ABSTRACT
Objective: Improvement of cognitive function in patients with major depressive disorder (MDD) is an important treatment outcome. REL-1017 (esmethadone HCl) is a novel N-methyl-d-aspartate receptor (NMDAR) channel blocker and a potentially rapidly acting antidepressant. The objective of this study was to define the effects of REL-1017 on subjective cognitive measures in patients with MDD.
Methods: Post hoc analysis was conducted of subjective cognitive measures from the Montgomery-Asberg Depression Rating Scale (MADRS) and the Symptoms of Depression Questionnaire (SDQ) from a randomized, double-blind, placebo-controlled, Phase 2a study. The study, designed to assess the safety, tolerability, and efficacy of 2 dosages (25 mg and 50 mg) of REL-1017 as an adjunctive treatment in patients with MDD unresponsive to standard antidepressants, included 62 patients. We analyzed subjective cognitive measures derived from the MADRS and SDQ scales at baseline and up to day 14, 7 days after the last dose of study drug. We developed 2 composite indexes that included subjective cognitive measures selected from the MADRS and SDQ.
Results: The subanalysis of single measures and the 2 composite indexes derived from the MADRS and SDQ measures showed clinically meaningful and statistically significant improvements in cognitive function (P < .05).
Conclusions: In a Phase 2a clinical trial, REL-1017 improved subjective measures of cognitive impairment, in addition to improving total MADRS and SDQ scores. These results need to be confirmed in larger and longer studies in MDD that include objective measures of cognitive function. Phase 3 studies of REL-1017 for MDD are currently underway.
Clinical Trials Registration: ClinicalTrials.gov identifier: NCT03051256
Prim Care Companion CNS Disord 2023;25(1):22m03267
To cite: Guidetti C, Serra G, Pani L, et al. Subanalysis of subjective cognitive measures from a phase 2, double-blind, randomized trial of REL-1017 in patients with major depressive disorder. Prim Care Companion CNS Disord. 2023;25(1):22m03267.
To share: https://doi.org/10.4088/PCC.22m03267
© 2023 Physicians Postgraduate Press, Inc.
aChild and Adolescent Neuropsychiatry Unit, Department of Neuroscience, Bambino Pediatric Hospital, IRCCS, Rome, Italy
bDepartment of Psychiatry and Behavioral Sciences, University of Miami, School of Medicine, Miami, Florida
cRelmada Therapeutics, Coral Gables, Florida
dDepartment of Anesthesiology, Albert Einstein College of Medicine, Bronx, New York
eDepartment of Pharmaceutical and Pharmacological Sciences, University of Padova, Padova, Italy
fDepartment of Health Science, University of Milan, Milan, Italy
gDepartment of Psychiatry, Massachusetts General Hospital, Boston, Massachusetts
*Corresponding author: Paolo L. Manfredi, MD, Relmada Therapeutics, 2222 Ponce de Leon Blvd Floor 3, Coral Gables, FL 33134 ([email protected]).
Major depressive disorder (MDD) is a prevalent, chronic, and highly disabling multidimensional psychiatric disorder representing a growing public health issue. MDD is the second leading cause of disability and chronic disease burden in the United States, among all medical conditions, as measured by “disability-adjusted life years.”1 By 2030, MDD is projected to become the leading cause of global disease burden according to the World Health Organization (WHO).2 Data from the National Epidemiologic Survey on Alcohol and Related Conditions III (NESARC-III) show that the 12-month prevalence and the lifetime prevalence of MDD were 10.4% and 20.6%, respectively, with most patients experiencing moderate (6 to 7 symptoms) or severe (8 to 9 symptoms) depression associated with comorbidity and functional impairment.3
MDD is a heterogeneous condition with a broad constellation of symptoms including cognitive/motivational, anxiety/irritability, and sleep domains, all of which heavily impact quality of life and global functioning. Many studies4 have shown that cognitive dysfunction is an important predictor of occupational and social functional impairment in adults with MDD. The importance of rapidly improving MDD functional outcomes, including cognitive measures, is increasingly recognized because the improvement of subjective cognitive impairment may be as important as the improvement of mood for patients with MDD.5
Up to 50%–60% of MDD patients do not achieve adequate response following the first antidepressant treatment.6 Moreover, the response to antidepressants is delayed by 4 to 8 weeks after initiation.7 Atypical antipsychotics are US Food and Drug Administration (FDA)–approved as adjunctive treatment of MDD for those patients with inadequate response to antidepressants, although they have modest efficacy and neurologic and metabolic side effects.8
