Using Measurement Strategies to Identify and Monitor Residual Symptoms
Major depressive disorder (MDD) is a persistent, pervasive, and chronic disorder that significantly affects patients’ functioning and quality of life. Most patients treated for MDD continue to have residual symptoms after acute treatment with pharmacotherapy. One of the most commonly encountered residual symptoms is cognitive dysfunction, which substantially affects patient outcomes. While antidepressant monotherapy is an effective first-line treatment for some patients with MDD, patients with residual symptoms (eg, cognitive dysfunction) will require an additional treatment intervention such as augmentation or switch to an alternative treatment strategy. Measurement-based care has been demonstrated to improve patient outcomes in MDD. The clinical importance of cognitive dysfunction in MDD invites the need to probe, screen, and measure the extent of cognitive impairment.
(J Clin Psychiatry 2013;74[suppl 2]:14-18)
From the Department of Psychiatry, University of Toronto, and the Mood Disorders Psychopharmacology Unit, University Health Network, Toronto, Ontario, Canada.
This article is derived from the planning teleconference series “Depression: Managing the Full Range of Symptoms to Achieve Lasting Remission,” which was held in May and June 2013 and supported by an educational grant from Takeda Pharmaceuticals International, Inc., US Region and Lundbeck.
Dr McIntyre has served on the advisory boards for AstraZeneca, Bristol-Myers Squibb, Eli Lilly, France Foundation, GlaxoSmithKline, Janssen-Ortho, Lundbeck, Merck, Organon, Pfizer, and Shire; has served on the speakers bureaus for AstraZeneca, Eli Lilly, Janssen-Ortho, Lundbeck, Merck, and Pfizer; has received grant/research support from AstraZeneca, Eli Lilly, Janssen-Ortho, Lundbeck, the National Alliance for Research on Schizophrenia and Depression, the National Institutes of Mental Health, Pfizer, Shire, and the Stanley Medical Research Institute; and has participated in CME activities for AstraZeneca, Bristol-Myers Squibb, CME Outfitters, Eli Lilly, France Foundation, I3CME, Merck, Optum Health, and Pfizer.
Corresponding author: Roger S. McIntyre, MD, 399 Bathurst St, MP 9-325, Toronto, Ontario, M5T 2S8, Canada ([email protected]).
doi:10.4088/JCP.12084su1c.03
© Copyright 2013 Physicians Postgraduate Press, Inc.
Major depressive disorder (MDD) is a prevalent condition associated with high rates of nonrecovery, recurrence, and illness-associated burden. The therapeutic objectives in MDD are to achieve symptom remission, functional recovery, and return to mental and physical health. Toward this aim, evidence-based treatment strategies that incorporate measurement-based care increase the probability of success. It is also important to incorporate patient reported outcomes (eg, subjective cognitive complaints, quality of life) when determining whether the therapeutic objectives have been achieved.
Treatment Objectives
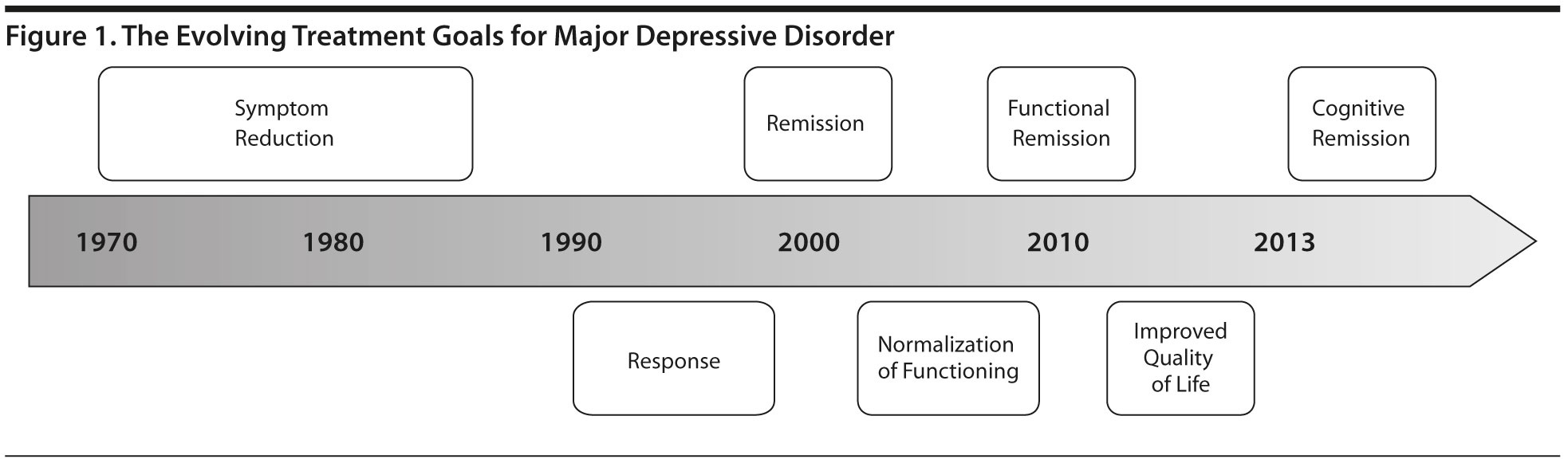
The treatment goals for MDD have evolved in the past 40 years (Figure 1). Soon after the introduction of antidepressant pharmacotherapy in the 1950s, the overarching goal of managing patients with depression was simply to improve symptoms. Subsequently, emphasis was placed on a categorical response, which typically was defined as a ≥ 50% improvement from baseline in depressive symptom severity. Further refinement of the therapeutic objectives resulted in the emphasis of full symptomatic remission defined as the abatement of active depressive symptoms. The impetus for this change was based on several lines of evidence indicating that subsyndromal depressive symptoms in MDD were associated with increased morbidity and mortality. Moreover, symptomatic remission increased the probability of functional recovery. Cost-of-illness studies1 have pressed the point that MDD is a highly costly disorder, largely due to impaired role performance in the workplace. A derivative of this observation is the testable hypothesis that improved outcomes in MDD would result in lower costs to individuals, families, and societies.

