Prim Care Companion CNS Disord 2021;23(3):20l02729
To cite: Das N, Kumar N. Suicidal vilazodone overdose presenting as serotonin syndrome in a young woman with major depressive disorder. Prim Care Companion CNS Disord. 2021;23(3):20l02729.
To share: https://doi.org/10.4088/PCC.20l02729
© Copyright 2021 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry and National Drug Dependence Treatment Centre, All India Institute of Medical Sciences, New Delhi, India
*Corresponding author: Nileswar Das, MD, Room 4096, Teaching Block, Office of Department of Psychiatry, All India Institute of Medical Sciences, New Delhi, India 110029 ([email protected]).
Vilazodone is a novel dual-acting selective serotonin reuptake inhibitor approved for treatment of adult depressive disorders.1 Serotonin syndrome is a rare but serious adverse drug reaction of vilazodone that produces toxic systemic effects and may lead to coma or death. The risk is higher when combined with other serotonergic drugs such as monoamine oxidase inhibitors (MAOIs).2–4 Available literature, however, is limited with regard to risk of serotonin syndrome in suicidal vilazodone overdose in adults.5 We hereby report a case of serotonin syndrome in a young woman with suicidal vilazodone overdose with no coingestion.
Case Report
A 27-year-old unmarried woman suffering from major depressive disorder for the last 10 years presented with a severe depressive episode since November 2018. In the current episode, she was given adequate trials of escitalopram, fluoxetine, venlafaxine, and bupropion (November 2018–May 2019). Augmentation was also tried (May 2019) with lithium and lamotrigine. Later, tablet vilazodone was started (July 2019) and was increased to 40 mg/day, as she tolerated it well. She also received additional brain stimulation therapies along with vilazodone but with no added benefit (August–September 2019). In October 2019, she consumed 30 tablets of vilazodone 20 mg (ie, 600 mg [2 mg/kg] in total). Approximately 4 hours after consumption, she developed delirium and was taken to the emergency department (ED).
In the ED, she developed marked agitation, restlessness, hyperactive delirium, profuse sweating, tachycardia up to 140 bpm, and hypotension up to 90/60 mm Hg and was given a 2-mg injection of intravenous (IV) lorazepam twice half an hour apart. Later, she was transferred to the intensive care unit (ICU) for further management given her deteriorating vital signs. During the detailed examination, her deep tendon reflexes (knee/ankle jerk) were brisk and planter reflex was flexor, and she developed spontaneous jaw clonus, which was confirmed by repeating inducible patellar clonus and ocular clonus. Urine drug screen was positive for only benzodiazepines. Other blood investigations were within normal limits. Electrocardiogram showed no abnormality (QTc of 390 ms). A diagnosis of serotonin syndrome was considered as per Hunter’s diagnostic algorithm,6 and supportive treatment (IV fluids and midazolam infusion 0.05 mg/kg/h) in the ICU was continued. After 24 hours, her delirium had resolved, and she was able to recognize her family members and the treating doctor.
She was transferred to the psychiatry inpatient unit after 72 hours of stabilization and was kept off psychotropic medications for the next 2 weeks. Later, she was started on sertraline (was not tried previously) on the psychiatry inpatient unit.
Discussion
Life-threatening suicide attempts with medication overdose are not uncommon among patients with depressive disorder. This case was challenging not only because the patient responded poorly to the treatment but also due to the uniqueness of presentation with suicidal vilazodone overdose and serious adverse reaction in the form of serotonin syndrome.