N-methyl-d-aspartate receptor (NMDAR) channel blockers are emerging as a new drug class with potentially rapid and effective antidepressant activity.9,10 Esketamine was recently approved by the FDA as an adjunctive treatment for MDD in patients with treatment-resistant depression, having been shown to be efficacious in MDD in a recent meta-analysis.11 Due to poor oral absorption, esketamine is administered intranasally. Furthermore, esketamine causes temporary dissociative side effects and changes in blood pressure, requiring monitoring after administration.12 The uncompetitive NMDAR blockers, memantine and the dextromethorphan-quinidine combination, have been approved by the FDA to treat Alzheimer’s disease and pseudobulbar affect, respectively. Dextromethorphan, another uncompetitive NMDAR channel blocker, in combination with bupropion, is in clinical development for the treatment of MDD.13
REL-1017 (esmethadone HCl, d-methadone HCl, dextromethadone HCl, (+)-methadone) is the opioid-inactive dextro-isomer of racemic methadone and a novel NMDAR channel blocker, with a preference for GluN1-GluN2D NMDAR subtypes.14,15 NMDARs are known to have a central role in directing neural plasticity.16,17 Impaired neural plasticity due to dysregulation of NMDARs has been implicated in a multiplicity of neuropsychiatric disorders and may play an important role in MDD.18 REL-1017 demonstrated rapid antidepressant-like effects in animal models of depressed behavior, including the forced swim test, the novelty-suppressed feeding test, the female-urine sniffing test, and the chronic unpredictable stress paradigm, with efficacy equivalent to ketamine.19,20 REL-1017 increased plasma levels of brain-derived neurotrophic factor (BDNF) in humans21 and induced neural plasticity in experimental models of depression via BDNF-dependent mechanisms.20 REL-1017 showed favorable safety, tolerability, and pharmacokinetic (PK) profiles in Phase 1 studies.22,23 Recently, it has been shown that REL-1017 is not neurotoxic on the central nervous system24 and does not cause withdrawal and dependence symptoms in rats.25 REL-1017 produced rapid, robust, and sustained antidepressant efficacy in a Phase 2 clinical trial in the absence of opioid or psychotomimetic effects.26 REL-1017 is currently undergoing testing in Phase 3 trials for MDD (ClinicalTrials.gov identifiers: NCT04688164, NCT05081167, NCT04855747). This subanalysis examined the potential effects of REL-1017 on subjective cognitive function.
METHODS
Study Design and Patients
We performed a post hoc subanalysis of subjective cognitive measures obtained from a double-blind, placebo-controlled, inpatient, 7-day treatment, 3-arm, Phase 2a trial (ClinicalTrials.gov identifier: NCT03051256).26 The safety, tolerability, and efficacy of 2 dosages of REL-1017 (25 mg or 50 mg/d once a day) as adjunctive treatment were examined in patients with MDD unresponsive to serotonergic antidepressants.
Assessments
The efficacy measures used in this study were select items from the Montgomery-Asberg Depression Rating Scale (MADRS) and the Symptoms of Depression Questionnaire (SDQ), respectively, which were the first and one of the secondary endpoints of the Phase 2 trial. The MADRS is a clinician-reported instrument frequently used to measure outcome in antidepressant efficacy studies.27 This 10-item scale evaluates core symptoms of depression, scored from 0 (symptom not present or normal) to 6 (severe or continuous presence of the symptom). A higher MADRS score indicates more severe depression. The overall score ranges from 0 to 60 with scores above 34 indicating severe depression. The SDQ is a 44-item self-report scale intended to capture the broad heterogeneity of symptoms of MDD.28 The SDQ items can be analyzed as a total score and can also be subdivided into 5 subscales. Subscale 1 measures common dimensions of depressive symptoms including lassitude, energy, mood, and cognitive and social functioning. Subscale 2 measures anxiety, anger, irritability, and agitation. Subscale 3 includes items on suicide, self-harm, and worthlessness. Subscales 4 and 5 measure physiologic features of depression, namely sleep difficulties and changes in appetite/weight, respectively. Each item is rated on a 6-point scale. Each item is rated based on a subject’s perception of what is normal for the individual (score = 2), what is better than normal (score = 1), and what is worse than normal (scores = 3–6).