- Monitoring patients’ symptoms from baseline with measurement tools increases the chances of success in MDD treatment.
- Using evidence-based treatments and intervening early in treatment with switching, combination, or adjunctive therapies can improve therapeutic outcomes for patients with MDD.
Several studies2,3 have documented that cognitive dysfunction in MDD is a principal mediator of functional impairment, notably in workplace settings. Notwithstanding cognitive impairment as a criterion item in a major depressive episode, cognitive dysfunction historically has received relatively little attention in MDD when compared with disturbances in mood or somatic domains. A pivot of sorts toward cognitive impairment is inspired by efforts to reduce cost of illness and to identify clinical pathological correlates that have prognosticative capability in depression.
Measurement-Based Care
Clinicians who provide care for individuals with MDD are encouraged to routinely measure not only symptoms and side effects but also functioning across the major domains.4 Measurement-based care involves the use of objective tools to quantify the presence and severity of depressive symptoms, to identify and measure functional outcomes, and to determine the presence and severity of adverse events. Evidence5 indicates that the incorporation of measurement-based care, a component of chronic disease management, increases the probability of remission, treatment persistence, and functional recovery (For a list of tools, see Supplementary eTable 1 at PSYCHIATRIST.COM.).
Screening Tools
Screening tools help clinicians determine whether to conduct a full diagnostic evaluation of an individual who may have depression. These questionnaires are often short and can be filled out by the patient before a clinical appointment. For example, the 2-item Patient Health Questionnaire (PHQ-2) asks patients 2 questions about decreased interest in activities and feelings of hopelessness or depression.6 If the PHQ-2 screen is positive, then the clinician can ask the patient to complete the PHQ-9, which queries patients on 9 features of depression.7 Both of these questionnaires require less than 3 minutes for patients to complete.8 Another brief and effective screening tool is the 7-item Hamilton Depression Rating Scale (HDRS-7), which takes less than 5 minutes to administer.9
While these tools are quick and efficient ways to screen for depressive symptoms in the clinical setting, screening tools should not be confused with diagnostic tools. Screening tools increase or decrease suspicion that an individual may have depression, but they do not diagnose depression.
Diagnostic Tools
After an initial screening, clinicians should employ diagnostic tools to identify a more precise diagnosis of MDD. The diagnosis is made clinically using the Diagnostic and Statistical Manual (DSM),10 but tools can supplement the manual. For example, the Structured Clinical Interview for DSM-IV Axis I Disorders has a clinician-rated version that is designed for clinical practice.11 The Mini-International Neuropsychiatric Interview, which is based on both DSM-IV-TR and International Classification of Diseases, Tenth Revision criteria, is also appropriate for clinical practice.12
Unfortunately, many diagnostic tools are better suited for the research setting and are not practical for the busy office practice because they are often lengthy and cumbersome. However, some screening tools may also function as diagnostic tools, such as the PHQ-9.
