose titration schedule (10 mg daily for 1 week, increased to 20 mg daily for 1 week and 40 mg daily thereafter) is important to minimize gastrointestinal adverse events and to achieve the full recommended dose of 40 mg per day.
Sexual dysfunction is a well-recognized side effect of some antidepressant drugs. However, patients often underreport sexual problems with the spontaneous adverse event collection methods used in traditional clinical trials. While most SSRI trials generally report a low incidence of sexual dysfunction adverse events, some studies that used specific metrics to evaluate sexual dysfunction have reported a significantly higher rate of sexual dysfunction (up to 63% and 41% of men and women, respectively) associated with SSRI treatment compared with placebo or non-SSRI antidepressants.74,75 Therefore, it is important to include validated, standardized evaluations of sexual function in trials of drug classes that are known to affect sexual functioning to ensure that this important side effect is systematically assessed.
In the phase III studies of vilazodone, sexual function was evaluated using validated scales; the Arizona Sexual Experiences Questionnaire was used in the first study68 and the Changes in Sexual Functioning Questionnaire was used in the second study.41 Changes in sexual function, as reported using these measures, were found to be minimal over the 8-week treatment period in the vilazodone group and were generally similar to placebo,76 although these studies did not include an active SSRI comparator and were not specifically designed to test for differences in sexual functioning. It is hypothesized that treatment-emergent sexual dysfunction was minimized due to the partial agonist activity of vilazodone at 5-HT1A receptors. This hypothesis is consistent with the amelioration of SSRI-induced sexual dysfunction observed in studies of coadministration of an SSRI with 5-HT1A receptor partial agonists (for example, buspirone or pindolol).77-80 Future clinical trials that include an active SSRI comparator would be necessary to test this hypothesis.81
The Role of Vilazodone in Clinical Practice
The pharmacologic profile of vilazodone may address several unmet needs inherent in the pharmacotherapy of depression. First, the proposed mechanism of action is consistent with a rapid onset of clinical efficacy, which is supported by the results of the pooled analysis suggesting effects of vilazodone as early as week 1.68,72 Perhaps equally important, vilazodone may fill the void for a serotonergic antidepressant that has a low incidence of sexual side effects; it has been recommended that this claim be tested using a noninferiority trial versus placebo and including an active comparator known to increase the incidence of sexual dysfunction.81 It also remains to be seen whether patients who develop significant sexual side effects on other SSRIs can be readily switched to vilazodone, with preservation of response and restoration of sexual function. If such advantages are confirmed, it would represent a significant advantage over current practice, which includes either switching to nonserotonergic antidepressants such as bupropion or adding “antidotes” such as sildenafil. Another unmet need for antidepressant therapy is better remediation of comorbid anxiety symptoms. Indeed, the negative impact of anxiety on treatment outcome for a range of first-line and second-line therapies was one of the strongest findings in the STAR*D study.82 Beyond the theoretical rationale underlying the pharmacologic effects of vilazodone on anxiety, clinical evidence has supported the efficacy of concomitant administration of an SSRI and 5-HT1A receptor partial agonist (ie, buspirone or pindolol) in MDD.42,43,47,50,56 It is important to note, however, that the completed clinical trials were not specifically designed to assess onset of efficacy, sexual side effects, or efficacy in patients with concomitant anxiety, so future studies will be needed to confirm these proposed mechanism-based benefits. Furthermore, the interpretation of the onset of clinical efficacy is confounded by the titration schedule; significant effects that were observed at week 1 in one of the clinical studies occurred at a dose of only 10 mg/d, although the recommended therapeutic dose is 40 mg/d.
