Objective: Glucagon-like peptide (GLP-1) analogs promote diabetes control and weight loss. Most GLP-1 analogs also lower adverse cardiovascular outcomes, making them ideal agents for patients with severe mental illness. The objective of this study was to analyze diabetic patients taking antipsychotic medications, comparing those on GLP-1 analogs with those on other diabetes treatments.
Methods: A total of 46 patients referred to outpatient diabetes clinics between July 2010 and April 2017 who were prescribed antipsychotic medications during the entire study period were included in this retrospective analysis. Eleven (24%) patients were started on a GLP-1 analog (cases), and 35 (76%) were treated with alternative antidiabetic agents (controls).
Results: Cases and controls did not differ in age, sex, height, weight, or medical therapies at the time of referral. Within 1 year, a reduction in mean ± SE glycosylated hemoglobin (HbA1c) levels was noted for both groups (cases: −1.26% ± 0.17%, controls: −1.47% ± 0.45%). However, while patients on GLP-1 analogs lost 7.07 ± 2.62 kg, control patients gained 1.93 ± 1.14 kg (P < .05). Blunted HbA1c reductions were also noted in patients who took antipsychotic medication in addition to antidepressant medication (on antidepressant medication [n = 22]: −0.77% ± 0.29%, off antidepressant medication [n = 9]: −2.97% ± 0.6%, P < .001). This observation did not apply to patients treated with GLP-1 analogs, as they had larger HbA1c reductions than patients on alternative regimens (controls [n = 15]: −0.46% ± 0.4%, cases [n = 7]: −1.43% ± 0.15%, P < .05).
Conclusions: GLP-1 analogs promote both diabetes and weight control in diabetic patients on antipsychotic medications with or without antidepressant medications.
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders
CME Background
Articles are selected for credit designation based on an assessment of the educational needs of CME participants, with the purpose of providing readers with a curriculum of CME articles on a variety of topics throughout each volume. Activities are planned using a process that links identified needs with desired results.
To obtain credit, read the article, correctly answer the questions in the Posttest, and complete the Evaluation. A $10 processing fee will apply.
CME Objective
After studying this article, you should be able to:
• Monitor patients with severe mental illness who are taking antipsychotic medications for metabolic syndrome and diabetes so that treatment can be implemented as appropriate to minimize adverse cardiovascular outcomes
Accreditation Statement
The CME Institute of Physicians Postgraduate Press, Inc., is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Credit Designation
The CME Institute of Physicians Postgraduate Press, Inc., designates this journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Note: The American Academy of Physician Assistants (AAPA) accepts certificates of participation for educational activities certified for AMA PRA Category 1 Credit™ from organizations accredited by ACCME or a recognized state medical society. Physician assistants may receive a maximum of 1.0 hour of Category I credit for completing this program.
Release, Expiration, and Review Dates
This educational activity was published in January 2020 and is eligible for AMA PRA Category 1 Credit™ through February 28, 2022. The latest review of this material was January 2020.
Financial Disclosure
All individuals in a position to influence the content of this activity were asked to complete a statement regarding all relevant personal financial relationships between themselves or their spouse/partner and any commercial interest. The CME Institute has resolved any conflicts of interest that were identified. In the past year, Larry Culpepper, MD, MPH, Editor in Chief, has been a consultant for Acadia, Allergan, Eisai, Merck, Supernus, and Takeda; has been a stock shareholder of M-3 Information; and has received royalties from UpToDate and Oxford University Press. No member of the CME Institute staff reported any relevant personal financial relationships. Faculty financial disclosure appears at the end of the article.
ABSTRACT
Objective: Glucagon-like peptide (GLP-1) analogs promote diabetes control and weight loss. Most GLP-1 analogs also lower adverse cardiovascular outcomes, making them ideal agents for patients with severe mental illness. The objective of this study was to analyze diabetic patients taking antipsychotic medications, comparing those on GLP-1 analogs with those on other diabetes treatments.
Methods: A total of 46 patients referred to outpatient diabetes clinics between July 2010 and April 2017 who were prescribed antipsychotic medications during the entire study period were included in this retrospective analysis. Eleven (24%) patients were started on a GLP-1 analog (cases), and 35 (76%) were treated with alternative antidiabetic agents (controls).
Results: Cases and controls did not differ in age, sex, height, weight, or medical therapies at the time of referral. Within 1 year, a reduction in mean ± SE glycosylated hemoglobin (HbA1c) levels was noted for both groups (cases: −1.26% ± 0.17%, controls: −1.47% ± 0.45%). However, while patients on GLP-1 analogs lost 7.07 ± 2.62 kg, control patients gained 1.93 ± 1.14 kg (P < .05). Blunted HbA1c reductions were also noted in patients who took antipsychotic medication in addition to antidepressant medication (on antidepressant medication [n = 22]: −0.77% ± 0.29%, off antidepressant medication [n = 9]: −2.97% ± 0.6%, P < .001). This observation did not apply to patients treated with GLP-1 analogs, as they had larger HbA1c reductions than patients on alternative regimens (controls [n = 15]: −0.46% ± 0.4%, cases [n = 7]: −1.43% ± 0.15%, P < .05).
Conclusions: GLP-1 analogs promote both diabetes and weight control in diabetic patients on antipsychotic medications with or without antidepressant medications.