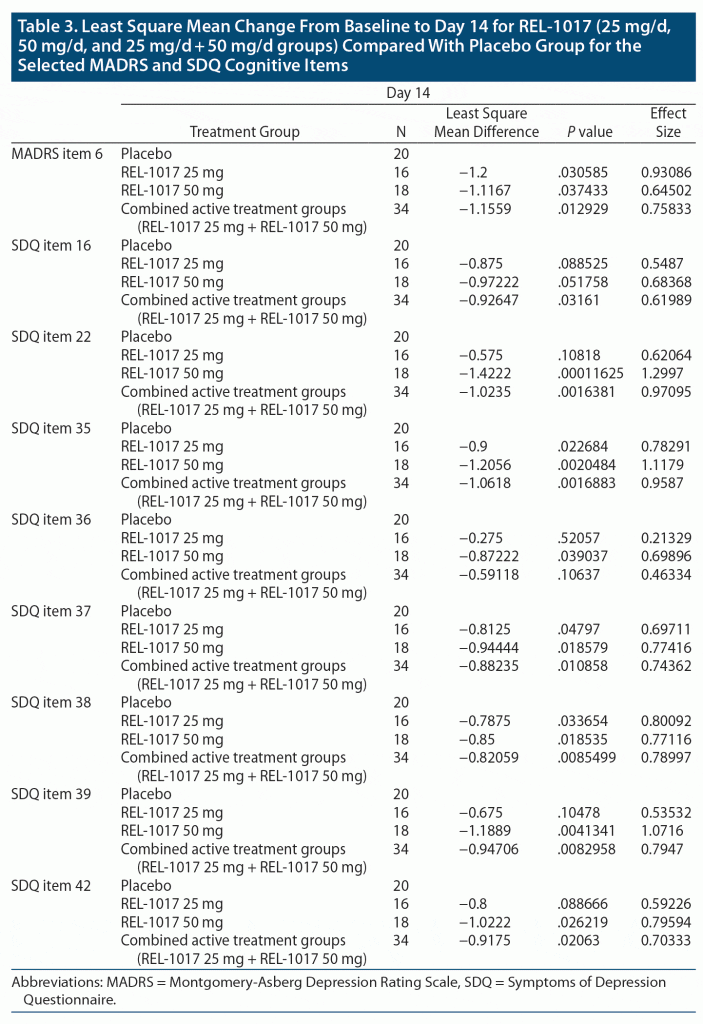
The MADRS and SDQ were administered predose on day 1 (at baseline) and on day 2, postdose on day 4 and on day 7 within 3 hours of dosing, and on day 14 (end of observation period). The MADRS was administered according to the Structured Interview Guide for the MADRS.29 Clinician-rated cognitive symptoms were assessed from item 6 of the MADRS, which measures subjective concentration difficulties. Patient-reported subjective cognitive symptoms were assessed using 8 SDQ items: item 16 (wakefulness), item 22 (slowed down feeling), item 35 (ability to focus/sustain attention), item 36 (ability to remember/recall information), item 37 (ability to find words), item 38 (sharpness/mental acuity), item 39 (ability to make decisions), and item 42 (ability to work/study/function at home), which are all included in SDQ subscale 1.
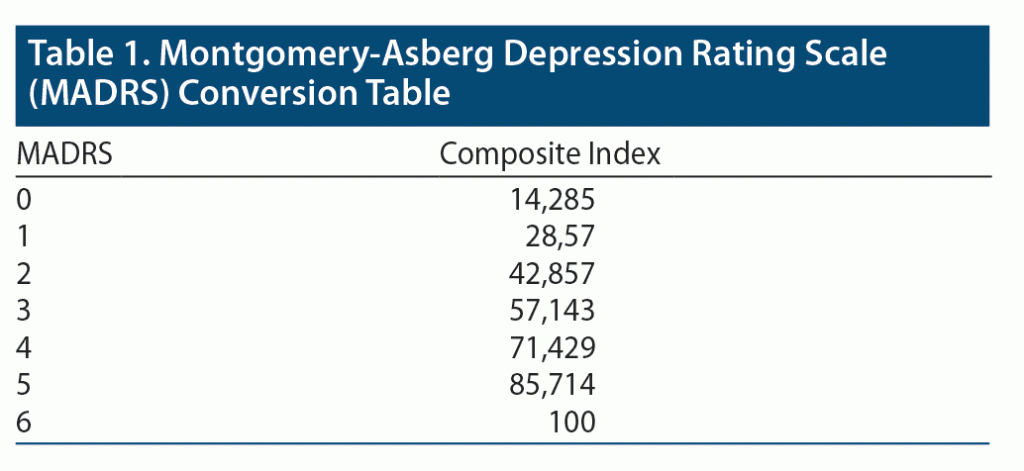
As an additional analysis of measures of overall subjective cognitive function, we also developed 2 SDQ/MADRS Composite Cognitive Indexes. To make the 2 measures comparable between each other, we converted the MADRS and SDQ score data (collected singularly from every person in each reporting day) following these rules: CI = (MADRS +1) * (100/7) CI = (SDQ −1) * (100/5).
Table 1 and Table 2 compare the 2 scales with the corresponding index values. The conversion aims at making the 2 scales comparable to synthesize a larger amount of data in a single composite index. The Composite Cognitive Index 1 is the equally weighted mean of the cognitive item scores from the 8 SDQ items and item 6 from MADRS. The Composite Cognitive Index 2 is computed by applying a 0.5 weight to the single MADRS question values and 0.5 weight to the mean values of the 8 SDQ items.
Statistical Analysis
Statistical analyses were performed using MATLAB software, version R2019. The change from baseline of the single cognitive items of the MADRS and the SDQ scores and of the Composite Cognitive Indexes 1 and 2 on day 14 was compared for placebo versus REL-1017 active treatment groups, 25 mg and 50 mg (analyzed separately and combined), using a mixed model analysis as described by Fava et al.26 The likelihood-based method applied was the mixed-effect model repeated measures with fixed-effect terms for treatment, visit (day 2, day 4, day 7, day 14), and the interaction between treatment and visit. The least square (LS) mean difference (between REL-1017 and placebo) was provided along with the P value for testing the null hypothesis and the Cohen’s effect size (calculated based on the LS mean differences and the pooled standard deviations). An inferential statistical analysis was applied to each treatment group (REL-1017 25 mg group, N = 16; REL-1017 50 mg group, N = 18) and to the combined active treatment sample (N = 34: REL-1017 25 mg group, N = 16 + REL-1017 50 mg group N = 18).