Symptom Severity and Adverse Effect Tools
As in other areas of chronic disease management (eg, hypertension, diabetes), clinicians are encouraged to systematically probe and measure depressive symptomatology over time in patients with MDD. The PHQ-9 and the HDRS-7 are suitable to use in clinical practice.9 The 16-item Quick Inventory of Depressive Symptomatology (QIDS)13 can be completed by the clinician or by the patient and is also effective in clinical settings. Additionally, adverse effects of treatment should be tracked at each follow-up visit, and the Frequency, Intensity, and Burden of Side Effects Rating (FIBSER) is a useful tool for this purpose.
REsidual Symptoms in Depression
The last 20 years have brought about substantial progress in the development of pharmacotherapy, psychosocial interventions, neurostimulation, and nutraceutical approaches to depression. Notwithstanding these developments, outcomes in depression remain disappointing. After acute pharmacotherapy, the majority of patients do not achieve the therapeutic objective of remission.14 Common ongoing symptoms reported by patients include fatigue, sleep difficulties, and cognitive problems.15
Remission and Residual Symptoms
Patients who experience full remission of depressive symptoms are more likely than those who do not to achieve premorbid levels of psychosocial functioning.16 Incomplete remission increases the risk of relapse and recurrence and predicts a chronic course of depression.17 Remission with residual symptoms also increases the use of health care services and disability benefits18 and the risk of suicide.19 Incomplete remission stands in the way of recovery, affects the patient’s children and other loved ones, and deprives employers and society of productive individuals.20
Many barriers can prevent full remission in depression, including lack of timely access to coordinated primary and/or preventative health care, lack of use of measurement-based care and evidence-based care, lack of resolution of comorbidities, nonadherence to and insufficient duration of treatment, and failure to address psychosocial matters.4,20 Clinicians must do their best to address as many barriers as possible to help patients achieve asymptomatic remission.4
Residual Cognitive Symptoms
Cognitive dysfunction in depression has been defined and operationalized in several ways. A convenient categorization includes the subcategories cold cognitive dysfunction, defined as nonemotionally valenced, and hot cognitive dysfunction, which is best recognized by information processing biases in MDD.
Cognitive domains such as memory, executive function, attention, and information processing speed are negatively affected by depression.21 Cognitive dysfunction is a principal mediator of functional impairment,22 can be a factor in nonadherence to therapy,23 and is associated with suicidal ideation.24
Cognitive impairment is common in patients with MDD. Conradi et al15 found that 94% of patients in a 3-year prospective study reported cognitive problems during a depressive episode. Of the patients whose depression remitted, 44% continued to complain of residual cognitive impairment. It has also been reported that residual cognitive impairment may predispose and portend recurrence as well as be a principal explanation for suboptimal functional recovery.25 Although cognitive complaints are frequent in people with depression, self-rated cognitive dysfunction might not be verified by objective measures.26 Nevertheless, the use of objective and/or subjective measures of cognition increases the likelihood of detecting cognitive dysfunction in patients with MDD.27