Prim Care Companion CNS Disord 2020;22(1):19m02504
To cite: Perlis LT, Lamberti JS, Miedlich SU. Glucagon-like peptide analogs are superior for diabetes and weight control in patients on antipsychotic medications: a retrospective cohort study. Prim Care Companion CNS Disord. 2020;22(1):19m02504.
To share: https://doi.org/10.4088/PCC.19m02504
© Copyright 2020 Physicians Postgraduate Press, Inc.
aDepartment of Family Medicine, University of Rochester School of Medicine and Dentistry, Rochester, New York
bDepartment of Psychiatry, University of Rochester School of Medicine and Dentistry, Rochester, New York
cDivision of Endocrinology and Metabolism, Department of Medicine, University of Rochester School of Medicine and Dentistry, Rochester, New York
*Corresponding author: Susanne U. Miedlich, MD, Division of Endocrinology and Metabolism, Department of Medicine, University of Rochester School of Medicine and Dentistry, Box 693, 601 Elmwood Ave, Rochester, NY 14642 ([email protected]).
Among US adults, obesity and diabetes incidence rates have dramatically increased during the last few decades. Both are associated with increased morbidity and mortality as well as costs of over 200 billion per year.1,2 Notably, the rates of both obesity and type 2 diabetes are considerably higher in patients with severe and persistent mental illnesses such as recurrent major depressive disorder or schizophrenia compared to the general population.3–5 While there is evidence that impaired glucose tolerance is more prevalent even in drug-naive patients with schizophrenia, treatment with antipsychotic medications (APMs) clearly furthers the risk of obesity and diabetes, as demonstrated by a number of studies in both rodents and humans (reviewed in Ballon et al6). Interestingly, a similar trend of increased rates of obesity and diabetes can be observed in patients with depression.7 The use of antidepressant medications (ADMs) has been proposed to promote metabolic abnormalities, although a definitive role of ADMs in mediating obesity and diabetes has not been demonstrated as consistently as for APMs.8,9
The 10-year risk for cardiovascular disease is about 1.5-fold to > 3-fold higher in patients with schizophrenia, which is most likely a consequence of both obesity and diabetes in addition to hyperlipidemia, hypertension, and nicotine abuse.3,10 Thus, utilization of antidiabetic agents, which have the potential to reduce cardiovascular events, must be a primary goal when caring for diabetic patients on APMs.
Currently available diabetes medications differentially impact weight and cardiovascular outcomes. For instance, both sulfonylureas and insulin promote weight gain,11,12 and although these medications reduce myocardial infarctions, they do so after a lag time of about 20 years.13 On the other hand, newer diabetes medications, for example glucagon-like peptide (GLP-1) analogs, not only promote diabetes control and weight loss, but also reduce cardiovascular events within only a few years.14,15
With regard to APMs, administration of clozapine in rats caused impaired glucose tolerance and insulin resistance and led to reduced endogenous GLP-1 levels in 1 study.16 Furthermore, acute administration of the GLP-1 analog exendin-4 reversed the findings of impaired glucose tolerance and insulin resistance.16 On the basis of these results, one could expect that GLP-1 analogs may be even more potent than other antidiabetic drugs in patients on APMs. Most studies,16–20 in both rats and humans, report some degree of hyperphagia, weight gain, increased adiposity, hyperglycemia, and insulin resistance after treatment with APMs and most markedly after treatment with olanzapine or clozapine. Notably, olanzapine-induced hyperphagia, weight gain, increased adiposity, and hyperglycemia in rats were mostly reversed by coadministration of liraglutide, a GLP-1 analog.21
To date, 3 placebo-controlled, prospective studies22–24 have investigated the effects of the GLP-1 analogs exenatide and liraglutide on weight control and glucose homeostasis in mostly prediabetic patients on APMs. While 2 studies23,24 demonstrated improved glycemic control and weight loss, 1 study22 did not. It is worth noting that the studies22–24 were conducted over a time period of only 12 to 24 weeks.
To our knowledge, there are no studies addressing the efficacy of GLP-1 analogs in diabetic patients on APMs. We thus sought to explore how therapy with GLP-1 analogs compares to alternative regimens in type 2 diabetes patients who are also treated with APMs. To inform these questions, we performed a retrospective data analysis of patients seen in outpatient diabetes clinics of the University of Rochester Medical Center (URMC), Rochester, New York.
METHODS
Design and Participants
A retrospective chart review was conducted of electronic medical records of patients seen between July 2010 and April 2017. Patients included were > 18 years old, had a body mass index (BMI) > 25 kg/m2, had a diagnosis of diabetes mellitus (diagnostic ICD-10 codes: E10 or E11), were on an APM, and had been seen in any of the outpatient endocrine clinics at URMC during the study time frame. The study protocol was reviewed and approved by the URMC Research Subjects Review Board (study no. RSRB00067259).
Exposures
Potential patients were identified through the URMC Clinical Analytics (eRecord Reporting) team using the inclusion criteria. Patients fulfilling the inclusion criteria who were treated with an APM for the entire study period and had at least 1 follow-up visit at 1 of our endocrine clinics were included in the final data set.