RESULTS
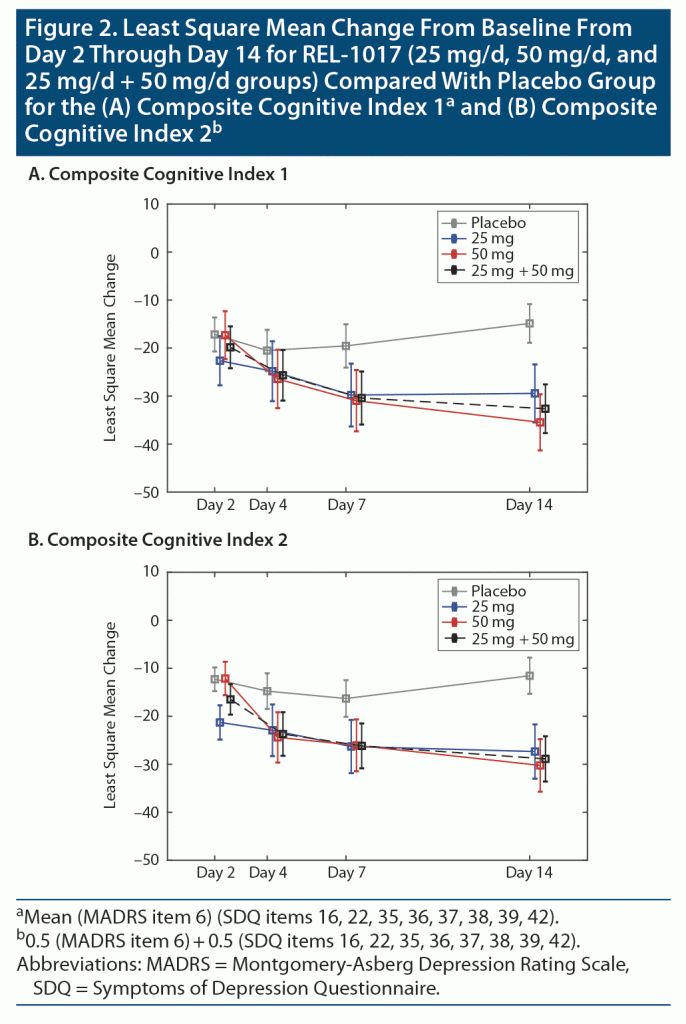
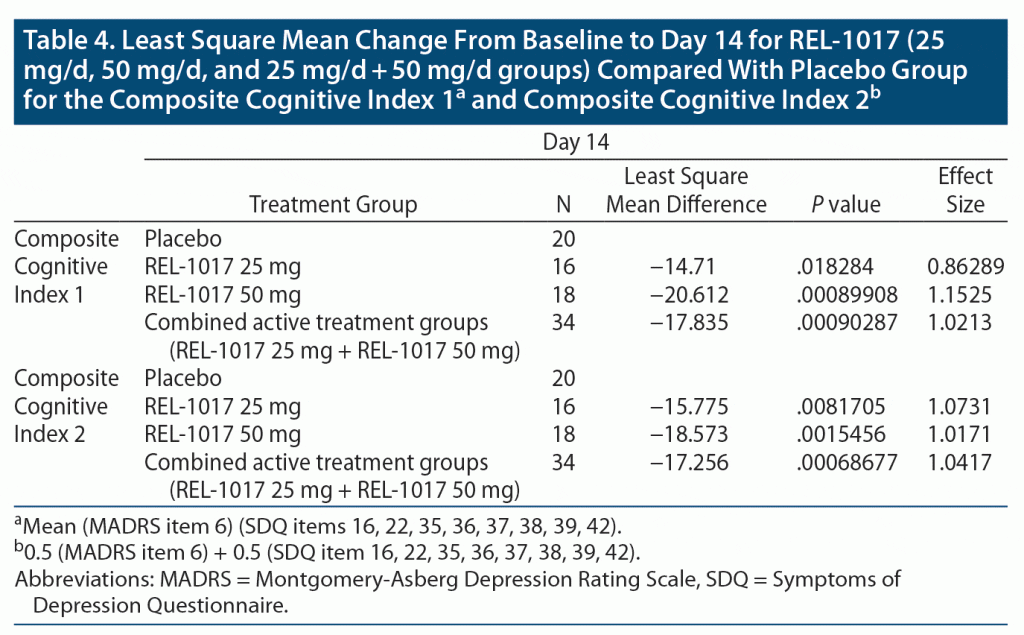
Several measures indicated marked difference in the subjective cognitive measures between patients treated with REL-1017 versus placebo. These changes were consistently in the direction of less severe cognitive symptoms in patients treated with REL-1017 on day 14, 7 days after treatment discontinuation. Statistically significant differences in the change from baseline were observed on day 14 for patients in the REL-1017 25 mg group compared to the placebo group on “concentration difficulties” (item 6 of the MADRS, P = .03, Table 3, Figure 1 panel A), “ability to focus/sustain attention” (item 35 of the SDQ, P = .02, Table 3, Figure 1 panel D), “ability to remember/find words” (item 37 of the SDQ, P = .04, Table 3, Figure 1 panel F), “sharpness/mental acuity” (item 38 of the SDQ, P = .03, Table 3, Figure 1 panel G), and the Composite Cognitive Index 1 and 2 (P = .01, Table 4, Figure 2A and P = .008, Table 2, Figure 2B, respectively).