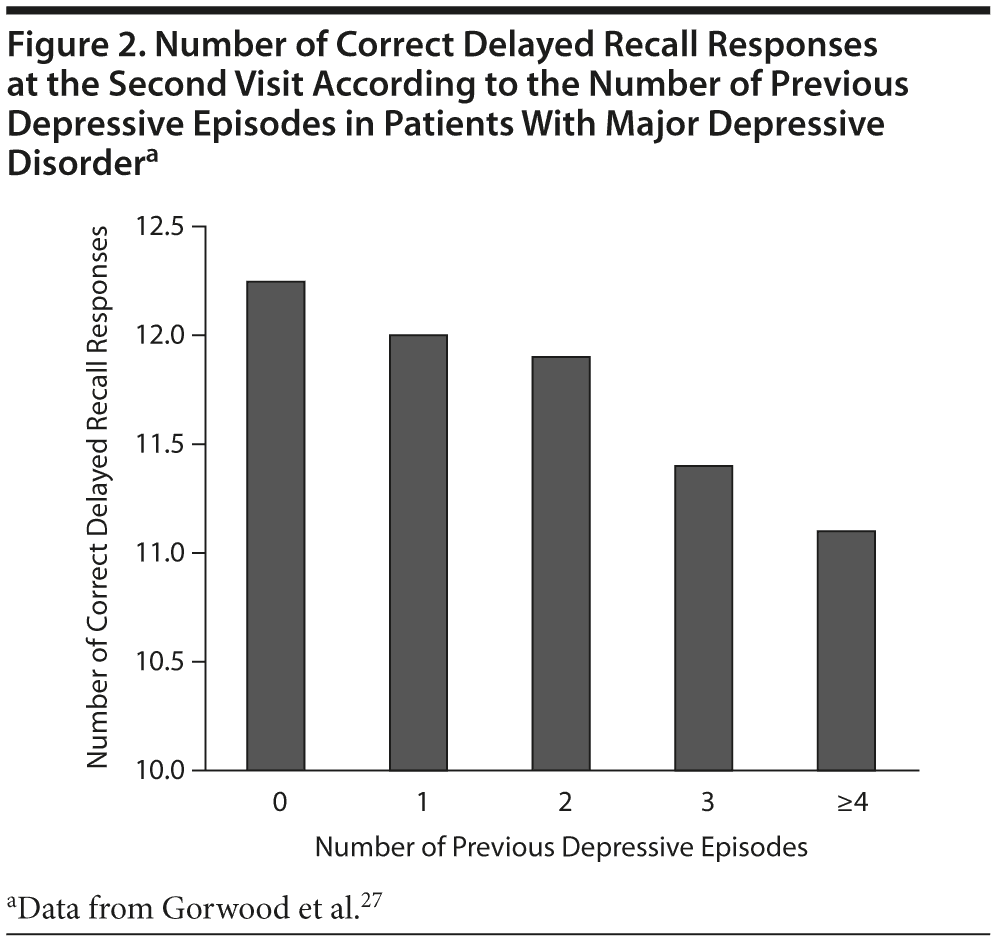
In subsets of individuals with MDD, cognitive dysfunction may be progressive as a function of the number of depressive episodes. For example, Gorwood and colleagues27 found a negative correlation between the number of correct responses on the Weschler paragraph recall index and the number of past depressive episodes (P < .001) and the length of depressive episodes (P < .012). Cognitive performance was reported to be decreased by 2% to 3% per depressive episode for the first 4 episodes (Figure 2).27
Information processing bias (hot cognitive dysfunction) in depression refers to the automatic bias that patients have toward negative stimuli; patients with depression have an attentional bias toward negative stimuli and a bias against positive stimuli. Erickson et al28 empirically measured this paradigm by comparing people with depression who are not medicated with healthy controls on the Affective Go/No-Go Task, in which subjects are asked to respond to sets of happy and sad words. Depressed individuals needed a longer time before reacting to the happy words than the sad words, whereas healthy subjects showed the opposite pattern. Because cognitive problems significantly affect patients’ functioning and quality of life, clinicians should work toward cognitive symptom remission. The forgoing series of observations has provided the basis for exploring the efficacy of some of the psychosocial treatments like mindfulness-based cognitive therapy.29 In some cases, pharmacotherapy can help treat not only the more traditional depressive symptoms but also the cognitive domains affected by depression.
Evidence-Based treatmentS
In a large meta-analysis of 182 clinical trials, Papakostas and Fava30 found that the rate of response with antidepressant treatment (53.8%) was higher than with placebo (37.3%) in patients with MDD. However, this response rate leaves much room for improvement, and no unequivocal gold standard exists in antidepressant treatment. Clinicians must use measurement tools and evidence to guide treatment selection.
Antidepressant treatments have differing success rates in treating various aspects of depression. In the process of treating patients, a particular aspect of their symptomatology (such as cognition) may need to be targeted. For example, a study by Raskin and colleagues31 found that duloxetine is effective in improving cognitive outcome measures in patients with recurrent MDD who are ≥ 65 years old. Four cognitive tests were selected as outcome measures to target the following aspects of cognitive functioning: verbal learning and memory, attention, executive function, and working memory. The composite scores of these 4 tests—the Verbal Learning and Recall Test, the Symbol Digit Substitution Test, the Two-Digit Cancellation Test, and the Letter-Number Sequencing Test—significantly improved in patients who were treated with duloxetine (60 mg/d) compared with placebo (P < .02). Scores on the 4 tests individually showed that significant improvements occurred in learning and memory via the Verbal Learning (P = .003) and Recall (P = .02) Tests, but the other tests did not show significant improvements in attention, information processing speed, and executive function. Duloxetine also significantly improved depression more than placebo according to HDRS total scores at 8 weeks (P < .001). The Verbal Learning and Recall Test, the Symbol Digit Substitution Test, the Two-Digit Cancellation Test, and the Letter-Number Sequencing Test can be completed in a short amount of time and may be practical tools for clinical practice.
