CASE CONFERENCE
The Banner Alzheimer’s Institute Case Conference is a weekly event in which physicians and staff discuss challenging and/or teaching cases of patients seen at the Institute’s Stead Family Memory Clinic. These conferences are attended by a multidisciplinary group that includes Banner Alzheimer’s Institute dementia specialists, community physicians (internal medicine, family medicine, and radiology), physician assistants, social workers, nurses, medical students, residents, and fellows. The Banner Alzheimer’s Institute, located in Phoenix, Arizona, has an unusually ambitious mission: to end Alzheimer’s disease without losing a generation, set a new standard of care for patients and families, and forge a model of collaboration in biomedical research. The Institute provides high-level care and treatment for patients affected by Alzheimer’s disease, dementia, and related disorders. In addition, the Institute offers extensive support services for families and many unique and rewarding research opportunities.
Prim Care Companion CNS Disord 2021;23(5):21alz03101
To cite: Gopalakrishna G, Patel N. Do not drink, do not drive: alcohol use disorder in the elderly. Prim Care Companion CNS Disord. 2021;23(5):21alz03101.
To share: https://doi.org/10.4088/PCC.21alz03101
© Copyright 2021 Physicians Postgraduate Press, Inc.
Ganesh Gopalakrishna, MD, is a geriatric psychiatrist and dementia specialist at Banner Alzheimer’s Institute and a clinical associate professor of psychiatry at the University of Arizona College of Medicine, Phoenix, Arizona.
Nisha Patel, DO, is a postgraduate year 4 resident physician in the Department of Psychiatry at the University of Arizona College of Medicine, Phoenix, Arizona.
Published online: October 21, 2021.
*Corresponding author: Ganesh Gopalakrishna, MD, Stead Memory Clinic, Banner Alzheimer’s Institute, 901 E Willetta St, Phoenix AZ 85006 ([email protected]).
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders
CME Background
Articles are selected for credit designation based on an assessment of the educational needs of CME participants, with the purpose of providing readers with a curriculum of CME articles on a variety of topics throughout each volume. This special series of case reports about dementia was deemed valuable for educational purposes by the Publisher, Editor in Chief, and CME Institute staff. Activities are planned using a process that links identified needs with desired results.
To obtain credit, read the article, correctly answer the questions in the Posttest, and complete the Evaluation. This activity is free.
CME Objective
After studying this article, you should be able to:
- Screen elderly patients for alcohol use disorder
Accreditation Statement
The CME Institute of Physicians Postgraduate Press, Inc., is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Release, Expiration, and Review Dates
This educational activity was published in October 2021 and is eligible for AMA PRA Category 1 Credit™ through October 31, 2023. The latest review of this material was October 2021.
Financial Disclosure
All individuals in a position to influence the content of this activity were asked to complete a statement regarding all relevant personal financial relationships between themselves or their spouse/partner and any commercial interest. The CME Institute has resolved any conflicts of interest that were identified. In the past year, Larry Culpepper, MD, MPH, Editor in Chief, has been a consultant for AbbVie, Acadia, Allergan, Eisai, Merck, and Takeda; has been a stock shareholder of M-3 Information; and has received royalties from UpToDate and Oxford University Press. No member of the CME Institute staff reported any relevant personal financial relationships. Faculty financial disclosure appears at the end of the article.
HISTORY OF PRESENTING ILLNESS
Mr A is an 82-year-old Hispanic man who presented with his family to the memory clinic at Banner Alzheimer’s Institute for evaluation of cognitive impairment. He was referred to the memory clinic by his primary care physician (PCP) after his memory symptoms persisted despite improvement of mood with a selective serotonin reuptake inhibitor. Although his mood was better controlled, Mr A had started drinking more between his PCP visits and the first memory clinic appointment. The PCP’s long-term follow-up plans included a family meeting with the social worker at Banner Alzheimer’s Institute to address additional resources available to the patient and family. The family was continuously involved in his care, and his wife was the primary caregiver.
Mr A was a poor historian due to limited insight. His family reported that, progressively over the last 2 years, he was forgetting details of recent events and conversations and had difficulty tracking dates and appointments. He had forgotten the names of his daughters and was unable to recognize the relationship between different family members. He was also repeating questions, had word-finding difficulty, and was having trouble understanding others and making judgements. He was misplacing items and thinking others were stealing from him.