Data were obtained through review of electronic medical records and included age, sex, height, weight, BMI, smoking status, glycosylated hemoglobin (HbA1c), systolic and diastolic blood pressure, and medications (subdivided into APMs, ADMs, antidiabetic medications, mood stabilizers, antihypertensives, and hormone preparations). Subjects were subsequently grouped into cases or controls based on the use of GLP-1 analogs (cases = on GLP-1 analog, controls = not on GLP-1 analog). The remaining antidiabetic medications were recorded as insulin or oral medications. Endocrine clinic provider progress notes and visit encounter documentation served as the main data source for vital signs and medication regimens. Data for weight and HbA1c were collected at the time points 0, 3, 6, and 12 months following introduction of the GLP-1 analog (cases) or referral to or follow-up in one of our clinics (controls). We only included data from patients for whom the use of ≥ 1 APM was documented for the time of observation (up to 12 months). On the basis of the nature of the study (electronic medical record review), no statements can be made regarding initiation of APMs prior to the study period or the individual patient’s adherence to the APM prescription. Data for systolic and diastolic blood pressure were recorded at time points 0 and 12 months. When appointments did not fall exactly at 3, 6, or 12 months, data were applied to the closest time points.
Outcomes and Measures
Differences in HbA1c and weight were the main recorded outcomes in both cases and controls as well as subgroups on or off ADMs. Weight and HbA1c changes were calculated for each patient by subtracting weight (in kg) and HbA1c (in %) at time 0 (time of referral for controls or start of GLP-1 analog for cases) from the respective values at 3, 6, or 12 months. BMI and blood pressure values at 0 and 12 months were also compared between the 2 groups.
Statistical Analysis
SPSS version 24 (IBM Corp, Armonk, New York) was used for descriptive analyses and statistical comparisons between groups. Kolmogorov-Smirnov tests were applied to determine whether the data were normally distributed (P > .1). The values of all continuous variables subjected to statistical comparisons were normally distributed. Thus, parametric tests (independent sample t tests) were carried out throughout the study to compare cases and controls. For comparisons of categorical variables, we applied χ2 tests. A P value < .05 was considered statistically significant.
RESULTS
A total of 46 patients were included in this retrospective analysis based on concurrent prescription of an APM and follow-up in our diabetes clinic with at least 1 follow-up visit. Of note, 1 of the 11 cases (9.1%) and 5 of the 35 controls (13.9%) were treated with either olanzapine or clozapine; the latter APMs are particularly known to mediate adverse metabolic outcomes compared to other APMs.5,25–27 On the other hand, ziprasidone, an APM with a metabolically more favorable side effect profile, was utilized in 2 cases (18.2%) and 2 controls (5.7%).
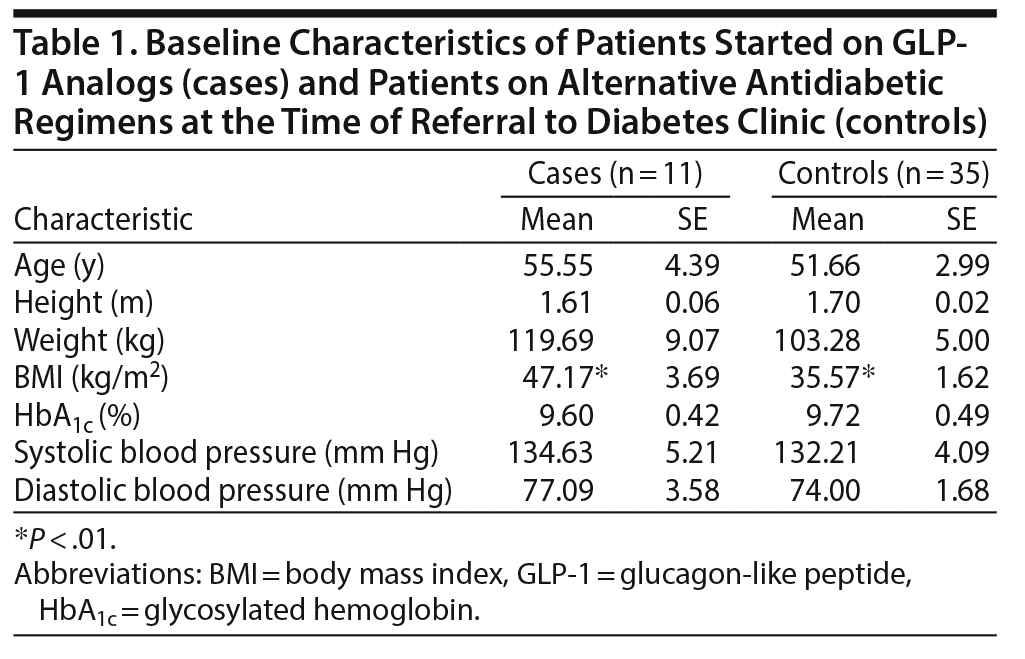
Regarding specific antidiabetic regimens, 11 patients (24%) were treated with GLP-1 analogs (cases), and 35 patients (76%) were on alternative antidiabetic regimens (controls). A closer look at the antidiabetic medication regimens in patients showed that in 89% of the control patients, insulin was either started or doses were adjusted. In 7 control patients, a dipeptidyl-peptidase-4 inhibitor or metformin was added or increased, and 1 patient was started on a sodium-glucose cotransporter-2 inhibitor. While most cases (9 of 11) were treated with the GLP-1 analog liraglutide, 1 patient was on exenatide and 1 patient was on dulaglutide. Of the cases, 90% were also treated with insulin. Baseline data are shown in Table 1. Overall, there were no significant differences in age, height, weight, medications, systolic and diastolic blood pressures, or HbA1c between cases and controls at the beginning of the observation period (see Table 1). However, cases had significantly higher BMIs than controls (P < .01, see Table 1). Note that 73% of the cases but only 40% of the controls were women; however, this difference was not statistically significant (P = .09).