The multimodal antidepressant vortioxetine has also been studied in older adults with recurrent MDD. Katona et al32 compared the efficacy of vortioxetine, duloxetine, and placebo in depression and cognitive impairment in elderly patients. Patients treated with vortioxetine (5 mg/d) showed significantly more improvement on total HDRS scores than placebo at 8 weeks (P = .0011); duloxetine was also superior to placebo. Secondary outcomes from this study also indicated that vortioxetine significantly improved measures of learning and memory as well as information processing speed when compared with placebo. The benefit in cognitive performance was determined to be largely a direct effect of the treatment rather than an indirect effect as a consequence of depressive symptoms mitigation. As a consequence of these findings with vortioxetine, buttressed by preclinical observations supporting a procognitive effect, this particular agent has been primarily studied to determine if it offers a direct procognitive effect in younger adults with MDD.
Modifying Treatment
Measurement-based care is important in determining whether the current course of treatment is effective or not. Some patients require longer exposure to antidepressants or psychosocial treatments before achieving remission, and clinicians have to decide how long to wait before modifying therapy. Recent data33 suggest that a lack of antidepressant efficacy can be predicted early in the course of treatment.
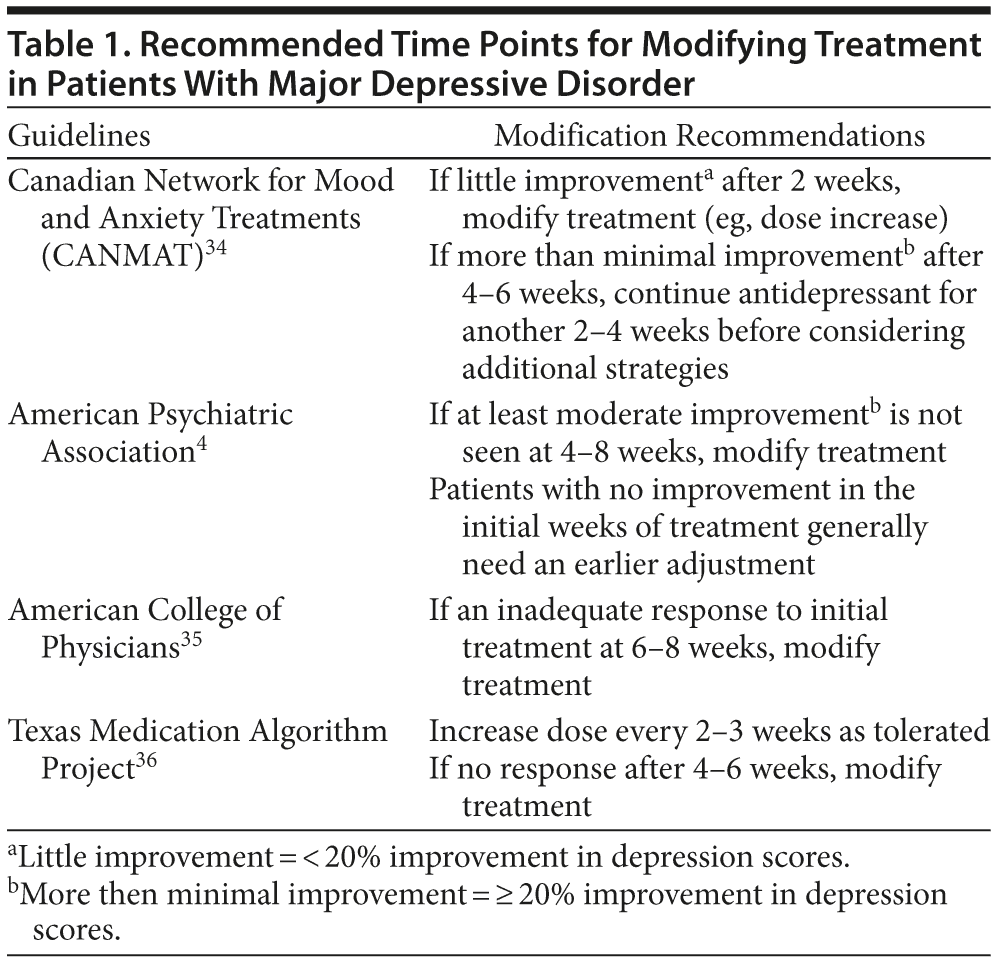
The results of a meta-analysis33 indicated that if patients experience little to no benefit (ie, < 20% improvement from baseline) with an adequate antidepressant dose within 2 weeks, they have a 4% to 18% chance of response and only a 0% to 5% chance of remission with that treatment. Full treatment benefit may take 6 to 8 weeks or longer, but an early lack of improvement can help the clinician decide to modify therapy. Evidence-based and consensus-based recommendations encourage clinicians to intervene by switching to another antidepressant, combining antidepressants, or adding another agent to the existing antidepressant within the first 4 to 6 weeks of treatment (Table 1).4,34-36 It is generally recommended that if insufficient benefit is seen in the first 2 weeks of treatment, then dose optimization can be considered.