CONCLUSIONS
Vilazodone, a novel FDA-approved antidepressant, was specifically designed to function both as an SSRI, by inhibiting SERT, and as a partial agonist at 5-HT1A receptors, similar to buspirone and pindolol. It has been hypothesized that this dual mechanism of action may shorten the onset of antidepressant activity, decrease side effects attributed to serotonin reuptake inhibition (eg, sexual dysfunction), and provide enhanced benefits for symptoms of anxiety. The efficacy of vilazodone in MDD has been demonstrated in 2 large, randomized, double-blind, placebo-controlled trials,41,68 in which patients treated with vilazodone compared with placebo-treated patients showed significant improvement after 8 weeks. In 1 study, significant improvement compared with placebo was observed as early as week 1,68 although this rapid time course of efficacy remains to be validated. If clinical studies corroborate the theoretical advantages attributed to its dual mechanism of action, vilazodone may become a unique treatment option with a rapid onset, minimal sexual side effects, and anxiolytic properties.
Drug names: bupropion (Wellbutrin, Aplenzin, and others), buspirone (BuSpar and others), citalopram (Celexa and others), duloxetine (Cymbalta), escitalopram (Lexapro and others), fluoxetine (Prozac and others), fluvoxamine (Luvox and others), paroxetine (Paxil, Pexeva, and others), sertraline (Zoloft and others), sildenafil (Viagra and Revatio), venlafaxine (Effexor and others), vilazodone (Viibryd).
Author affiliations: Clinical Data, Inc, New Haven, Connecticut (Dr Pierz), and Perelman School of Medicine, University of Pennsylvania, Philadelphia (Dr Thase). Dr Pierz is now with Purdue Pharma, Stamford, Connecticut.
Potential conflicts of interest: Dr Pierz is a former employee of Forest Research Institute, the current sponsor of vilazodone, and of Clinical Data Inc, the former sponsor of vilazodone, and has served as a consultant to Transgenomic Inc. Dr Thase has served as a consultant to or on the advisory boards of AstraZeneca, Bristol-Myers Squibb, Cerecor, Dey, Eli Lilly, Forest, Gerson Lehman Group, GlaxoSmithKline, Guidepoint Global, H. Lundbeck, MedAvante, Merck, Neuronetics, Novartis, Ortho McNeil, Otsuka, PamLab, Pfizer, Shire, Sunovion, Supernus, Takeda, and Transcept; has received grant/research support from Agency for Healthcare Research and Quality, Alkermes, Eli Lilly, Forest, GlaxoSmithKline, National Institute of Mental Health, Otsuka, PharmaNeuroboost, Roche, and Sepracor; has served on the speakers bureaus of AstraZeneca, Bristol-Myers Squibb, Dey, Eli Lilly, Merck, and Pfizer; has equity holdings in MedAvante; and has received royalties from American Psychiatric Association, Guilford Publications, Herald House, and W W Norton & Company. Dr Thase’s spouse is an employee of Embryon (formerly Advogent; Embryon does business with Bristol-Myers Squibb and Pfizer/Wyeth).
Funding/support: Support for this publication was funded by Forest Laboratories, Inc, New York, New York.
Role of the sponsor: Development of this article was supported by Forest Laboratories, Inc, New York, New York.
Acknowledgments: Writing and editorial support for the preparation of the manuscript was provided by Michael L. Miller, PhD, and Adam Ruth, PhD, of Prescott Medical Communications Group, Chicago, Illinois, a contractor of Forest Research Institute. Drs Miller and Ruth have no other conflicts of interest to disclose related to the subject of this article.