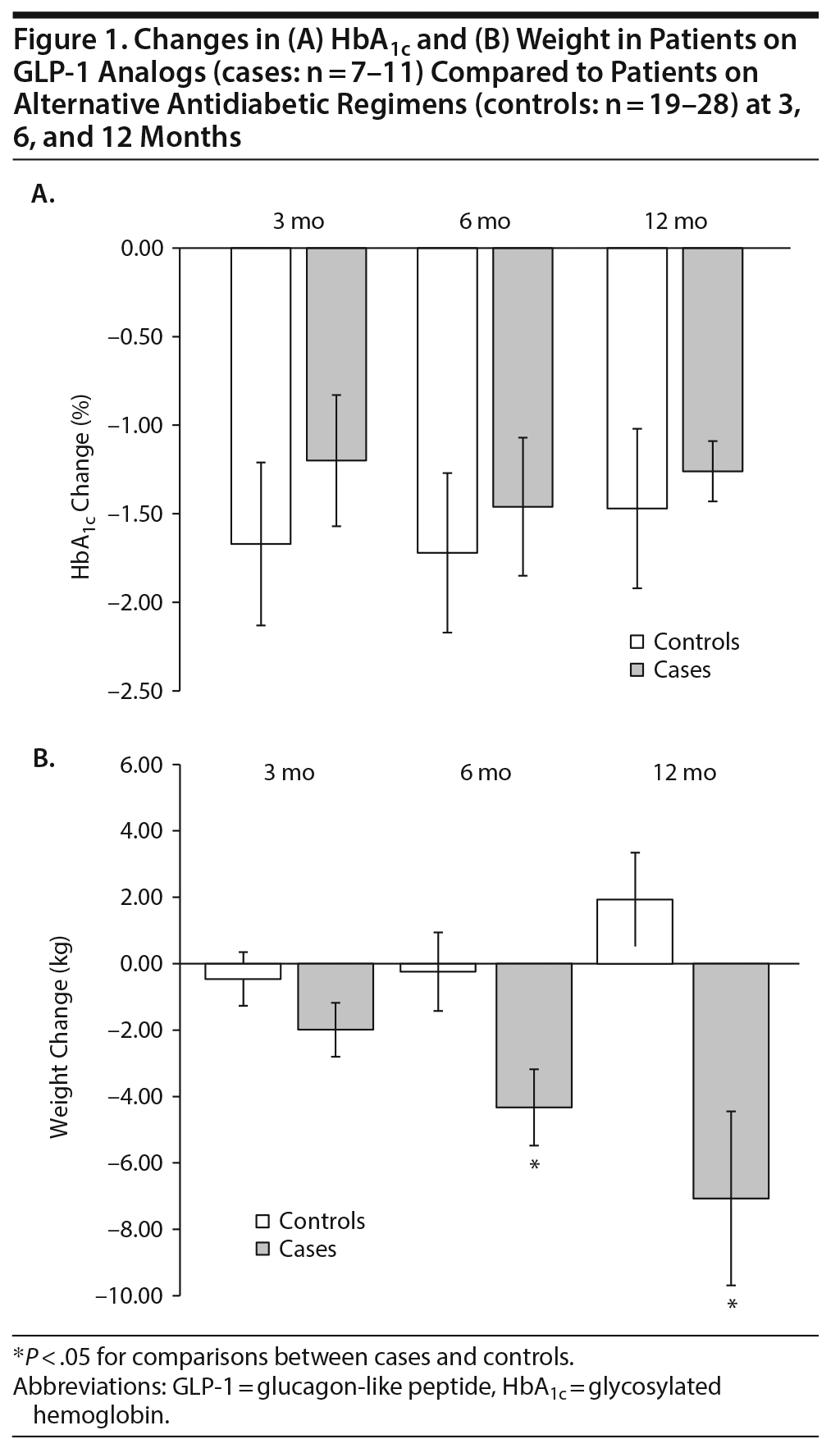
Over the course of 12 months, HbA1c levels improved similarly for both cases and controls. Specifically, compared to baseline HbA1c levels at 3, 6, and 12 months decreased a mean ± SE of −1.2% ± 0.37%, −1.46% ± 0.39%, and −1.26% ± 0.17%, respectively, for the cases. In control patients, mean ± SE HbA1c reductions were −1.67% ± 0.46%, −1.72% ± 0.45%, and −1.47% ± 0.45% at 3, 6, and 12 months, respectively (Figure 1A).
Significant differences were noted in patient weights (Figure 1B); patients on GLP-1 analogs lost weight over the course of 1 year, while patients on alternative regimens gained weight. At 3, 6, and 12 months, cases lost a mean ± SE of 1.99 ± 0.81 kg, 4.33 ± 1.15 kg, and 7.07 ± 2.62 kg, respectively. There was a slight downward trend in the weights of control patients at 3 and 6 months: 0.46 ± 0.81 kg and 0.24 ± 1.18 kg, respectively. However, after 1 year, control patients had gained 1.93 ± 1.14 kg. The differences in weight between controls and cases were significant at 6- and 12-month time points (P < .05). Overall, the impact of added GLP-1 analogs on weight control in a relatively small and diverse cohort of patients treated with a wide array of APMs, ranging from ziprasidone to clozapine, appears to be clinically robust. No differences were noted between controls and cases in regard to systolic or diastolic blood pressures after 12 months (data not shown).