REL-1017 50 mg was found to be superior to placebo for improving cognitive symptoms on day 14 as rated with the MADRS item 6 “concentration difficulties” (P = .03, Figure 1 Panel A), with all the cognitive items of the SDQ (items 16, 22, 35–39, 42; Table 3; Figure 1 panels B–I), and with the Composite Cognitive Index 1 and 2 (P = .0008, Table 4, Figure 2A and P = .001, Table 4, Figure 2B, respectively).
Also, in the combined REL-1017 25 mg + 50 mg group, REL1017 was found to be superior to placebo for improving cognitive symptoms as rated with the MADRS item 6 “concentration difficulties” (P = .01, Figure 1 Panel A), with all the cognitive items of the SDQ (items 16, 22, 35–39, 42; Table 3 and Figure 1 panels B–I), and with the Composite Cognitive Index 1 and 2 (P = .0009, Table 4, Figure 2A and P = .0006, Table 4, Figure 2B, respectively).
Moreover, as previously reported,26 there were clinically meaningful and statistically significant improvements on total MADRS score starting on day 4 in both REL-1017 treatment groups, and the improvement continued through day 7 and day 14, 7 days after study drug discontinuation. For the SDQ total score and subscales 1, 2, and 4, clinically meaningful and statistically significant improvements were shown on day 14 in both REL-1017 groups, as described in detail by Fava and colleagues.26
DISCUSSION
The mechanism of action of NMDAR uncompetitive antagonists in MDD may be related to the restoration of glutamatergic neural plasticity. Because of the relevance of cognitive symptomatology in patients with MDD, we performed a post hoc analysis of select MADRS and SDQ items that may inform on the effects of the novel uncompetitive NMDAR antagonist REL-1017 on subjective measures of cognitive function.
Emerging data on impaired neural plasticity as the potential pathophysiologic basis for MDD18,30 and the use of glutamatergic modulators for the treatment of MDD,9 taken together with the established pivotal role of NMDARs in neural plasticity,16,17 underscore the importance of assessing cognitive domains in MDD. This subanalysis of MADRS and SDQ data suggests that the rapid, robust, and sustained therapeutic effects of REL-1017 for MDD are not limited to improving mood but extend to subjective cognitive domains. These improvements in subjective cognitive measures support the postulated mechanism of action for REL-1017 as a promising rapid-acting antidepressant candidate that may restore impaired neural plasticity in patients with MDD and may signal disease-modifying potential for REL-1017 and other uncompetitive NMDAR channel blockers effective for MDD. Additionally, MDD symptomatology improvements extending to cognitive, motivational, anxiety, irritability, and sleep function as shown by our subanalysis and Fava and colleagues26 may have meaningful implications for patient quality of life and functioning, including working and social abilities, with a potentially positive socioeconomic impact. A better definition of the different areas of impairment may improve our understanding of the mechanism of disease of MDD and its impact on patient quality of life and function and the development of targeted interventions.
Among the domains affected in depression, cognition is an extremely important dimension related to loss of human capital. Cognitive impairment has been reported to affect function independent of mood symptoms and has been correlated with functional impairments. A significant percentage of patients successfully achieving conventional symptomatic remission do not return to premorbid functioning and experience decreases in working abilities and productivity.31 Clinical presentation of MDD, as defined by the DSM-5, includes cognitive impairment as a criterion item of a major depressive episode (MDE).32 Domain-specific cognitive deficits in learning and memory, executive function, processing speed, and attention and concentration are highly replicated findings in patients with MDD presenting with an MDE.33 It is important to note that improvements in mood may not translate into functional recovery.34
When treating MDD, cognitive impairments may persist during periods of symptomatic improvement, suggesting a lack of direct correlation between emotional and functional improvement.31 The persistence of cognitive impairments after remission of depressive symptoms has been shown to contribute to the failure in achieving full functional recovery in MDD.34,35 While efficacy testing of antidepressant drugs has generally focused on mood symptoms, emerging evidence indicates that a substantial percentage of patients may continue to exhibit clinically significant cognitive deficits that impact functional capacity despite remission of mood symptoms.4,36 The inherent disconnect between mood and functional improvements indicates that antidepressants should ideally also target functionally relevant domains in addition to mood symptoms to provide a successful long-term remission and a global functional recovery in MDD.