Mr A reported a problem using the phone but has always had problems using technology. His wife took over the finances 6 months prior to his first memory clinic appointment because he was not paying bills and was unable to balance the checkbook. He reported taking medications independently and goes shopping for alcohol by himself. He is independent in bathing, brushing his teeth, dressing, toileting, and eating. He does need reminders to shave, shower, and comb his hair. Mr A continued to drive, but his daughter had concern over his speed, turning in front of others, straddling lanes, running over curbs, getting lost, and getting angry while driving. He did get lost once while driving.
Mr A’s family reported that he had anxiety when separating from his wife but would still leave the home to obtain alcohol; he also had anhedonia and irritability, which had improved with citalopram. Mr A reported a good mood, but his sleep was disturbed, and he acted out in his dreams. He once even fell out of bed due to these sleep behaviors. He reported good energy levels, but his appetite was poor, and he often did not eat when drinking alcohol. He denied suicidal ideations, homicidal ideations, or auditory or visual hallucinations. He reported paranoia and delusions about people stealing from him, but no other delusions or paranoia. He often repeatedly checked locks and the mail, even on Sundays.
PAST MEDICAL HISTORY
Mr A denied any head trauma, stroke, or seizures. He had a history of hypertension, chronic kidney disease, vitamin B12 deficiency, and macrocytic anemia likely secondary to chronic alcohol use.
ALLERGIES
Mr A had no known drug allergies.
MEDICATIONS
Citalopram 10 mg/d
Ferrous sulfate 235 mg/d
Folic acid 1 mg/d
Hydralazine 25 mg twice/d
Metoprolol succinate extended release 25 mg/d
Sodium bicarbonate 10 g 3 times/d
Vitamin B12 1,000 mcg/d
Vitamin D2 50,000 IU/week
SOCIAL HISTORY
Mr A lived with his wife and grandson. He did not exercise on a routine basis but worked in the yard. He had a high school education. He was retired since 2004. He worked at a warehouse operating forklifts for Walmart for 15–20 years. He liked hunting, fishing, camping, playing the lottery, and boxing. He and his wife have been married for over 57 years. He had 4 daughters and 2 sons who were supportive but did not live with him. He learned English in grade school at age 7.
PSYCHIATRIC HISTORY
Mr A denied any psychiatric hospitalizations or suicide attempts. He was started on citalopram in 2018 for depression.
FAMILY HISTORY
Mr A stated that his sister developed dementia in her 80s, and his brothers developed dementia in their 70s. He stated that all his siblings and half siblings have or had dementia.
MENTAL STATUS EXAMINATION
Mr A appeared alert, well nourished, and in no acute distress. His rate, volume, and articulation of speech were normal. His thought process was sequential, coherent, and logical. His associations were intact and appropriate. He did not display loose associations and had no abnormal or psychotic thought processing, including hallucinations, delusions, obsessions, phobia, or compulsions. Mr A denied suicidal and homicidal ideation. He had impaired insight and judgement. He self-described his mood as good, and his affect was mood congruent, reactive, and appropriate.
The DSM-5 (American Psychiatric Association, 2013) defines a major neurocognitive disorder as follows:
- Evidence of significant cognitive decline from a previous level of performance in 1 or more areas of cognitive domains (complex attention, executive function, learning and memory, language, perceptual-motor or social cognition) based on:
- Concern of the individual, a knowledgeable informant, or the clinician that there has been a significant decline in cognitive function and
- Substantial impairment in cognitive performance, preferably documented by standardized neuropsychological testing or, in its absence, another quantified clinical assessment.
- The cognitive deficits interfere with independence in everyday activities.
- The cognitive deficits do not occur exclusively in the context of a delirium.
- The cognitive deficits are not better explained by another mental disorder.
It is important to rule out potentially reversible causes of cognitive decline, like substance-induced cognitive disorders, before considering neurodegenerative disorders. There is mention of alcohol use in Mr A’s history. It is important to obtain a comprehensive history of substance abuse when evaluating a patient for cognitive decline. Health care professionals frequently ignore screening elderly patients for problematic alcohol use (DiBartolo and Jarosinski, 2017).