REFERENCES
1. Kessler RC, Birnbaum H, Bromet E, et al. Age differences in major depression: results from the National Comorbidity Survey Replication (NCS-R). Psychol Med. 2010;40(2):225-237. PubMed doi:10.1017/S0033291709990213
2. Hirschfeld RM. The epidemiology of depression and the evolution of treatment. J Clin Psychiatry. 2012;73(suppl 1):5-9. PubMed doi:10.4088/JCP.11096su1c.01
3. Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163-2196. PubMed doi:10.1016/S0140-6736(12)61729-2
4. Stafford RS, Ausiello JC, Misra B, et al. National patterns of depression treatment in primary care. Prim Care Companion J Clin Psychiatry. 2000;2(6):211-216. PubMed doi:10.4088/PCC.v02n0603
5. Unützer J, Park M. Strategies to improve the management of depression in primary care. Prim Care. 2012;39(2):415-431. PubMed doi:10.1016/j.pop.2012.03.010
6. Wang PS, Demler O, Olfson M, et al. Changing profiles of service sectors used for mental health care in the United States. Am J Psychiatry. 2006;163(7):1187-1198. PubMed doi:10.1176/appi.ajp.163.7.1187
7. Hirschfeld RM, Keller MB, Panico S, et al. The National Depressive and Manic-Depressive Association consensus statement on the undertreatment of depression. JAMA. 1997;277(4):333-340. PubMed doi:10.1001/jama.1997.03540280071036
8. Ferguson JM. SSRI antidepressant medications: adverse effects and tolerability. Prim Care Companion J Clin Psychiatry. 2001;3(1):22-27. PubMed doi:10.4088/PCC.v03n0105
9. Remick RA, Froese C, Keller FD. Common side effects associated with monoamine oxidase inhibitors. Prog Neuropsychopharmacol Biol Psychiatry. 1989;13(3-4):497-504. PubMed doi:10.1016/0278-5846(89)90137-1
10. Wimbiscus M, Kostenko O, Malone D. MAO inhibitors: risks, benefits, and lore. Cleve Clin J Med. 2010;77(12):859-882. PubMed doi:10.3949/ccjm.77a.09103
11. Flockhart DA. Dietary restrictions and drug interactions with monoamine oxidase inhibitors: an update. J Clin Psychiatry. 2012;73(suppl 1):17-24. PubMed doi:10.4088/JCP.11096su1c.03
12. Mark TL, Levit KR, Buck JA. Datapoints: psychotropic drug prescriptions by medical specialty. Psychiatr Serv. 2009;60(9):1167. PubMed doi:10.1176/appi.ps.60.9.1167
13. Blier P, Saint-André E, Hébert C, et al. Effects of different doses of venlafaxine on serotonin and norepinephrine reuptake in healthy volunteers. Int J Neuropsychopharmacol. 2007;10(1):41-50. PubMed doi:10.1017/S1461145705006395
14. Stahl SM, Grady MM, Moret C, et al. SNRIs: their pharmacology, clinical efficacy, and tolerability in comparison with other classes of antidepressants. CNS Spectr. 2005;10(9):732-747. PubMed
15. Thase ME. Are SNRIs more effective than SSRIs? a review of the current state of the controversy. Psychopharmacol Bull. 2008;41(2):58-85. PubMed
16. Gartlehner G, Gaynes BN, Hansen RA, et al. Comparative benefits and harms of second-generation antidepressants: background paper for the American College of Physicians. Ann Intern Med. 2008;149(10):734-750. PubMed doi:10.7326/0003-4819-149-10-200811180-00008
17. Gaynes BN, Dusetzina SB, Ellis AR, et al. Treating depression after initial treatment failure: directly comparing switch and augmenting strategies in STAR*D. J Clin Psychopharmacol. 2012;32(1):114-119. PubMed doi:10.1097/JCP.0b013e31823f705d