The effect of antidepressant therapies on cognitive function remains controversial. Originally, selective serotonin reuptake inhibitors (SSRIs) did not appear to cause cognitive impairment in contrast to tricyclic antidepressants,37 but there is conflicting evidence regarding this side effect, and many patients complain of concentration and memory impairment during their course of therapy with SSRIs.38 A study reported that patients with obsessive-compulsive disorder and MDD, which were treated with SSRIs, experienced statistically significant memory loss after 8 weeks of treatment.39
To date, only 2 antidepressants (ie, duloxetine and vortioxetine) have demonstrated clinically relevant effects on cognitive dysfunction in MDD.35 Vortioxetine has shown clinically relevant improvements in cognitive dysfunction, independent of mood symptoms, in adults aged 18–65 years with MDD.40 Some studies have also shown a procognitive effect of duloxetine in patients with MDD, which may be secondary to the improvement in depressive symptoms.41–43 Additionally, some findings suggest that ketamine treatment may exert beneficial effects on measures of executive functioning in patients with treatment-resistant depression. It is a testable hypothesis that the antisuicide effects of ketamine may be in part mediated by improvements in executive function (eg, impulsivity).44 There is a need to improve measurements and targeting of cognitive dysfunction and overall performance in occupational and functional domains. We analyzed item 6 from the MADRS, which is the one item that is most closely related to subjective cognitive function. This additional MADRS analysis is consistent with the conclusion of the SDQ-1 subscale, suggesting that REL-1017 may induce subjective cognitive improvement.
Finally, therapeutic results that are sustained after treatment discontinuation may signal neural plasticity-related effects that are potentially disease-modifying effects.26 The goal of treatment in MDD is rapid and full functional recovery. Early assessment of MDD patients with standardized tools that evaluate a multiplicity of symptomatic domains and early treatment with drugs that potentially address these same domains may lead to improved functional outcomes.4,45 Integration of emotional assessment with assessments of cognitive/motivational domains may improve our understanding of the pathophysiology of MDD and the potentially wide spectrum of efficacy of NMDAR antagonists, beyond the improvement of mood.
This study has several limitations. First, cognitive function was not assessed with objective neuropsychological tests of executive function, processing speed, attention, and learning and memory, but with subjective cognitive measures. Therefore, further research looking at objective measures of cognitive performance would be helpful to obtain definitive results regarding the efficacy of therapeutics for cognitive impairment in MDD. Other limitations include a short-term follow-up (up to 2 weeks) and a limited sample size. These results need to be replicated in larger and longer Phase 3 studies, which are currently underway and also include objective measures of cognitive function.
Submitted: February 17, 2022; accepted July 22, 2022.
Published online: February 14, 2023.
Relevant financial relationships: Dr Guidetti has received consulting fees from MGGM, LLC. Dr Serra has a contract as a consultant psychiatrist at Bambino Gesu’ Children Hospital, IRCCS, Rome, Italy. Dr Pani has received consulting fees and stock or stock options from Relmada Therapeutics, Inc. Dr Pappagallo has received consulting fees from Relmada Therapeutics, Inc; has patents or patents pending for capsaicin and capsaicin analogues and for 5-HT2A agonists; and has stock or stock options from Acadia. Dr Maglio has a contract as a psychiatrist research fellow, child and adolescent psychiatry unit at Bambino Gesu’ Children Hospital, IRCCS, Rome, Italy. Dr De Martin has received grants and contracts from MGGM, LLC; has provided consulting services for Neuroarbor LLC; has patents or patents pending for esmethadone and its derivatives and for 5-HT2A agonists; and has received support for attending meetings and travel from Aesculapius Farmaceutici SpA. Dr Mattarei has received grants and contracts from MGGM, LLC, has provided consulting services for Neuroarbor LLC, and has patents or patents pending for esmethadone and its derivatives and for 5-HT2A agonists. Dr Folli has received consulting fees and stock or stock options from Relmada Therapeutics, Inc. Dr Manfredi has received consulting fees from Relmada Therapeutics, Inc and has patents or patents pending for esmethadone and its derivatives and for 5-HT2A agonists. Dr Fava’s disclosures can be viewed at https://mghcme.org/app/uploads/2022/04/MF-Disclosures-Lifetime-updated-April-2022.pdf.
Funding/support: This work was supported by Relmada Therapeutics, Inc, Coral Gables, Florida.
Role of the sponsor: Relmada Therapeutics participated in the design and conduct of the study; collection, management, analysis, and interpretation of data; and preparation, review, or approval of the manuscript.
Previous presentation: Poster presented at the Neuroscience Education Institute Congress; November 5, 2021; Colorado Springs, Colorado.
Acknowledgments: Richard Perry, PharmD, Richard Perry Consulting, assisted in the preparation of this manuscript and received fees from Relmada Therapeutics, Inc. Edoardo Chiech, MSc, statistical consultant, received consulting fees from MGGM, LCC. The acknowledged individuals report no other conflicts of interest related to the subject of this article.
Clinical Points
- The efficacy of REL-1017 in treating cognitive symptoms could be related to the neural plasticity mechanism of action with potential disease-modifying effect rather than symptomatic treatment, especially when used early after the onset of MDD.
- Residual cognitive symptoms have been identified as a predictor of poor outcomes when treating depression; accurate measuring of cognitive function may be considered a sign rather than a symptom and may provide the most accurate measurement of severity of MDD and response to treatment.
- A combination of esmethadone with cognitive-behavioral psychotherapy may help patients with MDD experiencing cognitive symptoms feel better.