Mr A initially reported drinking 2 beers per night. He noted that he had cut down his use, due to family pressure and initial behavioral interventions with his PCP, from half a bottle of wine daily. However, his family noted him averaging 21 cans of beer a week with some wine. He does have a history of a few citations for driving while intoxicated. He denied periods of sobriety. He had been drinking an 8 pack of beer per day for decades. He received a daily allowance of $20 from his wife, $15 of which he spent on alcohol. He walks to the store multiple times a day and has threatened to go to the bank if his wife does not give him money. He frequently stumbles when walking. He has had multiple falls and recently was found in the trash can, likely by falling over it. He sleeps poorly when intoxicated and will sleep until 2 pm. The following day, he does not remember his behaviors while intoxicated and apologizes for them. He reported smoking marijuana in the past for decades but has not used the substance in the last 5 years. He has never smoked tobacco cigarettes or used other tobacco products.
PHYSICAL AND NEUROLOGIC EXAMINATION
Mr A’s physical examination showed mild abnormalities in vision and hearing, but no localizing or lateralizing neurologic findings were present. Cranial nerve testing was symmetric, and his pupils were equal, round, and reactive to light. Sensation was grossly intact. Coordination testing was accurate. Deep tendon reflexes were symmetric with no pathologic reflexes. Station and gait were normal.
It is important to screen for any physical sequelae of continued alcohol use, which could include jaundice, anemia, hepatomegaly, pedal edema, ataxia, and neuropathy. No stigmata were noted in this patient.
Mr A scored 18 on the MMSE (Folstein et al, 1975), which is in the moderately impaired range. He missed points on orientation registration, attention, comprehension, copying the drawing, and recall.
Mr A scored 13 on the MoCA (Nasreddine et al, 2005), suggesting moderate impairment. He demonstrated impairment in visuospatial tasks, attention, orientation language, abstraction, and delayed recall.
For alcohol use disorder workup, especially when presenting with cognitive or memory concerns, it is imperative to check the patient’s thiamine level. Thiamine deficiency is common with chronic alcohol use and is caused by malnutrition, nausea, and vomiting. Alcohol also reduces the gastrointestinal tract’s ability to absorb thiamine appropriately. The liver, if diseased by alcohol, may also have difficulty storing thiamine. Although there is a possibility of reversing some effects of the deficiency, thiamine repletion will often just reduce the progress of brain changes associated with alcohol (Zahr and Pfefferbaum, 2017).
Imaging is important in the evaluation of memory decline, especially given its utility to rule out major organic etiologies such as tumors, cerebrovascular accident, or other pathologies. In this case, we can use imaging to look for brain change patterns and radiologic signatures associated with alcohol-related brain diseases. For example, in Wernicke-Korsakoff syndrome caused by chronic alcoholism and thiamine deficiency, brain imaging has shown volume deficits not only in mammillary bodies, but also in the hippocampus. Anterograde amnesia is one of the key findings related to Wernicke-Korsakoff syndrome (Zahr and Pfefferbaum, 2017).
Given that alcohol may be confounding the presentation of cognitive decline in Mr A, neuropsychological testing would be more appropriate following reduction or abstinence in alcohol use. In this case, the physician’s workup included obtaining folate level, complete blood count (CBC), comprehensive metabolic panel (CMP), vitamin B12 level, and thyroid-stimulating hormone (TSH) level to rule out reversible causes of dementia. Magnetic resonance imaging was also ordered. The physician provided psychoeducation on the effects of alcohol use on memory.
LABORATORY AND RADIOLOGY RESULTS
Mr A had recently had a RPR (rapid plasma reagin) test, which was negative. CBC results revealed low hemoglobin, and CMP showed kidney dysfunction. Liver function tests were within normal limits. His vitamin B12 level was low, and his folate and TSH levels were within normal limits.
THE TREATING PHYSICIAN’S IMPRESSION
Based on the history provided, the clinical presentation, and the results of the cognitive and physical examination, the treating physician felt that Mr A had a mild to moderate neurocognitive disorder (per DSM-5 criteria). The pattern of symptoms and signs was typical of Alzheimer’s disease, but in this case, mixed dementia remained a consideration—specifically, Alzheimer’s disease combined with cognitive impairment secondary to long-term alcohol use.