Adding atypical antipsychotics to antidepressant treatment is the most thoroughly studied augmentation approach in MDD.37 A meta-analysis by Nelson et al37 found that atypical antipsychotics used in conjunction with antidepressants were significantly more effective in reducing depressive symptoms than placebo (P < .00001). However, some of the possible disadvantages of atypical antipsychotics are weight gain, metabolic decompensation, sedation, and extrapyramidal side effects, making tolerability of these treatments a concern. Monitoring side effects closely can improve outcomes with these treatments.
More recently, the psychostimulant lisdexamfetamine has been shown to be an effective adjunctive therapy. In a multicenter trial of 173 adults with residual depressive symptoms after 8 weeks of escitalopram treatment, Trivedi et al38 found that nonremitted patients treated with adjunctive lisdexamfetamine for 6 weeks were significantly improved according to Montgomery-Asberg Depression Rating Scale (P = .0902) and QIDS-SR (P = .0774) scores when compared with subjects who received placebo.
Other medications have also proven to be effective adjunctive therapy in treatment-resistant depression. Patients with MDD who had not responded to selective serotonin reuptake inhibitor (SSRI) treatment had significantly greater HDRS response and remission rates when treated with add-on S-adenosyl methionine compared with placebo (P < .05).39 Adjunctive l-methylfolate also improved response rates and depression symptom scores in patients with partial response or no response to SSRI treatment.40 Likewise, the N-methyl-d-aspartate (NMDA) antagonist ketamine has been shown to improve depressive symptoms and suicidal ideation in treatment-resistant depression.41 At this time, however, ketamine is considered to be experimental as questions regarding its safe use without inducing psychosis and its long-term therapeutic benefits have not been determined.
Finally, the Treatment with Exercise Augmentation for Depression (TREAD) study42 found that adding high-dose exercise (16 kcal/kg/wk expenditure) to SSRI treatment in nonremitted patients improved remission rates. However, low-dose exercise (4 kcal/kg/wk expenditure) did not exhibit the same degree of improvement. Along with medications, aerobic exercise may benefit some people with treatment-resistant depression.
Conclusion
Major depressive disorder is a common, often severe, and persistent mental disorder and is a leading cause of disability globally. Unfortunately, most individuals with MDD do not achieve full symptom remission, which is the first step toward functional recovery and improved quality of life. Cognitive dysfunction, one of the most common residual symptoms in MDD, plays an important role in functional outcome, compliance, and illness complexity, making resolution of cognitive symptoms an important therapeutic objective.
Using measurement tools and assigning patients to treatments that have been shown to be effective in large, randomized, controlled trials help to improve therapeutic outcomes. Monitoring symptomatology with measurement tools, such as depression symptom inventories and cognitive testing, increases the chance of success in MDD treatment. With careful monitoring, clinicians can determine within 4 to 6 weeks of treatment (or earlier) whether treatment modification is necessary. Treatment selection and sequencing will need to consider both the beneficial and detrimental effects a chosen strategy has on cognitive dysfunction. Most people with MDD will require treatment beyond the initial first-line choice to reach the therapeutic objectives of remission, premorbid functioning, and asymptomatic recovery.
Drug names: duloxetine (Cymbalta), escitalopram (Lexapro and others), ketamine (Ketalar and others), lisdexamfetamine (Vyvanse).
Disclosure of off-label usage: Dr McIntyre has determined that, to the best of his knowledge, ketamine and vortioxetine are not approved by the US Food and Drug Administration for the treatment of depression.
Supplementary material: Available at PSYCHIATRIST.COM.
References
1. Luppa M, Heinrich S, Angermeyer MC, et al. Cost-of-illness studies of depression: a systematic review. J Affect Disord. 2007;98(1-2):29-43. PubMed doi:10.1016/j.jad.2006.07.017
2. Kennedy N, Foy K, Sherazi R, et al. Long-term social functioning after depression treated by psychiatrists: a review. Bipolar Disord. 2007;9(1-2):25-37. PubMed doi:10.1111/j.1399-5618.2007.00326.x
3. Hammar A, Ardal G. Cognitive functioning in major depression: a summary. Front Hum Neurosci. 2009;3(26):1-7. PubMed
4. American Psychiatric Association. Practice Guideline for the Treatment of Patients With Major Depressive Disorder, Third Edition. Washington, DC: American Psychiatric Association; 2010. http://psychiatryonline.org/content.aspx?bookid=28§ionid=1667485. Accessed August 27, 2013.