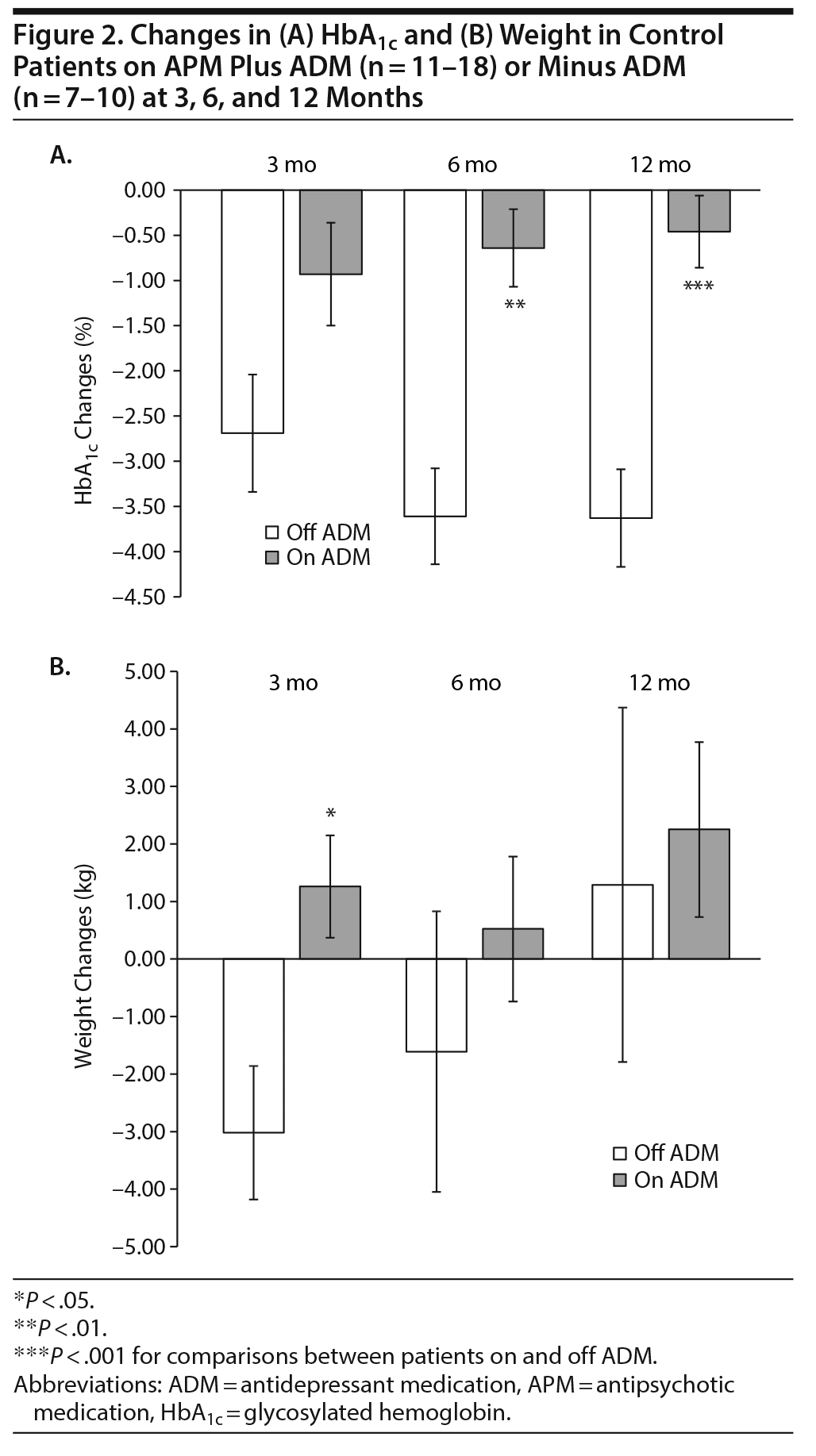
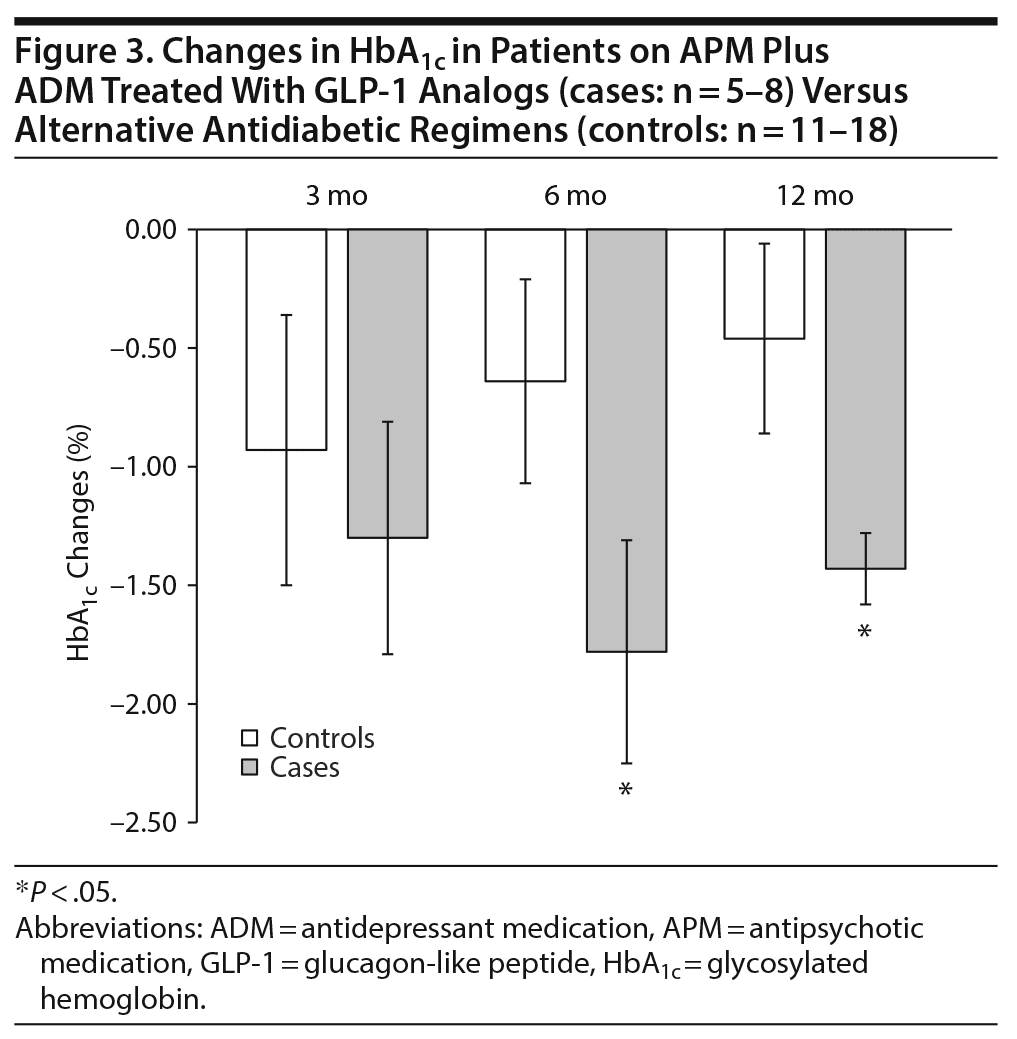
Next, we explored whether any of the concurrent medications (ADMs, antihypertensive medications, mood stabilizers) impacted HbA1c or weight changes over time. Within the entire cohort, we noted a significant effect of ADM on HbA1c changes after 6 and 12 months of follow-up (HbA1c changes in patients off ADMs at 3 months: −2.34% ± 0.57%, at 6 months: −3.02% ± 0.58%, at 12 months: −2.97% ± 0.6%; HbA1c changes in patients on ADMs at 3 months: −1.04% ± 0.41%, at 6 months: −0.94% ± 0.36%, at 12 months: −0.77% ± 0.29%. In other words, patients who were on both APM and ADM had significantly blunted HbA1c reductions compared to patients not on ADM. Taking a closer look, the discrepancy in HbA1c changes was most apparent in control patients on or off ADM (Figure 2A). We reasoned that an increase in weight could be considered a potential contributing factor for this result, perhaps by worsening insulin resistance. However, when analyzed, there were no differences in baseline weight or weight changes in control patients on or off ADM after 6 and 12 months (Figure 2B). Alternatively, we reasoned that patients on ADM (ie, depressed patients or patients with negative symptoms) may be less adherent to their respective antidiabetic regimens. If that was the case, it should be applicable to patients on GLP-1 analogs as well. Thus, we compared patients on APM, ADM, and GLP-1 analogs (cases on ADM) to patients on APM, ADM, and alternative antidiabetic regimens (controls on ADM). Surprisingly, we found that only controls but not cases had blunted HbA1c reductions when also on ADM, a difference that reached statistical significance after 6 and 12 months, respectively (HbA1c changes in controls after 6 months: –0.64% ± 