While previous literature has provided a cautionary note for tricyclic antidepressants and MAOIs, most studies have argued that selective serotonin reuptake inhibitors are safe in suicidal overdoses.3,4 However, this may not be true for vilazodone. Being a relatively newer molecule, literature is sparse about assessing risk associated with vilazodone in the context of overdose. It has been hypothesized in the literature that the possible higher risk of serotonin syndrome with vilazodone is due to partial agonism of serotonergic 5-HT1A receptors in addition to selective serotonin reuptake inhibition.5 And, it is possible that the risk increases many fold when combined with other serotonergic drugs.2,3,7
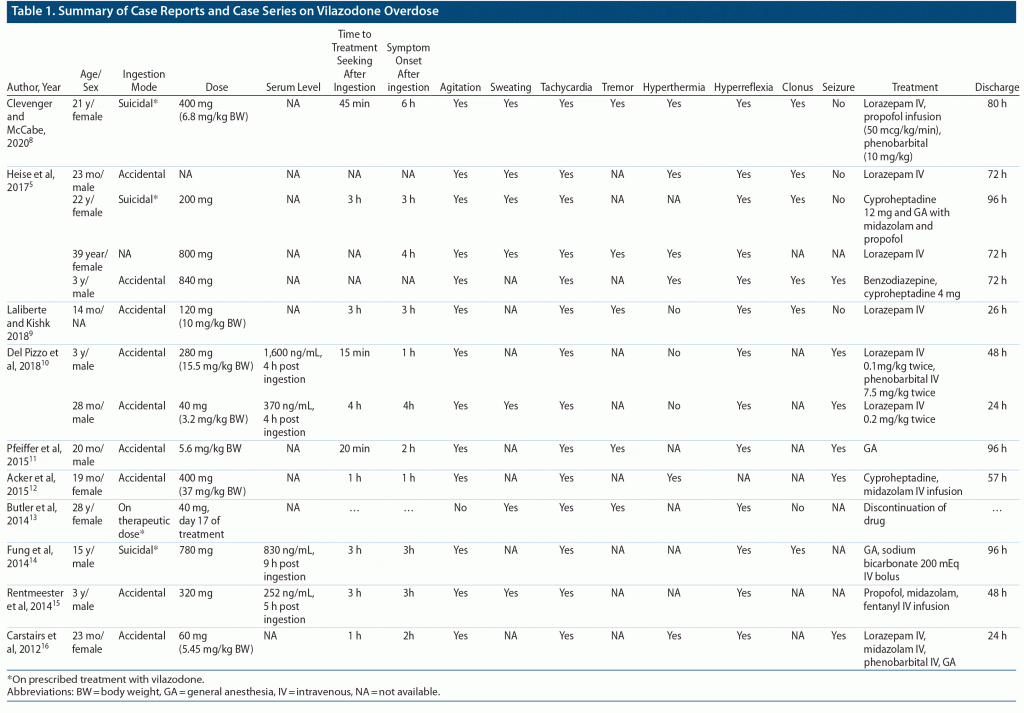
At present, available literature predominantly (9 of 14 cases) consists of case reports with accidental overdoses in the pediatric population (Table 1), with a dose range of 40–800 mg and symptoms of serotonin syndrome usually appearing after 1–6 hours. Most common symptoms reported in these cases were tachycardia, agitation, and hyperreflexia. Treatment seeking was prompt and often before appearance of any symptoms in cases of accidental ingestion. Most patients (11 of 14) required ICU admission and were frequently treated conservatively with parenteral benzodiazepines, general anesthetics, or oral cyproheptadine (because of its 5-HT receptor–blocking properties). All patients were discharged after 24 to 96 hours of ICU admission.8–16
Vilazodone is metabolized predominantly by hepatic cytochrome P450 enzymes, peak plasma level is reached in 3–5 hours (explaining the time of appearance of the symptoms), and it usually has a half-life of 25 hours (explains usual time to recovery after initial supportive care).1,5
In this case, we could not obtain a serum vilazodone level, but the patient denied consuming any other medication and she was not on any other prescription medication during that time (also, family members retrieved 3 empty strips of vilazodone). The appearance of serotonin syndrome coincided with reported plasma peak level (ie, 3–5 hours after ingestion), and the consumed dose was also similar (12 mg/kg body weight) to the average reported in cases.9,10
This case highlights the risk of serotonin syndrome in suicidal overdose (where treatment seeking may not be as prompt as in the case of accidental ingestion) of vilazodone, even with no coingestion in a patient with major depressive disorder, raising the issue about safety of vilazodone as an antidepressant in patients with higher suicide risk. Hence, it is necessary to inform family members about risk of serotonin syndrome in case of overdose and the need to give medications under supervision. Further studies are needed to establish the risk versus benefit to guide the real-world decision making.