References (45)

- Vos T, Barber RM, Bell B, et al; Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(9995):743–800. PubMed CrossRef
- Malhi GS, Mann JJ. Depression. Lancet. 2018;392(10161):2299–2312. PubMed CrossRef
- Hasin DS, Sarvet AL, Meyers JL, et al. Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the United States. JAMA Psychiatry. 2018;75(4):336–346. PubMed CrossRef
- Knight MJ, Baune BT. Cognitive dysfunction in major depressive disorder. Curr Opin Psychiatry. 2018;31(1):26–31. PubMed CrossRef
- Zuckerman H, Pan Z, Park C, et al. Recognition and treatment of cognitive dysfunction in major depressive disorder. Front Psychiatry. 2018;9:655. PubMed CrossRef
- Fava M, Davidson KG. Definition and epidemiology of treatment-resistant depression. Psychiatr Clin North Am. 1996;19(2):179–200. PubMed CrossRef
- Miller L, Campo JV. Depression in adolescents. N Engl J Med. 2021;385(5):445–449. PubMed CrossRef
- Zhou X, Keitner GI, Qin B, et al. Atypical antipsychotic augmentation for treatment-resistant depression: a systematic review and network meta-analysis. Int J Neuropsychopharmacol. 2015;18(11):pyv060. PubMed CrossRef
- Henter ID, Park LT, Zarate CA Jr. Novel glutamatergic modulators for the treatment of mood disorders: current status. CNS Drugs. 2021;35(5):527–543. PubMed
- Pochwat B, Pałucha-Poniewiera A, Szewczyk B, et al. NMDA antagonists under investigation for the treatment of major depressive disorder. Expert Opin Investig Drugs. 2014;23(9):1181–1192. PubMed CrossRef
- Papakostas GI, Salloum NC, Hock RS, et al. Efficacy of esketamine augmentation in major depressive disorder: a meta-analysis. J Clin Psychiatry. 2020;81(4):19r12889. PubMed CrossRef
- Molero P, Ramos-Quiroga JA, Martin-Santos R, et al. Antidepressant efficacy and tolerability of ketamine and esketamine: a critical review. CNS Drugs. 2018;32(5):411–420. PubMed CrossRef
- Iosifescu DV, Jones A, O’Gorman C, et al. Efficacy and safety of AXS-05 (dextromethorphan-bupropion) in patients with major depressive disorder: a phase 3 randomized clinical trial (GEMINI). J Clin Psychiatry. 2022;83(4):21m14345. PubMed CrossRef
- Bettini E, Nola S, De Martin S, et al. Esmethadone (REL-1017) reduces glutamate-induced currents in NMDA receptors with the GluN2D subunit. Biol Psychiatry. 2021;89(9):S198–S199. CrossRef
- Gorman AL, Elliott KJ, Inturrisi CE. The d- and l-isomers of methadone bind to the non-competitive site on the N-methyl-d-aspartate (NMDA) receptor in rat forebrain and spinal cord. Neurosci Lett. 1997;223(1):5–8. PubMed CrossRef
- Hansen KB, Yi F, Perszyk RE, et al. Structure, function, and allosteric modulation of NMDA receptors. J Gen Physiol. 2018;150(8):1081–1105. PubMed CrossRef
- Nicoll RA. A brief history of long-term potentiation. Neuron. 2017;93(2):281–290. PubMed CrossRef
- Boku S, Nakagawa S, Toda H, et al. Neural basis of major depressive disorder: beyond monoamine hypothesis. Psychiatry Clin Neurosci. 2018;72(1):3–12. PubMed CrossRef
- Hanania T, Manfredi P, Inturrisi C, et al. The N-methyl-d-aspartate receptor antagonist d-methadone acutely improves depressive-like behavior in the forced swim test performance of rats. Exp Clin Psychopharmacol. 2020;28(2):196–201. PubMed CrossRef
- Fogaça MV, Fukumoto K, Franklin T, et al. N-Methyl-d-aspartate receptor antagonist d-methadone produces rapid, mTORC1-dependent antidepressant effects. Neuropsychopharmacology. 2019;44(13):2230–2238. PubMed CrossRef
- De Martin S, Gabbia D, Folli F, et al. REL-1017 (Esmethadone) increases circulating BDNF levels in healthy subjects of a phase 1 clinical study. Front Pharmacol. 2021;12:671859. PubMed CrossRef
- Henningfield J, Apseloff G, Gorodestsky C, et al. No meaningful opioid abuse liability of REL-1017 (esmethadone; D-methadone), a rapid-acting antidepressant in clinical development: a human abuse potential study. Neuropsychopharmacology. 2021;46(suppl 1):315.