18. Kornstein SG, Li D, Mao Y, et al. Escitalopram versus SNRI antidepressants in the acute treatment of major depressive disorder: integrative analysis of four double-blind, randomized clinical trials. CNS Spectr. 2009;14(6):326-333. PubMed
19. Kennedy SH, Andersen HF, Thase ME. Escitalopram in the treatment of major depressive disorder: a meta-analysis. Curr Med Res Opin. 2009;25(1):161-175. PubMed doi:10.1185/03007990802622726
20. Frampton JE. Vilazodone: in major depressive disorder. CNS Drugs. 2011;25(7):615-627. PubMed doi:10.2165/11207550-000000000-00000
21. Coppen A. The biochemistry of affective disorders. Br J Psychiatry. 1967;113(504):1237-1264. PubMed doi:10.1192/bjp.113.504.1237
22. Coppen A, Shaw DM, Malleson A, et al. Tryptamine metabolism in depression. Br J Psychiatry. 1965;111(479):993-998. PubMed doi:10.1192/bjp.111.479.993
23. Prange AJ Jr. The pharmacology and biochemistry of depression. Dis Nerv Syst. 1964;25:217-221. PubMed
24. Owens MJ. Selectivity of antidepressants: from the monoamine hypothesis of depression to the SSRI revolution and beyond. J Clin Psychiatry. 2004;65(suppl 4):5-10. PubMed
25. Stahl SM. Mechanism of action of serotonin selective reuptake inhibitors: serotonin receptors and pathways mediate therapeutic effects and side effects. J Affect Disord. 1998;51(3):215-235. PubMed doi:10.1016/S0165-0327(98)00221-3
26. Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev Med. 2009;60(1):355-366. PubMed doi:10.1146/annurev.med.60.042307.110802
27. Eisch AJ, Petrik D. Depression and hippocampal neurogenesis: a road to remission? Science. 2012;338(6103):72-75. PubMed doi:10.1126/science.1222941
28. Carr GV, Lucki I. The role of serotonin receptor subtypes in treating depression: a review of animal studies. Psychopharmacology (Berl). 2011;213(2-3):265-287. PubMed doi:10.1007/s00213-010-2097-z
29. Pytliak M, Vargová V, Mech×rová V, et al. Serotonin receptors—from molecular biology to clinical applications. Physiol Res. 2011;60(1):15-25. PubMed
30. McDevitt RA, Neumaier JF. Regulation of dorsal raphe nucleus function by serotonin autoreceptors: a behavioral perspective. J Chem Neuroanat. 2011;41(4):234-246. PubMed doi:10.1016/j.jchemneu.2011.05.001
31. Savitz J, Lucki I, Drevets WC. 5-HT(1A) receptor function in major depressive disorder. Prog Neurobiol. 2009;88(1):17-31. PubMed doi:10.1016/j.pneurobio.2009.01.009
32. Drevets WC, Thase ME, Moses-Kolko EL, et al. Serotonin-1A receptor imaging in recurrent depression: replication and literature review. Nucl Med Biol. 2007;34(7):865-877. PubMed doi:10.1016/j.nucmedbio.2007.06.008
33. Sargent PA, Kjaer KH, Bench CJ, et al. Brain serotonin 1A receptor binding measured by positron emission tomography with [11C]WAY-100635: effects of depression and antidepressant treatment. Arch Gen Psychiatry. 2000;57(2):174-180. PubMed doi:10.1001/archpsyc.57.2.174
34. Stahl S. 5-HT1A receptors and pharmacotherapy: is serotonin receptor down-regulation linked to the mechanism of action of antidepressant drugs? Psychopharmacol Bull. 1994;30(1):39-43. PubMed
35. Blier P, Ward NM. Is there a role for 5-HT1A agonists in the treatment of depression? Biol Psychiatry. 2003;53(3):193-203. PubMed doi:10.1016/S0006-3223(02)01643-8