5. Trivedi MH, Daly EJ. Measurement-based care for refractory depression: a clinical decision support model for clinical research and practice. Drug Alcohol Depend. 2007;88(suppl 2):S61-S71. PubMed doi:10.1016/j.drugalcdep.2007.01.007
6. Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41(11):1284-1292. PubMed doi:10.1097/01.MLR.0000093487.78664.3C
7. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613. PubMed doi:10.1046/j.1525-1497.2001.016009606.x
8. Gelenberg AJ. Using assessment tools to screen for, diagnose, and treat major depressive disorder in clinical practice. J Clin Psychiatry. 2010;71(suppl E1):e01.
9. McIntyre RS, Konarski JZ, Mancini DA, et al. Measuring the severity of depression and remission in primary care: validation of the HAMD-7 scale. CMAJ. 2005;173(11):1327-1334. PubMed
10. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association; 2000.
11. First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P). New York: Biometrics Research, New York State Psychiatric Institute; November, 2002.
12. Sheehan DV, LeCrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22-33. PubMed
13. Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), Clinician Rating (QIDS-C), and Self-Report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54(5):573-583. PubMed doi:10.1016/S0006-3223(02)01866-8
14. Rush AJ, Warden D, Wisniewski SR, et al. STAR*D: revising conventional wisdom. CNS Drugs. 2009;23(8):627-647. PubMed
15. Conradi HJ, Ormel J, de Jonge P. Presence of individual (residual) symptoms during depressive episodes and periods of remission: a 3-year prospective study. Psychol Med. 2011;41(6):1165-1174. PubMed doi:10.1017/S0033291710001911
16. Miller IW, Keitner GI, Schatzberg AF, et al. The treatment of chronic depression, part 3: psychosocial functioning before and after treatment with sertraline or imipramine. J Clin Psychiatry. 1998;59(11):608-619. PubMed doi:10.4088/JCP.v59n1108
17. Judd LL, Paulus MJ, Schettler PJ, et al. Does incomplete recovery from first lifetime major depressive episode herald a chronic course of illness? Am J Psychiatry. 2000;157(9):1501-1504. PubMed doi:10.1176/appi.ajp.157.9.1501
18. Frasure-Smith N, Lespérance F. Depression and anxiety as predictors of 2-year cardiac events in patients with stable coronary artery disease. Arch Gen Psychiatry. 2008;65(1):62-71. PubMed doi:10.1001/archgenpsychiatry.2007.4
19. Tranter R, O’ Donovan C, Chandarana P, et al. Prevalence and outcome of partial remission in depression. J Psychiatry Neurosci. 2002;27(4):241-247. PubMed
20. Stotland NL. Recovery from depression. Psychiatr Clin North Am. 2012;35(1):37-49. PubMed doi:10.1016/j.psc.2011.11.007
21. Millan MJ, Agid Y, Br×¼ne M, et al. Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat Rev Drug Discov. 2012;11(2):141-168. PubMed doi:10.1038/nrd3628
22. Jaeger J, Berns S, Uzelac S, et al. Neurocognitive deficits and disability in major depressive disorder. Psychiatry Res. 2006;145(1):39-48. PubMed doi:10.1016/j.psychres.2005.11.011
23. Wetherell JL, Un×¼tzer J. Adherence to treatment for geriatric depression and anxiety. CNS Spectr. 2003;8(suppl 3):48-59. PubMed
24. Keilp JG, Gorlyn M, Russell M, et al. Neuropsychological function and suicidal behavior: attention control, memory and executive dysfunction in suicide attempt. Psychol Med. 2013;43(3):539-551. PubMed doi:10.1017/S0033291712001419
25. McIntyre RS, Cha DS, Soczynska JK, et al. Cognitive deficits and functional outcomes in major depressive disorder: determinants, substrates, and treatment interventions. Depress Anxiety. 2013;30(6):515-527. PubMed doi:10.1002/da.22063
26. Naismith SL, Longley WA, Scott EM, et al. Disability in major depression related to self-rated and objectively-measured cognitive deficits: a preliminary study. BMC Psychiatry. 2007;7(1):32. PubMed doi:10.1186/1471-244X-7-32
27. Gorwood P, Corruble E, Falissard B, et al. Toxic effects of depression on brain function: impairment of delayed recall and the cumulative length of depressive disorder in a large sample of depressed outpatients. Am J Psychiatry. 2008;165(6):731-739. PubMed doi:10.1176/appi.ajp.2008.07040574