- Bernstein G, Davis K, Mills C, et al. Characterization of the safety and pharmacokinetic profile of D-methadone, a novel N-methyl-d-aspartate receptor antagonist in healthy, opioid-naïve subjects: results of two phase 1 studies. J Clin Psychopharmacol. 2019;39(3):226–237. PubMed CrossRef
- Bifari F, Pappagallo M, Bleavins M, et al. REL-1017 (Esmethadone), a novel NMDAR blocker for the treatment of MDD is not neurotoxic in sprague-dawley rats. Front Pharmacol. 2022;13:863959. PubMed CrossRef
- Henningfield J, Gauvin D, Bifari F, et al. REL-1017 (esmethadone; D-methadone) does not cause reinforcing effect, physical dependence and withdrawal signs in Sprague Dawley rats. Sci Rep. 2022;12(1):11389. PubMed CrossRef
- Fava M, Stahl S, Pani L, et al. REL-1017 (esmethadone) as adjunctive treatment in patients with major depressive disorder: a phase 2a randomized double-blind trial. Am J Psychiatry. 2022;179(2):122–131. PubMed CrossRef
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134(4):382–389. PubMed CrossRef
- Pedrelli P, Blais MA, Alpert JE, et al. Reliability and validity of the Symptoms of Depression Questionnaire (SDQ). CNS Spectr. 2014;19(6):535–546. PubMed CrossRef
- Williams JBW, Kobak KA. Development and reliability of a structured interview guide for the Montgomery Asberg Depression Rating Scale (SIGMA). Br J Psychiatry. 2008;192(1):52–58. PubMed CrossRef
- Mathews DC, Henter ID, Zarate CA. Targeting the glutamatergic system to treat major depressive disorder: rationale and progress to date. Drugs. 2012;72(10):1313–1333. PubMed CrossRef
- Lam RW, Endicott J, Hsu MA, et al. Predictors of functional improvement in employed adults with major depressive disorder treated with desvenlafaxine. Int Clin Psychopharmacol. 2014;29(5):239–251. PubMed CrossRef
- American Psychiatric Association. Depressive Disorders. In: Diagnostic and Statistical Manual of Mental Disorders, 5th Edition. Washington, DC: American Psychiatric Association; 2013.
- Fava M, Mahableshwarkar AR, Jacobson W, et al. What is the overlap between subjective and objective cognitive impairments in MDD? Ann Clin Psychiatry. 2018;30(3):176–184. PubMed
- Fava M, Graves LM, Benazzi F, et al. A cross-sectional study of the prevalence of cognitive and physical symptoms during long-term antidepressant treatment. J Clin Psychiatry. 2006;67(11):1754–1759. PubMed CrossRef
- Pan Z, Park C, Brietzke E, et al. Cognitive impairment in major depressive disorder. CNS Spectr. 2019;24(1):22–29. PubMed CrossRef
- Bortolato B, Miskowiak KW, Köhler CA, et al. Cognitive remission: a novel objective for the treatment of major depression? BMC Med. 2016;14(1):9. PubMed CrossRef
- Biringer E, Rongve A, Lund A. A review of modern antidepressants’ effects on neurocognitive function. Curr Psychiatry Rev. 2009;5(3):164–174. CrossRef
- Popovic D, Vieta E, Fornaro M, et al. Cognitive tolerability following successful long term treatment of major depression and anxiety disorders with SSRi antidepressants. J Affect Disord. 2015;173:211–215. PubMed CrossRef
- Sayyah M, Eslami K, AlaiShehni S, et al. Cognitive function before and during treatment with selective serotonin reuptake inhibitors in patients with depression or obsessive-compulsive disorder. Psychiatry J. 2016;2016:5480391. PubMed CrossRef
- Lundbeck FDA Updates Trintellix (vortioxetine) Label to Include Data Showing Improvement in Processing Speed, an Important Aspect of Cognitive Function in acute Major Depressive Disorder (MDD). Accessed Assessed May 17, 2018. https://globenewswire.com/news-release/2018/05/02/1495434/0/en/FDA-updates-Trintellix-vortioxetine-label-to-include-data-showing-improvement-in-processing-speed-an-important-aspect-of-cognitive-function-in-acute-Major-Depressive-Disorder-MDD.html
- Raskin J, Wiltse CG, Siegal A, et al. Efficacy of duloxetine on cognition, depression, and pain in elderly patients with major depressive disorder: an 8-week, double-blind, placebo-controlled trial. Am J Psychiatry. 2007;164(6):900–909. PubMed CrossRef
- Greer TL, Sunderajan P, Grannemann BD, et al. Does duloxetine improve cognitive function independently of its antidepressant effect in patients with major depressive disorder and subjective reports of cognitive dysfunction? Depress Res Treat. 2014;2014:627863. PubMed CrossRef
- Mahableshwarkar AR, Zajecka J, Jacobson W, et al. A randomized, placebo-controlled, active-reference, double-blind, flexible-dose study of the efficacy of vortioxetine on cognitive function in major depressive disorder. Neuropsychopharmacology. 2015;40(8):2025–2037. PubMed CrossRef
- Lee Y, Syeda K, Maruschak NA, et al. A new perspective on the anti-suicide effects with ketamine treatment: a procognitive effect. J Clin Psychopharmacol. 2016;36(1):50–56. PubMed CrossRef
- Culpepper L, Lam RW, McIntyre RS. Cognitive impairment in patients with depression: awareness, assessment, and management. J Clin Psychiatry. 2017;78(9):1383–1394. PubMed CrossRef
This PDF is free for all visitors!