Prim Care Companion CNS Disord 2021;23(4):20l02772
To cite: Castanheira L, Santos R, Lemos M, et al. Aripiprazole-induced skin reaction. Prim Care Companion CNS Disord. 2021;23(4):20l02772.
To share: https://doi.org/10.4088/PCC.20l02772
© Copyright 2021 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry, Hospital de Santa Maria, Centro Hospitalar Universitário Lisboa Norte EPE, Lisbon, Portugal
bClínica Universitária de Psicologia e Psiquiatria, Faculdade de Medicina, Universidade de Lisboa, Lisbon, Portugal
*Corresponding author: Lígia Castanheira, MD, Centro Hospitalar Universitário Lisboa Norte, Avenida Professor Egas Moniz, Lisbon, 1649-028, Portugal ([email protected]).
Adverse skin reactions are reported in approximately 5% of those for whom antipsychotics are prescribed, occurring with both typical and atypical antipsychotics.1 Reports of aripiprazole-induced skin reactions are sparse. We report a case of a patient in a manic episode treated with aripiprazole who experienced a skin rash reaction that remitted after the drug was stopped.
Case Report
A 43-year-old woman with a 25-year history of bipolar affective disorder, supposedly asymptomatic in the last 10 years with no psychopharmacologic treatment (previously treated only with valproic acid in an unknown dosage), was admitted to the emergency department with a 2-week history of elated humor, increased vital energy, accelerated thinking, decreased need to sleep, disinhibition, and delusional ideas of grandiose and mystic content.
There were no known comorbid medical conditions or history of drug use or allergies. Physical and neurologic examinations were within normal limits. Routine laboratory tests at admission and the electrocardiogram result revealed no significant abnormalities. The patient was diagnosed with a maniac episode with mood-congruent psychotic features according to DSM-5 criteria and admitted to the psychiatry department.
Treatment with valproic acid was initiated and titrated to 1,000 mg per day and diazepam 20 mg per day. After 6 days, there was no improvement in the patient’s condition, namely of psychotic symptoms, so ziprasidone was added at a dosage of 80 mg per day, administrated in 2 doses after lunch and dinner. Unfortunately, it was not tolerated due to extrapyramidal symptoms and had to be suspended 13 days later. Treatment with aripiprazole was initiated and titrated up to 30 mg per day.
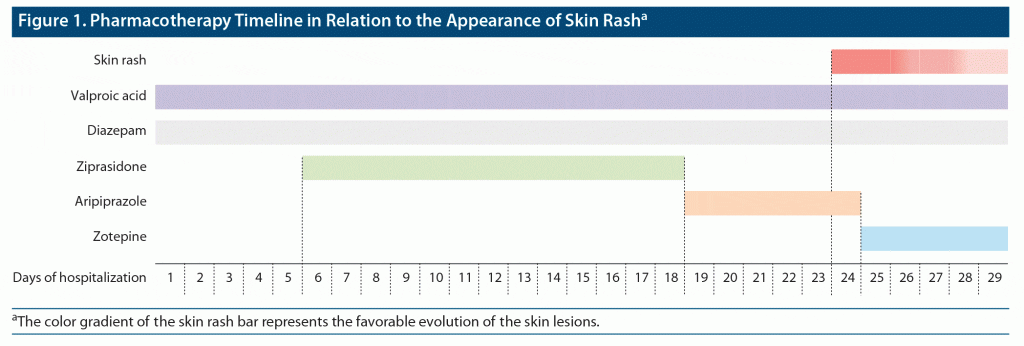
After 6 days of aripiprazole therapy, the patient developed an erythematous morbilliform maculopapular skin rash, pruritic but not scaly. Initially, the distribution was limited to both legs and forearms but progressively spread to the thoraco-abdominal region. Edema of the upper and lower extremities was also present. Infectious and other systemic diseases were excluded. The laboratory tests showed no abnormal results, including normal renal function, ionogram, and N-terminal pro–b-type natriuretic peptide. Valproic acid serum concentration never reached toxicity, and a dermatologist observation confirmed the diagnosis of a toxycodermal reaction. Aripiprazole was suspended, and zotepine 100 mg was added. There was a significant improvement of the skin rash, disappearing within 6 days (Figure 1).