0.43%, after 12 months: –0.46% ± 0.40%; HbA1c changes in cases after 6 months: –1.78% ± 0.47%, after 12 months: –1.43% ± 0.15%), despite the small sample size (n = 5–7, Figure 3). While this finding appears to be robust, it will need further exploration by larger controlled, prospective studies.
DISCUSSION
The development of APM has greatly impacted mental health outcomes in patients with schizophrenia and schizoaffective disorders. Second-generation APMs have emerged as a standard of care for patients with schizophrenia and schizoaffective disorders. Second-generation APMs are also increasingly being used as adjunctive therapy in patients with mood disorders and even in children and adolescents with attention-deficit/hyperactivity and disruptive behavior disorders.28,29
Compared to first-generation APMs, second-generation APMs have variable affinities to dopamine D2 receptors and also target serotonergic, histaminergic, and adrenergic neurotransmission.30 This pharmacologic profile results in fewer extrapyramidal symptoms compared to first-generation APMs.30 Also, the second-generation APMs clozapine and olanzapine have demonstrated superiority in controlling psychotic symptoms, and patients are more likely to adhere to those 2 medications.27 Unfortunately, this advantage needs to be weighed against a much more unfavorable metabolic side effect profile. Patients on olanzapine or clozapine are at a very high risk of developing obesity, metabolic syndrome, and type 2 diabetes,25–27 a profile that translates to increased cardiovascular morbidity and mortality.3,10 That said, newer diabetes medications, for example GLP-1 analogs, not only control diabetes and weight but also reduce cardiovascular events.14,15 Therefore, they might be particularly promising in diabetic patients on APMs.
