Abstract
Importance: GLP-1 receptor agonists (GLP-1 RAs) are widely recognized for their antidiabetic properties, primarily due to their insulin secretagogue action. However, their potential therapeutic effects extend beyond the endocrine system, suggesting a promising role in the treatment of psychiatric disorders. Despite this potential, there is limited consolidation of evidence regarding their psychiatric applications. This scoping review aims to address this gap by exploring the effects of GLP-1 RAs across various psychiatric conditions, highlighting their therapeutic promise and the need for further investigation.
Observations: A comprehensive literature search was conducted using PubMed, Web of Science, Scopus, ClinicalTrials.gov, and PsycNet to identify studies examining the effects of GLP-1 RAs on psychiatric disorders. Original articles were included in the analysis. The findings suggest that GLP-1 RAs show efficacy in several areas, including autism-related disorders and depression. Conversely, GLP-1 RAs demonstrated no significant benefits in improving neurocognition or psychosocial functioning in schizophrenia, cocaine addiction, or dementia. Limited evidence also points to their effects on anxiety and cognitive functioning in dementia, but these findings were inconclusive.
Conclusions and Relevance: The current evidence underscores the potential of GLP-1 RAs as a novel therapeutic approach for certain psychiatric disorders, particularly alcohol-related disorders, depression, and autism-related food behaviors. However, their utility in other conditions, such as schizophrenia, cocaine addiction, and dementia, remains unsupported by robust evidence. The findings highlight the importance of conducting well-designed, long-term studies to elucidate the mechanisms and clinical applications of GLP-1 RAs in psychiatry. With further research, GLP-1 RAs could expand the therapeutic arsenal for psychiatric care, offering new hope for conditions with limited treatment options.
Prim Care Companion CNS Disord 2025;27(3):24nr03828
Author affiliations are listed at the end of this article.
Glucagon-like peptide-1 (GLP-1) is categorized as an incretin hormone secreted from the endocrine cells located within the intestines, pancreas, and central nervous system (CNS).1 Its primary functions constitute the regulation of glucose homeostasis and weight management, in addition to its integral neuroprotective role.2,3 The GLP-1 receptor agonists (GLP-1 RAs) including exenatide, liraglutide, semaglutide, and dulaglutide mirror these roles that are achieved through their interaction with GLP-1 receptors.4 In recent years, there has been a significant increase in attention, research, and clinical advancements delving into the intricate mechanisms underlying the effects of GLP-1 agonists in various disorders.5
GLP-1 RAs are used as antidiabetic drugs owing to their role in controlling insulin secretions, improving β cell functions, and suppressing glucagon secretions.4 GLP-1 RAs have been documented to stimulate brown fat metabolism, delay gastric emptying, and dampen satiety center’s activity in the hypothalamus, advocating its potential use in the treatment of obesity and diabetes.6,7 Recent investigations have revealed that GLP 1 RAs possess the ability to suppress the PI3K/AKT/ mTOR and ERK/MAPK pathways, resulting in the suppression of prostate cancer growth.8 Moreover, these agonists have demonstrated significant contributions in reducing cardiovascular risks by diminishing inflammation, lowering blood pressure, and enhancing microvascular functions.9
GLP-1 synthesis is not exclusive to the endocrine system, as it can also originate from neurons within the CNS. The presence of GLP-1 receptors has been recognized across various CNS regions, including the hippocampus, neocortex, hypothalamus, and cerebellum, suggesting for their role in neurodegenerative diseases.5,10 Moreover, the multifaceted functions of GLP-1 RAs extend into the realm of brain activity, particularly exhibiting neuroprotective effects. These effects are hypothesized to stem from classical growth factor mechanisms, involving increased gene expression linked to cell growth, repair, and replacement, along with an elevation in cellular metabolism. The mitigation of apoptosis and dampening of inflammatory responses further contribute to this protective aspect.11,12
Notably, the potential neuroprotective properties of GLP-1 RAs extend to conditions like Alzheimer disease (AD) and Parkinson disease by ameliorating motor impairment and curtailing oxidative stress, inflammation, and apoptosis.13,14 Intriguingly, emerging research proposes that GLP-1 RA treatments might hold the potential to treat addictive disorders, although this area remains under investigation.15 Moreover, GLP-1 RAs in diabetic patients might yield benefits beyond glycemic control, as their use decreases the risk of dementia and anxiety associated with diabetes.16
The emerging evidence strongly suggests that GLP-1 RAs hold significant promise not only in addressing diabetes and obesity but also in the realm of treating psychiatric disorders. Ongoing research endeavors seek to validate their efficacy in managing psychiatric comorbidities. This comprehensive review aims to fill a notable gap by discussing the potential effects of GLP-1 RAs across various psychiatric disorders. Notably absent from prior literature, this review synthesizes their role cohesively while incorporating the latest findings from recent studies.
METHODS
Studies that included the use of GLP-1 analogs and their effects on psychiatric disorders (including dementia), reported either as primary or secondary outcomes, were included. Only case reports, case series, retrospective chart reviews, open-label trials, and randomized controlled trials (RCTs) were considered.