36. Blier P, de Montigny C. Possible serotonergic mechanisms underlying the antidepressant and anti-obsessive-compulsive disorder responses. Biol Psychiatry. 1998;44(5):313-323. PubMed doi:10.1016/S0006-3223(98)00114-0
37. Celada P, Puig M, Amargós-Bosch M, et al. The therapeutic role of 5-HT1A and 5-HT2A receptors in depression. J Psychiatry Neurosci. 2004;29(4):252-265. PubMed
38. Blier P. Pharmacology of rapid-onset antidepressant treatment strategies. J Clin Psychiatry. 2001;62(suppl 15):12-17. PubMed
39. Artigas F, Adell A, Celada P. Pindolol augmentation of antidepressant response. Curr Drug Targets. 2006;7(2):139-147. PubMed doi:10.2174/138945006775515446
40. Martiny K, Lunde M, Bech P, et al. A short-term double-blind randomized controlled pilot trial with active or placebo pindolol in patients treated with venlafaxine for major depression. Nord J Psychiatry. 2012;66(3):147-154. PubMed doi:10.3109/08039488.2012.674553
41. Khan A, Cutler AJ, Kajdasz DK, et al. A randomized, double-blind, placebo-controlled, 8-week study of vilazodone, a serotonergic agent for the treatment of major depressive disorder. J Clin Psychiatry. 2011;72(4):441-447. PubMed doi:10.4088/JCP.10m06596
42. Trivedi MH, Fava M, Wisniewski SR, et al; STAR*D Study Team. Medication augmentation after the failure of SSRIs for depression. N Engl J Med. 2006;354(12):1243-1252. PubMed doi:10.1056/NEJMoa052964
43. Gelenberg AJ, Freeman MP, Markowitz JC, et al. Practice Guideline for the Treatment of Patients With Major Depressive Disorder, Third Edition. Arlington, VA: American Psychiatric Association. http://psychiatryonline.org/content.aspx?bookid=28§ionid=1667485#657567. Accessed October 14, 2013.
44. Bouwer C, Stein DJ. Buspirone is an effective augmenting agent of serotonin selective reuptake inhibitors in severe treatment-refractory depression. S Afr Med J. 1997;87(suppl):534-537, 540. PubMed
45. Jacobsen FM. Possible augmentation of antidepressant response by buspirone. J Clin Psychiatry. 1991;52(5):217-220. PubMed
46. Joffe RT, Schuller DR. An open study of buspirone augmentation of serotonin reuptake inhibitors in refractory depression. J Clin Psychiatry. 1993;54(7):269-271. PubMed
47. Appelberg BG, Syvפlahti EK, Koskinen TE, et al. Patients with severe depression may benefit from buspirone augmentation of selective serotonin reuptake inhibitors: results from a placebo-controlled, randomized, double-blind, placebo wash-in study. J Clin Psychiatry. 2001;62(6):448-452. PubMed doi:10.4088/JCP.v62n0608
48. Onder E, Tural U. Faster response in depressive patients treated with fluoxetine alone than in combination with buspirone. J Affect Disord. 2003;76(1-3):223-227. PubMed doi:10.1016/S0165-0327(02)00090-3
49. Rush AJ, Fava M, Wisniewski SR, et al; STAR*D Investigators Group. Sequenced Treatment Alternatives to Relieve Depression (STAR*D): rationale and design. Control Clin Trials. 2004;25(1):119-142. PubMed doi:10.1016/S0197-2456(03)00112-0
50. Portella MJ, de Diego-Adeli×±o J, Ballesteros J, et al. Can we really accelerate and enhance the selective serotonin reuptake inhibitor antidepressant effect? a randomized clinical trial and a meta-analysis of pindolol in nonresistant depression. J Clin Psychiatry. 2011;72(7):962-969. PubMed doi:10.4088/JCP.09m05827blu