In this retrospective chart review, we explored the efficacy of GLP-1 analogs compared to alternative regimens in diabetic patients on APMs. We speculated that GLP-1 analogs may be more advantageous. We found that GLP-1 analogs may indeed be superior, as they target both diabetes and weight control. The mean weight loss on GLP-1 analogs in our diabetic patients on APM was quite remarkable: 7.07 ± 2.62 kg. The mean weight loss observed in larger studies14,31,32 of diabetic patients tends to be somewhat less (between 2–4 kg). This finding could point to an increased efficacy of GLP-1 analogs in patients on APM, although it clearly needs to be explored further due to the small sample size of this cohort. Previously published trials22–24,33 in patients on APM treated with GLP-1 analogs were carried out in mostly prediabetic, mildly-moderately obese patients. The weight loss reported in those studies,22–24,33 achieved with the GLP-1 analogs (exenatide, liraglutide), ranged from 2 to 5 kg. Interestingly, weight loss was greater in patients treated with olanzapine or clozapine, which are the APMs with the most unfavorable metabolic side effect profile.33
Surprisingly, in our cohort, patients on APM and ADM achieved superior glycemic control with GLP-1 analogs compared to alternative regimens (see Figure 3). Unfortunately, based on the nature of this retrospective cohort study, we cannot rule out medication nonadherence or worsening mental illness as causative or contributing factors for this finding. It is tempting though to speculate that GLP-1 analogs may at least in part counteract potentially additive adverse effects of APM and ADM. For instance, both APM and ADM can similarly affect serotonergic and histaminergic neurotransmission. In this context, it is noteworthy that in patients with schizophrenia, a significantly increased risk of type 2 diabetes was associated with the administration of ≥ 3 psychotropic medications (APM and ADM) compared to 1 or 2.34 Simply speaking, more psychotropic drugs may lead to more adverse metabolic outcomes. Since GLP-1 analogs act at least in part centrally, and more specifically in hypothalamic areas where serotonin and also histamine, norepinephrine, and dopamine neurotransmission are at play (and targeted by second-generation APMs and ADMs), a modulatory effect of GLP-1 analogs on neurotransmitter signaling is at least conceivable and could contribute to their efficacy in patients on psychotropic medications.
Our study has several limitations. First, it was conducted retrospectively, thus we cannot rule out confounders on the level of the provider selectively targeting patients with a higher BMI for therapy with a GLP-1 analogue. Second, medication adherence was not monitored and may have been different between controls and cases, especially in the setting of potentially active depression. Third, we also noted an imbalance with regard to sex between cases (73% women) and controls (40% women), which may have impacted the results. Finally, while we tried to be as comprehensive as possible in the collection of relevant patient data, due to the study being done by e-record review, we may have missed potential covariates.
In summary, our retrospective cohort study suggests that GLP-1 analogs might be superior to alternative antidiabetic regimens in patients on APM, especially in patients on both APM and ADM. However, controlled, prospective trials in this high-risk patient population are greatly needed to investigate these promising trends further.
Clinical Points
- Patients with severe mental illness on second-generation antipsychotic medications are at high risk for metabolic syndrome, diabetes, and, most importantly, adverse cardiovascular outcomes.
- Glucagon-like peptide analogs are promising antidiabetic agents for patients on antipsychotic or antidepressant medications and should be considered early in this patient population.
Submitted: June 19, 2019; accepted August 20, 2019.
Published online: February 6, 2020.
Disclosure of off-label usage: The authors have determined that, to the best of their knowledge, no investigational information about pharmaceutical agents or device therapies that is outside US Food and Drug Administration–approved labeling has been presented in this article.
Financial disclosure: Drs Perlis, Lamberti, and Miedlich have no personal affiliations or financial relationships with any commercial interest to disclose relative to this article.
Funding/support: None.
Previous presentation: These data were presented as a published abstract at the Annual Meeting of the American Diabetes Association; June 22–26, 2018; Orlando, Florida.
Acknowledgments: The authors wish to thank Stephen R. Hammes, MD, PhD, and Steven D. Wittlin, MD, for their critical advice and review of the manuscript. Drs Hammes and Wittlin report no conflicts of interest related to the subject of this article.
REFERENCES
1. National Diabetes Statistics Report, 2017. CDC website. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed December 31, 2019.
2. Maps of Trends in Diagnosed Diabetes. April 2017. CDC website. https://www.cdc.gov/diabetes/statistics/slides/maps_diabetes_trends.pdf. Accessed December 31, 2019.