28. Erickson K, Drevets WC, Clark L, et al. Mood-congruent bias in affective go/no-go performance of unmedicated patients with major depressive disorder. Am J Psychiatry. 2005;162(11):2171-2173. PubMed doi:10.1176/appi.ajp.162.11.2171
29. Bostanov V, Keune PM, Kotchoubey B, et al. Event-related brain potentials reflect increased concentration ability after mindfulness-based cognitive therapy for depression: a randomized clinical trial. Psychiatry Res. 2012;199(3):174-180. PubMed doi:10.1016/j.psychres.2012.05.031
30. Papakostas GI, Fava M. Does the probability of receiving placebo influence clinical trial outcome? a meta-regression of double-blind, randomized clinical trials in MDD. Eur Neuropsychopharmacol. 2009;19(1):34-40. PubMed doi:10.1016/j.euroneuro.2008.08.009
31. Raskin J, Wiltse CG, Siegal A, et al. Efficacy of duloxetine on cognition, depression, and pain in elderly patients with major depressive disorder: an 8-week, double-blind, placebo-controlled trial. Am J Psychiatry. 2007;164(6):900-909. PubMed doi:10.1176/appi.ajp.164.6.900
32. Katona C, Hansen T, Olsen CK. A randomized, double-blind, placebo-controlled, duloxetine-referenced, fixed-dose study comparing the efficacy and safety of Lu AA21004 in elderly patients with major depressive disorder. Int Clin Psychopharmacol. 2012;27(4):215-223. PubMed doi:10.1097/YIC.0b013e3283542457
33. Szegedi A, Jansen WT, van Willigenburg APP, et al. Early improvement in the first 2 weeks as a predictor of treatment outcome in patients with major depressive disorder: a meta-analysis including 6562 patients. J Clin Psychiatry. 2009;70(3):344-353. PubMed doi:10.4088/JCP.07m03780
34. Lam RW, Kennedy SH, Grigoriadis S, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT). Canadian Network for Mood and Anxiety Treatments (CANMAT) clinical guidelines for the management of major depressive disorder in adults, pt 3: pharmacotherapy. J Affect Disord. 2009;117(suppl 1):S26-S43. PubMed doi:10.1016/j.jad.2009.06.041
35. Qaseem A, Snow V, Denberg TD, et al, for the Clinical Efficacy Assessment Subcommittee of American College of Physicians. Using second-generation antidepressants to treat depressive disorders: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2008;149(10):725-733. PubMed doi:10.7326/0003-4819-149-10-200811180-00007
36. Suehs BT, Argo TR, Bendele SD, et al, for the Texas Medication Algorithm Project Procedural Manual: Major Depressive Disorder Algorithms. Austin, TX: Texas Department of State Health Services; 2008. http://www.pbhcare.org/pubdocs/upload/documents/TMAP%20Depression%202010.pdf. Accessed August 26, 2013.
37. Nelson JC, Papakostas GI. Atypical antipsychotic augmentation in major depressive disorder: a meta-analysis of placebo-controlled randomized trials. Am J Psychiatry. 2009;166(9):980-991. PubMed doi:10.1176/appi.ajp.2009.09030312
38. Trivedi MH, Cutler AJ, Richards C, et al. Efficacy and safety of lisdexamfetamine dimesylate as augmentation therapy in adults with major depressive disorder treated with an antidepressant [Poster]. Presented at 63rd Institute on Psychiatric Services; October 2011; San Francisco, CA.
39. Papakostas GI, Mischoulon D, Shyu I, et al. S-adenosyl methionine (SAMe) augmentation of serotonin reuptake inhibitors for antidepressant nonresponders with major depressive disorder: a double-blind, randomized clinical trial. Am J Psychiatry. 2010;167(8):942-948. PubMed doi:10.1176/appi.ajp.2009.09081198
40. Papakostas GI, Shelton RC, Zajecka JM, et al. l-methylfolate as adjunctive therapy for SSRI-resistant major depression: results of two randomized, double-blind, parallel-sequential trials. Am J Psychiatry. 2012;169(12):1267-1274. PubMed doi:10.1176/appi.ajp.2012.11071114
41. Howland RH. Ketamine for the treatment of depression. J Psychosoc Nurs Ment Health Serv. 2013;51(1):11-14. PubMed doi:10.3928/02793695-20121219-01
42. Trivedi MH, Greer TL, Church TS, et al. Exercise as an augmentation treatment for nonremitted major depressive disorder: a randomized, parallel dose comparison. J Clin Psychiatry. 2011;72(5):677-684. PubMed doi:10.4088/JCP.10m06743