Published online: April 29, 2021.
Potential conflicts of interest: None.
Funding/support: None.
Acknowledgments: The authors thank Siddharth Sarkar, MD, and Preethy K, MD, both from the Department of Psychiatry and National Drug Dependence Treatment Centre, All India Institute of Medical Sciences, New Delhi, India, for their constant support and help in improving the quality of the manuscript. Drs Sarkar and K report no conflicts of interest related to the subject of this report.
Patient consent: Consent was received from the patient to publish this case report, and information and dates have been de-identified to protect anonymity.
References (16)

- Laughren TP, Gobburu J, Temple RJ, et al. Vilazodone: clinical basis for the US Food and Drug Administration’s approval of a new antidepressant. J Clin Psychiatry. 2011;72(9):1166–1173. PubMed CrossRef
- Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352(11):1112–1120. PubMed CrossRef
- Barbey JT, Roose SP. SSRI safety in overdose. J Clin Psychiatry. 1998;59(suppl 15):42–48. PubMed
- Peretti S, Judge R, Hindmarch I. Safety and tolerability considerations: tricyclic antidepressants vs selective serotonin reuptake inhibitors. Acta Psychiatr Scand suppl. 2000;403(s403):17–25. PubMed CrossRef
- Heise CW, Malashock H, Brooks DE. A review of vilazodone exposures with focus on serotonin syndrome effects. Clin Toxicol (Phila). 2017;55(9):1004–1007. PubMed CrossRef
- Dunkley EJC, Isbister GK, Sibbritt D, et al. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM. 2003;96(9):635–642. PubMed CrossRef
- Russell JL, Spiller HA, Chounthirath T, et al. Pediatric ingestion of vilazodone compared to other selective serotonin reuptake inhibitor medications. Clin Toxicol (Phila). 2017;55(5):352–356. PubMed CrossRef
- Clevenger J, McCabe D. Development of severe serotonin syndrome from acute ingestion of vilazodone without co-ingestion. Am J Emerg Med. 2020;38(5):1045.e1–1045.e2. PubMed
- Laliberte B, Kishk OA. Serotonin syndrome in a pediatric patient after vilazodone ingestion. Pediatr Emerg Care. 2018;34(12):e226–e228. PubMed CrossRef
- Del Pizzo J, Fernandez EK, Kopec KT, et al. Seizures after pediatric vilazodone Ingestion: a case series. Pediatr Emerg Care. 2018;34(3):e51–e54. PubMed CrossRef
- Pfeiffer S, Gunkelman S, Blackford M. Psychotropic exposures in pediatric patients: symptomatic iloperidone and vilazodone ingestions. Clin Toxicol (Phila). 2015;53(3):188. PubMed CrossRef
- Acker EC, Sinclair EA, Beardsley AL, et al. Acute vilazodone toxicity in a pediatric patient. J Emerg Med. 2015;49(3):284–286. PubMed CrossRef
- Butler TW, Lucas RA, Barghouthy W. Serotonin syndrome with standard-dose vilazodone (viibryd ) monotherapy. J Med Cases. 2014;5(11):567–569. CrossRef
- Fung BJ, Yanta JH, King AM, et al. Vilazodone may cause sodium channel blockade in overdose. J Med Toxicol. 2014;10(1):81. PubMed
- Rentmeester L, Saitman A, Chindarkar N, et al. Severe pediatric vilazodone toxicity with laboratory confirmation. Clin Toxicol. 2014;52(7):694–695.
- Carstairs SD, Griffith EA, Alayin T, et al. Recurrent seizure activity in a child after acute vilazodone ingestion. Ann Emerg Med. 2012;60(6):819–820. PubMed CrossRef
Enjoy this premium PDF as part of your membership benefits!