The DSM-5 (American Psychiatric Association, 2013) defines alcohol use disorder as follows:
Requires a problematic pattern of alcohol use leading to clinically significant impairment or distress as manifested by at least 2 of the following in a 12-month period:
- Drinking more alcohol over a longer period than intended
- Persistent desire or unsuccessful effort to reduce alcohol use
- Great deal of time spent obtaining and drinking alcohol, recovering from use
- Cravings
- Failure to fulfill major role obligations
- Continued use despite awareness of interpersonal or social problems
- Giving up activities for alcohol
- Use in hazardous situations
- Continued use despite awareness of physical or psychological problems
- Tolerance noted by markedly increased amount of use or diminished effects of use
- Characteristic withdrawal syndrome or needing to take substance to prevent withdrawal.
There are some challenges when applying the DSM-5 criteria to older adults. Socio-occupational dysfunction may be difficult to apply to elderly patients who are retired and living alone at home. Tolerance is also difficult to define, as impairment may happen at lower doses without need for escalation. In the case of Mr A, he clearly meets multiple criteria, validating a diagnosis of alcohol use disorder.
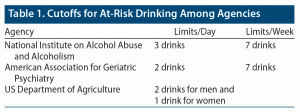
There are multiple ways to define excessive alcohol use. Heavy drinking is defined as alcohol consumption that has resulted in 1 or more adverse problems (medical, legal, family, psychological, financial, or social). The National Institute on Alcohol Abuse and Alcoholism defines heavy drinking as more than 4 drinks in men and more than 3 drinks in women in a given day. At-risk drinking is defined as alcohol consumption that increases the chance of developing alcohol-related problems and complications. The cutoffs defined for at-risk drinking vary among different agencies (Table 1).
The likelihood of having an alcohol-related discussion with patients declines as the patient ages. There are reliable and easy-to-administer screening instruments for ambulatory settings. The MAST-G (Blow et al, 1992) is specifically designed for older adults. It is a 24-item questionnaire with yes/no responses. More than 5 affirmative responses on the scale indicate problematic use. Sensitivity of this tool is 91%–93%, and specificity is 65%–84%. The AUDIT (Saunders et al, 1993) is a validated 10-item screening tool developed by the World Health Organization that can be self-administered. Scores can range from 0 to 40, with higher scores indicating greater use. The recommended cutoff is 8, but in older adults, the cutoff is lowered to 5. The CAGE (Ewing, 1984) is not a great screening tool for older adults, and it does not detect binge drinking well. The SASSI (Miller, 1985) is a widely used tool that is effective in identifying those who are in denial or purposefully hiding their substance use.
Older adults have demonstrated good or even better outcomes compared to younger adults with regard to alcohol use treatment. An age-specific, supportive, nonconfrontational approach is more successful than assertive styles. Outcomes are better in older women compared to men. Outcomes are also better with longer treatment duration. Some barriers to timely treatment do exist including lack of awareness, time constraints, hesitancy among providers, burden of comorbidities, access to transportation, coexisting depression and anxiety, solitude, and extent of family support.
Alcohol withdrawal should be considered as a differential cause for any older patient presenting with confusion. Elderly patients may have atypical withdrawal symptoms. Onset of withdrawal may not occur until several days after cessation of drinking. Confusion, rather than tremor and tachycardia, is often the predominant clinical sign in the elderly. The severity and duration of withdrawal tend to increase with age. Interview of family members can be crucial in establishing the diagnosis. Uncomplicated withdrawal can be managed at home, with family present at all times. Similar to the younger adult patient, benzodiazepines are used for symptomatic treatment during withdrawal.
Psychosocial interventions focus on overcoming isolation and establishing social support through increased family visits or having the patient visit a senior center. Psychosocial interventions can be challenging, especially given the ongoing cognitive decline.
Pharmacologic treatment has not been studied adequately in older adults, but it must be considered. The final goal of treatment is abstinence, but reducing drinking can be a reasonable goal with risk mitigation. Pharmacologic treatment should always be combined with behavioral treatment. In this case, Mr A was started on naltrexone 25 mg. The next appropriate step is to increase the dose to 50 mg if the patient can tolerate the lower dose (Kranzler and Soyka, 2018).
Naltrexone is a nonselective opioid antagonist, and it limits heavy drinking by curbing the craving for alcohol, which can reduce the risk of heavy drinking. There is minimal evidence supporting its use in the elderly. Two small randomized controlled trials (RCTs) with subjects aged 50 to 70 years showed reduced rate of relapse with naltrexone treatment (Kranzler and Soyka, 2018). This medication is especially useful since it can be started once daily while the patient is still drinking; however, the patient should not be receiving opioid pain medications. Eventually, the patient can transition to a monthly injection.
