Because this piece does not have an abstract, we have provided for your benefit the first 3 sentences of the full text.
Duloxetine is a commonly used antidepressant, with over 10 million prescriptions written per month in 2014 according to IMS Health. The medication acts by inhibiting the reuptake of serotonin and norepinephrine, increasing the concentration of these neurotransmitters in the brain. This dual mechanism enables duloxetine to be efficacious in the treatment of many disease states.

Dangers of Rapid Dosing: A Case of Dose-Dependent Drug-Induced Liver Injury From Duloxetine
Duloxetine1 is a commonly used antidepressant, with over 10 million prescriptions written per month in 2014 according to IMS Health.2 The medication acts by inhibiting the reuptake of serotonin and norepinephrine, increasing the concentration of these neurotransmitters in the brain. This dual mechanism enables duloxetine to be efficacious in the treatment of many disease states. It is approved by the US Food and Drug Administration for major depressive disorder as well as generalized anxiety disorder, diabetic neuropathy, fibromyalgia, and chronic musculoskeletal pain. Duloxetine is generally well tolerated, but can potentially elevate liver enzymes and in rare cases cause liver damage. While cases of hepatotoxicity associated with duloxetine use have been documented by Vuppalanchi et al,3 no studies have demonstrated this relationship to be dose-dependent.
Case Report
We present the case of a 37-year-old woman with a known history of major depressive disorder, unspecified anxiety disorder, alcohol use disorder, and opioid use disorder. She initially presented with depressive symptoms, including suicidal ideation, and was admitted to the inpatient psychiatric unit for acute stabilization. Given her uncontrolled symptoms of depression and anxiety and history of chronic pain, the patient was started on duloxetine 40 mg daily (9 am). She had no other significant comorbidities, and duloxetine was the only scheduled medication she was receiving while an inpatient. She tolerated the medication well and reported an improvement in depressive symptoms. After 3 doses of duloxetine 40 mg, her dose was subsequently increased from 40 mg to 60 daily (day 4 at 9 am) to target residual depressive symptoms. Twenty-four hours after the dose increase, the patient presented with significant anxiety, bilateral tremor, hyperthermia, diaphoresis, and autonomic instability. Her physical examination was remarkable for a mild right upper quadrant tenderness. A comprehensive inpatient cardiac evaluation identified no evidence of myocardial ischemia or dysfunction. Tests results for viral hepatitis and autoantibodies were negative.
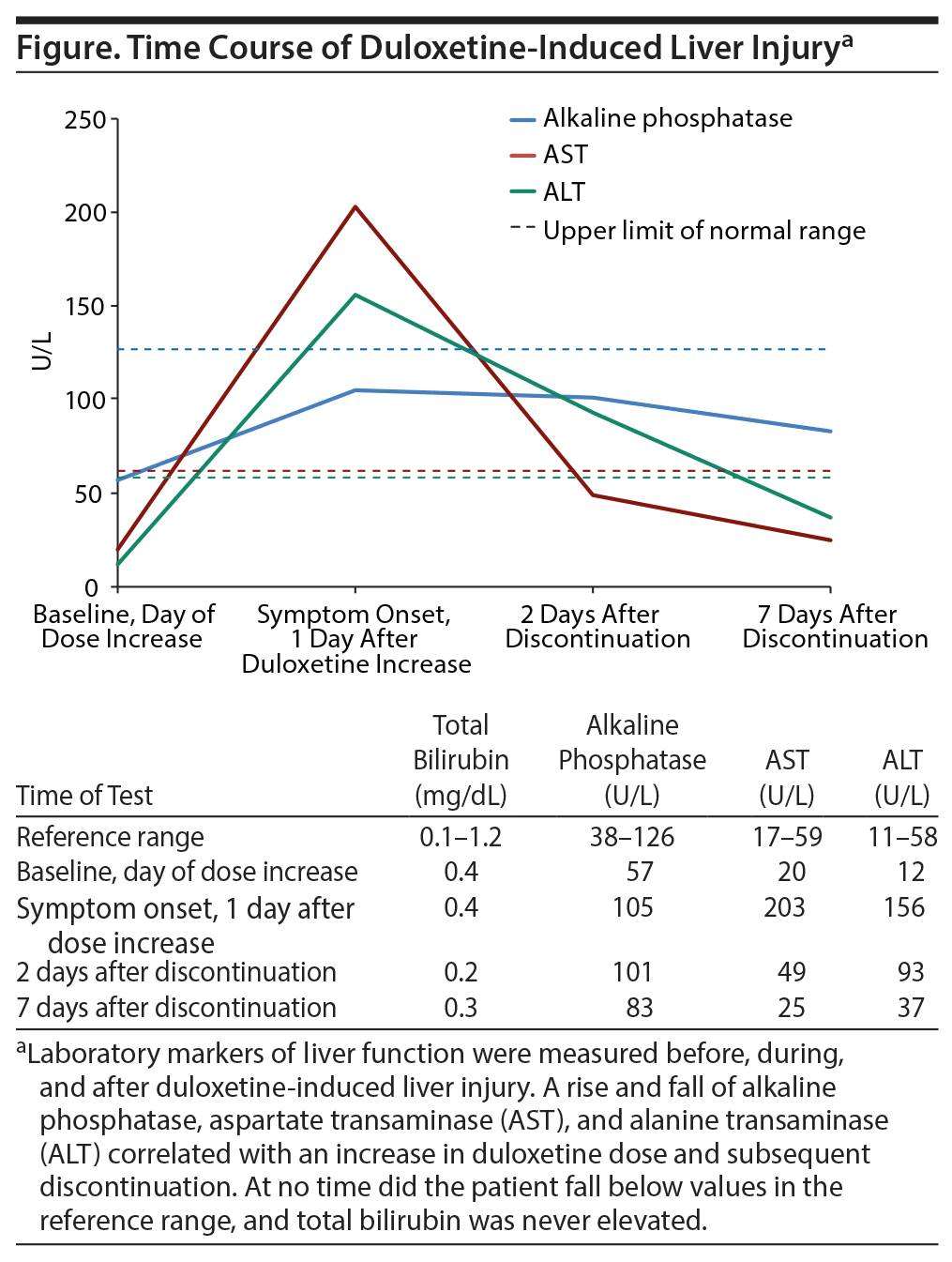
Laboratory markers of liver function were measured before, during, and after duloxetine-induced liver injury. A rise and fall of alkaline phosphatase, AST (aspartate transaminase), and ALT (alanine transaminase) correlated with an increase in duloxetine dose and subsequent discontinuation.