The patient was discharged on the 29th day after admission with improvement in her psychiatric symptomatology, and there were no skin lesions or edema remaining. Valproic acid 1,000 mg per day, zotepine 100 mg per day, and diazepam 10 mg per day were maintained. At the time of this writing, the patient is undergoing follow-up treatment at our outpatient clinic and maintains remission of psychiatric symptoms.
Discussion
Aripiprazole is an atypical antipsychotic with a partial agonistic action at dopamine (D2) and serotonin (5-HT1a) receptors and an antagonistic action at serotonin (5-HT2a) receptors.2 Through this combination of mechanisms, it stabilizes the dopamine and serotonin systems.3 The most commonly reported adverse effects at recommended doses (10–30 mg per day) include restlessness, sedation, extrapyramidal symptoms, blurred vision, headache, fatigue, and nausea.4
Dermatologic reactions were not reported during clinical trials, as stated in the Summary of Product Characteristics for aripiprazole.5 During postmarketing surveillance, rash, photosensitivity reaction, alopecia, and hyperhidrosis were noticed as adverse reactions with undetermined incidence.3 A literature search using MEDLINE identified 2 case reports3,6 of cutaneous reactions with aripiprazole treatment.
One of the most common types of drug-induced skin rash is the morbilliform rash. It frequently appears within 1 to 2 weeks after the drug initiation, disappearing after the drug discontinuation.6,7 It is worth stating that diazepam’s label advises about the possibility to cause skin reactions, and there are some skin rash reports that occur mainly due to the hypersensitivity to this drug.8,9
In the present case, the patient had already taken valproic acid with no adverse reactions. Diazepam was introduced at the hospital admission and maintained during the treatment course despite the evolution of the skin rash, so it seems unlikely to be the culprit. Thus, aripiprazole was the only plausible cause for the rash, which was confirmed by the remission of the skin lesions after discontinuation.
Published online: July 15, 2021.
Potential conflicts of interest: None.
Funding/support: None.
Patient consent: Consent was received from the patient to publish the case report, and information has been de-identified to protect anonymity.
References (9)

- Warnock JK, Morris DW. Adverse cutaneous reactions to antipsychotics. Am J Clin Dermatol. 2002;3(9):629–636. PubMed CrossRef
- de Bartolomeis A, Tomasetti C, Iasevoli F. Update on the mechanism of action of aripiprazole: translational insights into antipsychotic strategies beyond dopamine receptor antagonism. CNS Drugs. 2015;29(9):773–799. PubMed CrossRef
- Parker C. Aripiprazole induced severe and extensive skin reaction: a case report. Ther Adv Psychopharmacol. 2012;2(5):195–198. PubMed CrossRef
- Argo TR, Carnahan RM, Perry PJ. Aripiprazole, a novel atypical antipsychotic drug. Pharmacotherapy. 2004;24(2):212–228. PubMed CrossRef
- Summary of Product Characteristics. Abilify (aripiprazole) [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc; 2014.
- Nath S, Rehman S, Kalita KN, et al. Aripiprazole-induced skin rash. Ind Psychiatry J. 2016;25(2):225–227. PubMed CrossRef
- Svensson CK, Cowen EW, Gaspari AA. Cutaneous drug reactions. Pharmacol Rev. 2001;53(3):357–379. PubMed
- Ancuceanu R, Dinu M, Furtunescu F, et al. An inventory of medicinal products causing skin rash: clinical and regulatory lessons. Exp Ther Med. 2019;18(6):5061–5071. PubMed CrossRef
- Haybarger E, Young AS, Giovannitti JA Jr. Benzodiazepine allergy with anesthesia administration: a review of current literature. Anesth Prog. 2016;63(3):160–167. PubMed CrossRef
Enjoy this premium PDF as part of your membership benefits!