Acamprosate modulates glutaminergic transmission. The US Food and Drug Administration approved acamprosate for sustaining abstinence; however, there is limited evidence for effectiveness in the elderly. Gastrointestinal symptoms, especially diarrhea, are the most common side effect. However, this medication has 3-times-daily dosing, which can lead to noncompliance (Kranzler and Soyka, 2018).
Disulfiram is an acetaldehyde dehydrogenase inhibitor. If a patient taking disulfiram consumes alcohol, they may experience a severe reaction including diaphoresis, flushing, and hypotension. Disulfiram should be discontinued if the patient continues to drink while receiving treatment. Disulfiram has better results in open-label studies than RCTs. It works better in supervised settings, and the most common side effect is drowsiness. However, drug interactions and coexisting medical conditions limit the use of disulfiram in the elderly.
Topiramate is an anticonvulsant that has shown some evidence to reduce binge drinking; however, it has significant central nervous system side effects, including impaired attention, paresthesia, weight loss, and headache. There is limited evidence for gabapentin use in treating alcohol disorder. Negative side effects include sedation, dizziness, or gait disturbances (Kranzler and Soyka, 2018).
DISCUSSION
Alcohol is the most-used substance. As with the younger population, drinking is a part of social engagement for older adults, and people often adopt or share drinking habits of their peers and partners. Rates of substance use are generally lower among older adults than younger people. According to a 2015 study by the National Survey on Drug Use and Health (NSDUH), half of individuals aged ≥ 65 years and a quarter of those ≥ 85 years drink alcohol (Lehmann and Fingerhood, 2018). The young old, defined as those aged 65–74 years, have been found to use alcohol more frequently. Binge drinking for adults aged ≥ 65 years is defined as occasional periods of loss of control drinking characterized by 4 or more drinks on 1 occasion and enough to produce a blood alcohol level > 0.08. Binge drinking is more prevalent among elderly alcohol users. The prevalence rates for at-risk drinking are estimated to be 16% in men and 10.9% in women. Rates of older adult binge drinking are 19.6% for men and 6.3% for women, as seen in 2005–2008 NSDUH data (Lehmann and Fingerhood, 2018).
Physiologic changes affect pharmacokinetics, leading to increased susceptibility to harmful effects of heavy chronic alcohol use in the elderly. There is no evidence that alcohol metabolism is significantly changed with aging, but the ability of the liver to process alcohol can diminish. Blood-brain barrier permeability and neuronal receptor sensitivity to alcohol in the brain also increase with age. The elderly often have decreased lean body mass, total body water, and gastric enzymes (Lehmann and Fingerhood, 2018; Moore et al, 2003; National Institute on Alcohol Abuse and Alcoholism).
To further add to these challenges, older adults are more likely to have multiple chronic health conditions necessitating use of prescription medications that can interact with alcohol and other substances. They already have an increased risk of falls, confusion, cognitive impairment, and medical comorbidity, leading to frequent hospitalizations, increased health care costs, loss of independence, and social isolation. This risk increases further with alcohol use (American Association for Geriatric Psychiatry).
History of heavy alcohol use is a well-established risk factor for development of dementia. Interestingly, moderate alcohol consumption (not heavy chronic use) is associated with decreased morbidity and mortality among older adults. Light to moderate use also has been suggested to lower risk of dementia compared to abstainers and heavy users. A U-shaped or J-shaped relationship between alcohol use and risk of dementia is demonstrated in multiple epidemiologic studies (Rehm et al, 2019). Notably, a 23-year prospective cohort study with more than 9,000 participants aged 35–55 years showed that risk of dementia was increased in people who abstained from alcohol in midlife or consumed greater than 14 units per week (Sabia et al, 2018). This relationship is far from being settled, with other imaging studies showing negative consequences on brain volume and white matter changes. Identification and management of alcohol use among people with dementia is poorly studied.
Those caring for the elderly often assume or ignore substance use screening. However, it is imperative to target and address potential risk factors or contributors to cognitive changes. Further research with substance use in the elderly is warranted to enhance knowledge, approaches, and treatment for this population.
FUNDING/SUPPORT
None.