51. Zanardi R, Artigas F, Franchini L, et al. How long should pindolol be associated with paroxetine to improve the antidepressant response? J Clin Psychopharmacol. 1997;17(6):446-450. PubMed doi:10.1097/00004714-199712000-00002
52. Bordet R, Thomas P, Dupuis B. Effect of pindolol on onset of action of paroxetine in the treatment of major depression: intermediate analysis of a double-blind, placebo-controlled trial: Réseau de Recherche et d’ Expérimentation Psychopharmacologique. Am J Psychiatry. 1998;155(10):1346-1351. PubMed
53. Maes M, Libbrecht I, van Hunsel F, et al. Pindolol and mianserin augment the antidepressant activity of fluoxetine in hospitalized major depressed patients, including those with treatment resistance. J Clin Psychopharmacol. 1999;19(2):177-182. PubMed doi:10.1097/00004714-199904000-00014
54. Geretsegger C, Bitterlich W, Stelzig R, et al. Paroxetine with pindolol augmentation: a double-blind, randomized, placebo-controlled study in depressed in-patients. Eur Neuropsychopharmacol. 2008;18(2):141-146. PubMed doi:10.1016/j.euroneuro.2007.09.002
55. Pérez V, Gilaberte I, Faries D, et al. Randomised, double-blind, placebo-controlled trial of pindolol in combination with fluoxetine antidepressant treatment. Lancet. 1997;349(9065):1594-1597. PubMed doi:10.1016/S0140-6736(96)08007-5
56. Portella MJ, de Diego-Adeli×±o J, Puigdemont D, et al. Pindolol augmentation enhances response outcomes in first depressive episodes. Eur Neuropsychopharmacol. 2009;19(7):516-519. PubMed doi:10.1016/j.euroneuro.2009.04.004
57. Berman RM, Anand A, Cappiello A, et al. The use of pindolol with fluoxetine in the treatment of major depression: final results from a double-blind, placebo-controlled trial. Biol Psychiatry. 1999;45(9):1170-1177. PubMed doi:10.1016/S0006-3223(98)00383-7
58. Berman RM, Darnell AM, Miller HL, et al. Effect of pindolol in hastening response to fluoxetine in the treatment of major depression: a double-blind, placebo-controlled trial. Am J Psychiatry. 1997;154(1):37-43. PubMed
59. Pérez V, Soler J, Puigdemont D, et al. A double-blind, randomized, placebo-controlled trial of pindolol augmentation in depressive patients resistant to serotonin reuptake inhibitors: Grup de Recerca en Trastorns Afectius. Arch Gen Psychiatry. 1999;56(4):375-379. PubMed doi:10.1001/archpsyc.56.4.375
60. Perry EB, Berman RM, Sanacora G, et al. Pindolol augmentation in depressed patients resistant to selective serotonin reuptake inhibitors: a double-blind, randomized, controlled trial. J Clin Psychiatry. 2004;65(2):238-243. PubMed doi:10.4088/JCP.v65n0215
61. Segrave R, Nathan PJ. Pindolol augmentation of selective serotonin reuptake inhibitors: accounting for the variability of results of placebo-controlled double-blind studies in patients with major depression. Hum Psychopharmacol. 2005;20(3):163-174. PubMed doi:10.1002/hup.672
62. Ballesteros J, Callado LF. Effectiveness of pindolol plus serotonin uptake inhibitors in depression: a meta-analysis of early and late outcomes from randomised controlled trials. J Affect Disord. 2004;79(1-3):137-147. PubMed doi:10.1016/S0165-0327(02)00404-4
63. Whale R, Terao T, Cowen P, et al. Pindolol augmentation of serotonin reuptake inhibitors for the treatment of depressive disorder: a systematic review. J Psychopharmacol. 2010;24(4):513-520. PubMed doi:10.1177/0269881108097714