The patient’s initial laboratory work revealed normal liver function tests upon admission (see the table in the Figure). Repeated laboratory tests performed at the time of symptom onset revealed significant increases in alkaline phosphatase, AST, and ALT over baseline. All medications were held, and the patient was given a 1-liter bolus of normal saline. Forty-eight hours after the cessation of medication and fluids, the patient showed improvement of physical symptoms, and hepatic enzymes began to normalize. Symptoms fully resolved and laboratory markers returned to baseline within 1 week of duloxetine discontinuation (see Figure).
Discussion
This case offers an example of acute drug-induced liver injury occurring after a dosage increase of duloxetine. Risk of hepatotoxicity with duloxetine has been reported in case reports before3; however, this case is important because the injury appears after the patient did well initially on duloxetine. A dose increase appears to have resulted in rapid autonomic dysfunction and liver injury. The mechanism by which duloxetine causes hepatotoxicity remains unknown. Some case studies identified by Vuppalanchi et al3 have noted that biopsies indicate primary hepatocellular pattern of injury versus cholestatic. These lesions are quite likely caused by a metabolite of duloxetine, though further research is needed in this area. Until such time, the mechanism remains idiosyncratic in nature. Our patient possessed significant risk factors for hepatic dysfunction including female sex and alcohol consumption; however, no underlying disease process was identified in this case. It further should be noted that providers should have a heightened level of cognizance in individuals with several risk factors for liver injury. Also, she did not present with jaundice or elevated bilirubin. The onset of symptoms 24 hours after a duloxetine dose increase and a corresponding significant elevation in hepatic enzymes support the etiology of a dose-dependent drug-induced liver injury in this patient.
Published online: March 21, 2019.
Potential conflicts of interest: None.
Funding/support: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Patient consent: The patient gave permission for submission of this case for publication, and all information has been de-identified to protect anonymity.
REFERENCES
1. Cymbalta (duloxetine hydrochloride) delayed-release capsules for oral use [package insert]. Indianapolis, IN: Eli Lilly and Company; 2008.
2. Health IMS. Most Prescribed Psychiatric Drugs Monthly (July 2013 – June 2014). Mental Health Daily. November 29, 2014. http://www.mentalhealthdaily.com/2014/08/11/most-prescribed-psychiatric-drugs-monthly-june-2013-july-2014. Accessed November 20, 2017.
3. Vuppalanchi R, Hayashi PH, Chalasani N, et al; Drug-Induced Liver Injury Network (DILIN). Duloxetine hepatotoxicity: a case-series from the Drug-Induced Liver Injury Network. Aliment Pharmacol Ther. 2010;32(9):1174-1183. PubMed CrossRef
aDepartment of Psychiatry, Community Health Network, Indianapolis, Indiana
bPsychiatry Residency Program, Community Health Network, Indianapolis, Indiana
cDepartment of Pharmacy, Community Health Network, Indianapolis, Indiana
dDepartment of Pharmacy Practice, Butler University College of Pharmacy and Health Sciences, Indianapolis, Indiana
*Corresponding author: Areef S. Kassam, MD, MPA, Department of Psychiatry, Community Health Network, 7165 Clearvista Way, Indianapolis, IN 46256 ([email protected]).
Prim Care Companion CNS Disord 2019;21(2):18l02333
To cite: Kassam AS, Cunningham EA, Musco SE. Dangers of rapid dosing: a case of dose-dependent drug-induced liver injury from duloxetine. Prim Care Companion CNS Disord. 2019;21(2):18l02333.
To share: https://doi.org/10.4088/PCC.18l02333
© Copyright 2019 Physicians Postgraduate Press, Inc.
Please sign in or purchase this PDF for $40.00.