3. Goff DC, Sullivan LM, McEvoy JP, et al. A comparison of ten-year cardiac risk estimates in schizophrenia patients from the CATIE study and matched controls. Schizophr Res. 2005;80(1):45–53. PubMed CrossRef
4. Allison DB, Fontaine KR, Heo M, et al. The distribution of body mass index among individuals with and without schizophrenia. J Clin Psychiatry. 1999;60(4):215–220. PubMed CrossRef
5. Allison DB, Mentore JL, Heo M, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry. 1999;156(11):1686–1696. PubMed
6. Ballon JS, Pajvani U, Freyberg Z, et al. Molecular pathophysiology of metabolic effects of antipsychotic medications. Trends Endocrinol Metab. 2014;25(11):593–600. PubMed CrossRef
7. Farr SL, Hayes DK, Bitsko RH, et al. Depression, diabetes, and chronic disease risk factors among US women of reproductive age. Prev Chronic Dis. 2011;8(6):A119. PubMed
8. Serretti A, Mandelli L. Antidepressants and body weight: a comprehensive review and meta-analysis. J Clin Psychiatry. 2010;71(10):1259–1272. PubMed CrossRef
9. Barnard K, Peveler RC, Holt RI. Antidepressant medication as a risk factor for type 2 diabetes and impaired glucose regulation: systematic review. Diabetes Care. 2013;36(10):3337–3345. PubMed CrossRef
10. Crump C, Winkleby MA, Sundquist K, et al. Comorbidities and mortality in persons with schizophrenia: a Swedish national cohort study. Am J Psychiatry. 2013;170(3):324–333. PubMed CrossRef
11. Kahn SE, Haffner SM, Heise MA, et al; ADOPT Study Group. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355(23):2427–2443. PubMed CrossRef
12. Gerstein HC, Bosch J, Dagenais GR, et al; ORIGIN Trial Investigators. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med. 2012;367(4):319–328. PubMed CrossRef
13. Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–1589. PubMed CrossRef
14. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–322. PubMed CrossRef
15. Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–1844. PubMed CrossRef
16. Smith GC, Vickers MH, Cognard E, et al. Clozapine and quetiapine acutely reduce glucagon-like peptide-1 production and increase glucagon release in obese rats: implications for glucose metabolism and food choice behaviour. Schizophr Res. 2009;115(1):30–40. PubMed CrossRef
17. Vidarsdottir S, de Leeuw van Weenen JE, Frölich M, et al. Effects of olanzapine and haloperidol on the metabolic status of healthy men. J Clin Endocrinol Metab. 2010;95(1):118–125. PubMed CrossRef
18. Hegedűs C, Kovács D, Kiss R, et al. Effect of long-term olanzapine treatment on meal-induced insulin sensitization and on gastrointestinal peptides in female Sprague-Dawley rats. J Psychopharmacol. 2015;29(12):1271–1279. PubMed CrossRef
19. van der Zwaal EM, Janhunen SK, la Fleur SE, et al. Modelling olanzapine-induced weight gain in rats. Int J Neuropsychopharmacol. 2014;17(1):169–186. PubMed Crosf
20. Smith GC, Chaussade C, Vickers M, et al. Atypical antipsychotic drugs induce derangements in glucose homeostasis by acutely increasing glucagon secretion and hepatic glucose output in the rat. Diabetologia. 2008;51(12):2309–2317. PubMed CrossRef
21. Lykkegaard K, Larsen PJ, Vrang N, et al. The once-daily human GLP-1 analog, liraglutide, reduces olanzapine-induced weight gain and glucose intolerance. Schizophr Res. 2008;103(1–3):94–103. PubMed CrossRef
22. Ishøy PL, Knop FK, Broberg BV, et al. Effect of GLP-1 receptor agonist treatment on body weight in obese antipsychotic-treated patients with schizophrenia: a randomized, placebo-controlled trial. Diabetes Obes Metab. 2017;19(2):162–171. PubMed CrossRef
23. Larsen JR, Vedtofte L, Jakobsen MSL, et al. Effect of liraglutide treatment on prediabetes and overweight or obesity in clozapine- or olanzapine-treated patients with schizophrenia spectrum disorder: a randomized clinical trial. JAMA Psychiatry. 2017;74(7):719–728. PubMed CrossRef
24. Siskind DJ, Russell AW, Gamble C, et al. Treatment of clozapine-associated obesity and diabetes with exenatide in adults with schizophrenia: a randomized controlled trial (CODEX). Diabetes Obes Metab. 2018;20(4):1050–1055. PubMed CrossRef
25. Lamberti JS, Costea GO, Olson D, et al. Diabetes mellitus among outpatients receiving clozapine: prevalence and clinical-demographic correlates. J Clin Psychiatry. 2005;66(7):900–906. PubMed CrossRef
26. Lamberti JS, Olson D, Crilly JF, et al. Prevalence of the metabolic syndrome among patients receiving clozapine. Am J Psychiatry. 2006;163(7):1273–1276. PubMed CrossRef
27. Lieberman JA, Stroup TS, McEvoy JP, et al; Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209–1223. PubMed CrossRef
28. Spielmans GI, Berman MI, Linardatos E, et al. Adjunctive atypical antipsychotic treatment for major depressive disorder: a meta-analysis of depression, quality of life, and safety outcomes. PLoS Med. 2013;10(3):e1001403. PubMed CrossRef
29. Olfson M, King M, Schoenbaum M. Treatment of young people with antipsychotic medications in the United States. JAMA Psychiatry. 2015;72(9):867–874. PubMed CrossRef
30. Miyamoto S, Duncan GE, Marx CE, et al. Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol Psychiatry. 2005;10(1):79–104. PubMed CrossRef
31. Garber A, Henry R, Ratner R, et al; LEAD-3 (Mono) Study Group. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373(9662):473–481. PubMed CrossRef
32. Buse JB, Rosenstock J, Sesti G, et al; LEAD-6 Study Group. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet. 2009;374(9683):39–47. PubMed CrossRef
33. Siskind D, Hahn M, Correll CU, et al. Glucagon-like peptide-1 receptor agonists for antipsychotic-associated cardio-metabolic risk factors: a systematic review and individual participant data meta-analysis. Diabetes Obes Metab. 2019;21(2):293–302. PubMed CrossRef
34. Mamakou V, Hackinger S, Zengini E, et al. Combination therapy as a potential risk factor for the development of type 2 diabetes in patients with schizophrenia: the GOMAP study. BMC Psychiatry. 2018;18(1):249. PubMed CrossRef
Enjoy this premium PDF as part of your membership benefits!