64. Viibryd [package insert]. St Louis, MO: Forest Pharmaceuticals Inc; 2012.
65. Ashby CR Jr, Kehne JH, Bartoszyk GD, et al. Electrophysiological evidence for rapid 5-HT1A autoreceptor inhibition by vilazodone, a 5-HT1A receptor partial agonist and 5-HT reuptake inhibitor. Eur J Pharmacol. 2013;714(1-3):359-365. PubMed doi:10.1016/j.ejphar.2013.07.014
66. Page ME, Cryan JF, Sullivan A, et al. Behavioral and neurochemical effects of 5-(4-[4-(5-Cyano-3-indolyl)-butyl)-butyl]-1-piperazinyl)-benzofuran-2-carboxamide (EMD 68843): a combined selective inhibitor of serotonin reuptake and 5-hydroxytryptamine(1A) receptor partial agonist. J Pharmacol Exp Ther. 2002;302(3):1220-1227. PubMed doi:10.1124/jpet.102.034280
67. Hughes ZA, Starr KR, Langmead CJ, et al. Neurochemical evaluation of the novel 5-HT1A receptor partial agonist/serotonin reuptake inhibitor, vilazodone. Eur J Pharmacol. 2005;510(1-2):49-57. PubMed doi:10.1016/j.ejphar.2005.01.018
68. Rickels K, Athanasiou M, Robinson DS, et al. Evidence for efficacy and tolerability of vilazodone in the treatment of major depressive disorder: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2009;70(3):326-333. PubMed doi:10.4088/JCP.08m04637
69. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association; 2000.
70. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134(4):382-389. PubMed doi:10.1192/bjp.134.4.382
71. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56-62. PubMed doi:10.1136/jnnp.23.1.56
72. Jain R, Chen D, Edwards J, et al. Early and sustained improvement with vilazodone in adult patients with major depressive disorder: post hoc analyses of two phase III trials [published online ahead of print October 31, 2013]. Curr Med Res Opin. PubMed doi:10.1185/03007995.2013.855188</jrn>
73. Citrome L. Vilazodone for major depressive disorder: a systematic review of the efficacy and safety profile for this newly approved antidepressant: what is the number needed to treat, number needed to harm and likelihood to be helped or harmed? Int J Clin Pract. 2012;66(4):356-368. PubMed doi:10.1111/j.1742-1241.2011.02885.x</jrn>
74. Clayton AH, Pradko JF, Croft HA, et al. Prevalence of sexual dysfunction among newer antidepressants. J Clin Psychiatry. 2002;63(4):357-366. PubMed doi:10.4088/JCP.v63n0414
75. Clayton A, Kornstein S, Prakash A, et al. Changes in sexual functioning associated with duloxetine, escitalopram, and placebo in the treatment of patients with major depressive disorder. J Sex Med. 2007;4(4, pt 1):917-929. PubMed doi:10.1111/j.1743-6109.2007.00520.x
76. Clayton AH, Kennedy SH, Edwards JB, et al. The effect of vilazodone on sexual function during the treatment of major depressive disorder. J Sex Med. 2013 Oct;10(10):2465-2476. PubMed doi: 10.1111/jsm.12004
77. Norden M. Buspirone treatment of sexual dysfunction associated with selective serotonin reuptake inhibitors. Depression. 1994;2(2):109-112. doi:10.1002/depr.3050020208
78. Landén M, Eriksson E, Agren H, et al. Effect of buspirone on sexual dysfunction in depressed patients treated with selective serotonin reuptake inhibitors. J Clin Psychopharmacol. 1999;19(3):268-271. PubMed doi:10.1097/00004714-199906000-00012
79. Michelson D, Bancroft J, Targum S, et al. Female sexual dysfunction associated with antidepressant administration: a randomized, placebo-controlled study of pharmacologic intervention. Am J Psychiatry. 2000;157(2):239-243. PubMed doi:10.1176/appi.ajp.157.2.239
80. Othmer E, Othmer SC. Effect of buspirone on sexual dysfunction in patients with generalized anxiety disorder. J Clin Psychiatry. 1987;48(5):201-203. PubMed
81. Laughren TP, Gobburu J, Temple RJ, et al. Vilazodone: clinical basis for the US Food and Drug Administration’s approval of a new antidepressant. J Clin Psychiatry. 2011;72(9):1166-1173. PubMed doi:10.4088/JCP.11r06984
82. Fava M, Rush AJ, Alpert JE, et al. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Am J Psychiatry. 2008;165(3):342-351. PubMed doi:10.1176/appi.ajp.2007.06111868