DISCLOSURE OF OFF-LABEL USAGE
The authors have determined that, to the best of their knowledge, no investigational information about pharmaceutical agents or device therapies that is outside US Food and Drug Administration–approved labeling has been presented in this article.
FINANCIAL DISCLOSURE
Drs Gopalakrishna and Patel have no personal affiliations or financial relationships with any commercial interest to disclose relative to this article.
DISCLAIMER
The opinions expressed are those of the authors, not of Banner Health or Physicians Postgraduate Press.
CLINICAL POINTS
- When older adult patients present with cognitive decline, it is important to rule out any organic, substance-induced, or mood-related etiology.
- It is imperative to perform alcohol use screening in primary care settings for older adults who often are not screened properly, as this could provide insight into limitations of activities of daily living and cognitive issues.
- The Alcohol Use Disorders Identification Test and Michigan Alcohol Screening Test–Geriatrics Version are good screening tools for older adults with heavy alcohol use.
- Speaking to older adults about excessive alcohol use is best addressed by nonconfrontational, supportive conversation and use of long-term treatment.
- Family support can lead to better outcomes for older adults with alcohol use disorder.
References
American Association for Geriatric Psychiatry. Alcohol/Drug Abuse/Misuse. https://www.aagponline.org/index.php?src=gendocs&ref=substanceabuse&category=Foundation
American Association for Geriatric Psychiatry–Alcohol/Drug Abuse/Misuse. American Association for Geriatric Psychiatry–Alcohol/Drug Abuse/Misuse website. Accessed September 1, 2021. https://www.aagponline.org/index.php?src=gendocs&ref=substanceabuse&category=Foundation
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fifth Edition. DSM-5. Washington, DC: American Psychiatric Publishing; 2013.
Blow F, Brower K, Schulenberg J, et al. The Michigan Alcoholism Screening Test–Geriatric Version (MAST-G): A new elderly specific screening instrument. Alcohol Clin Exp Res. 1992;16:372.
DiBartolo MC, Jarosinski JM. Alcohol use disorder in older adults: challenges in assessment and treatment. Issues Ment Health Nurs. 2017;38(1):25–32. PubMed CrossRef
Ewing JA. Detecting alcoholism. The CAGE questionnaire. JAMA. 1984;252(14):1905–1907. PubMed CrossRef
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. PubMed CrossRef
Kranzler HR, Soyka M. Diagnosis and pharmacotherapy of alcohol use disorder: a review. JAMA. 2018;320(8):815–824. PubMed CrossRef
Lehmann SW, Fingerhood M. Substance-use disorders in later life. N Engl J Med. 2018;379(24):2351–2360. PubMed CrossRef
Miller GA. The Substance Abuse Subtle Screening Inventory (SASSI) Manual, Second Edition. Springville, IN: The SASSI Institute;1985, 1999.
Moore AA, Endo JO, Carter MK. Is there a relationship between excessive drinking and functional impairment in older persons? J Am Geriatr Soc. 2003;51(1):44–49. PubMed CrossRef
Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. PubMed CrossRef
National Institute on Alcohol Abuse and Alcoholism. Drinking Levels Defined. Accessed September 20, 2021. https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking
National Institute on Alcohol Abuse and Alcoholism. Older Adults. NIAAA website. Accessed September 1, 2021. https://www.niaaa.nih.gov/alcohols-effects-health/special-populations-co-occurring-disorders/older-adults
Rehm J, Hasan OSM, Black SE, et al. Alcohol use and dementia: a systematic scoping review. Alzheimers Res Ther. 2019;11(1):1. PubMed CrossRef
Sabia S, Fayosse A, Dumurgier J, et al. Alcohol consumption and risk of dementia: 23-year follow-up of Whitehall II cohort study. BMJ. 2018;362:k2927. PubMed
Saunders JB, Aasland OG, Babor TF, et al. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons With Harmful Alcohol Consumption–II. Addiction. 1993;88(6):791–804. PubMed CrossRef
US Department of Agriculture. Dietary Guidelines for Americans 2015–2020. Eighth Edition. Accessed September 30, 2021. https://health.gov/sites/default/files/2019-09/2015-2020_Dietary_Guidelines.pdf
Zahr NM, Pfefferbaum A. Alcohol’s effects on the brain: neuroimaging results in humans and animal models. Alcohol Res. 2017;38(2):183–206. PubMed
Enjoy this premium PDF as part of your membership benefits!