All books, conference papers, theses, editorials, review articles, meta-analyses, in-vitro studies, laboratory studies, and animal studies were excluded. Studies of participants without psychiatric disorders and abstract only articles were excluded as well.
Search Strategy
Five electronic databases, PubMed, Scopus, Web of Science, ClinicalTrials.gov, and PsycNet, were searched on June 10, 2023, using the search terms “(semaglutide OR GLP-1 analog OR GLP-1 agonist) AND (psychiat* OR depress* OR anxiety OR psycho* OR schizo* OR bipolar OR substance OR ADHD OR attention OR dementia OR autism OR alcohol use disorder OR AUD OR Alzheimer’s disease) AND (treatment).” No restrictions on language, country, publication year, or patient age, gender, or ethnicity were applied.
Study Selection
The search results obtained from the 5 databases were imported into Endnote version 20 to eliminate any duplicate entries. The titles and abstracts were screened by 4 separate reviewers (M.A., A.A., B.A.K., S.T.S.) followed by full-text screening. After the completion of full-text screening, a manual search was conducted to identify additional articles for inclusion. Discrepancies were resolved through consensus achieved via discussions among the reviewers.
Data Extraction and Grading
The data were extracted independently by the authors and were cross-checked by discussion among the 4 reviewers (M.A., A.A., B.A.K., S.T.S.), with guidance from the senior authors (S.N., N.A.) in case of discrepancy. The data were categorized as the reported diagnosis of the included study, outcome measures, and study design. The Oxford Centre for Evidence-Based Medicine 2011 Levels of Evidence17 was used to grade the quality of evidence (OCEBM). According to the OCEBM, level 1 evidence pertains to systematic reviews of RCTs or individual RCTs with a narrow CI. Level 2 evidence corresponds to cohort studies or systematic reviews of cohort studies. Level 3 evidence is assigned to case control studies or systematic reviews of case-control studies. Level 4 evidence is relevant to case-series studies that focus on therapy, prevention, etiology, and harm. The tiers of evidence are employed in the process of formulating grades of recommendation. Grade A is assigned to studies that consistently demonstrate a level 1 standard. Grade B is assigned to studies that consistently demonstrate a level 2 or 3 standard or are extrapolations based on level 1 studies. Grade C is assigned to studies that are at a level 4 standard or are extrapolations based on level 2 or 3 studies. According to the OCEBM, a grade D classification is assigned to evidence that consists of inconsistent or inconclusive studies of any level.
RESULTS
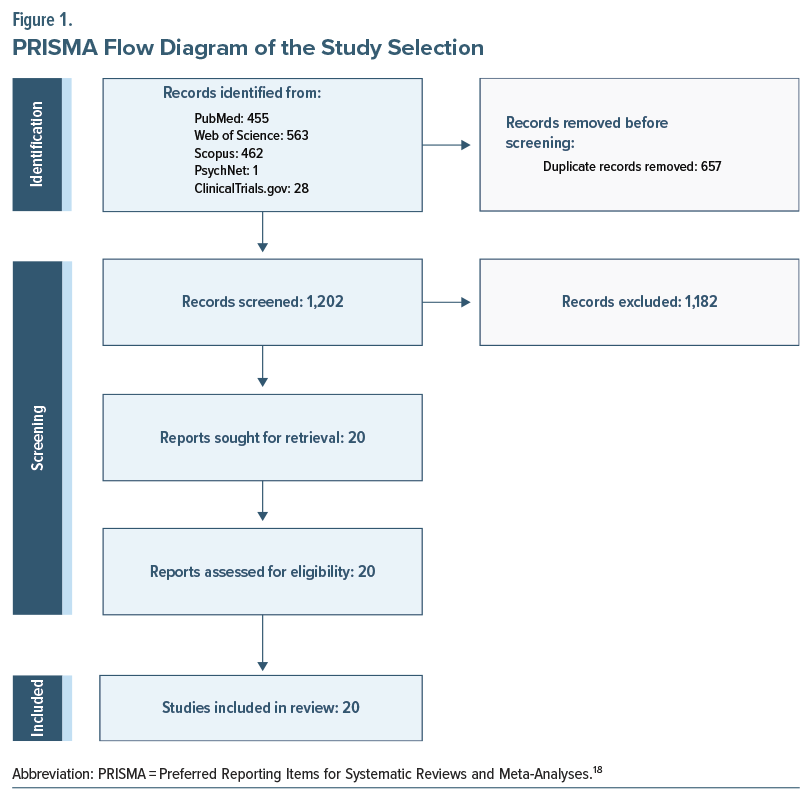
The initial electronic search for this review yielded 1,509 studies. After the removal of duplicates, title and abstract screening was performed, following which 657 articles were excluded. Full-text screening of 48 articles was subsequently performed, and 20 articles met the inclusion criteria (Figure 1).
The studies focused on the use of GLP-1 agonists and their potential effects if any on psychiatric illnesses. Studies that were analyzed evaluated the effects of these agents on anxiety, depression, psychosocial functioning in schizophrenia, dementia, cognitive functioning, substance use disorders such as cocaine and alcohol, and behavioral problems in autism. The summary is provided in Tables 1–3.
Depression
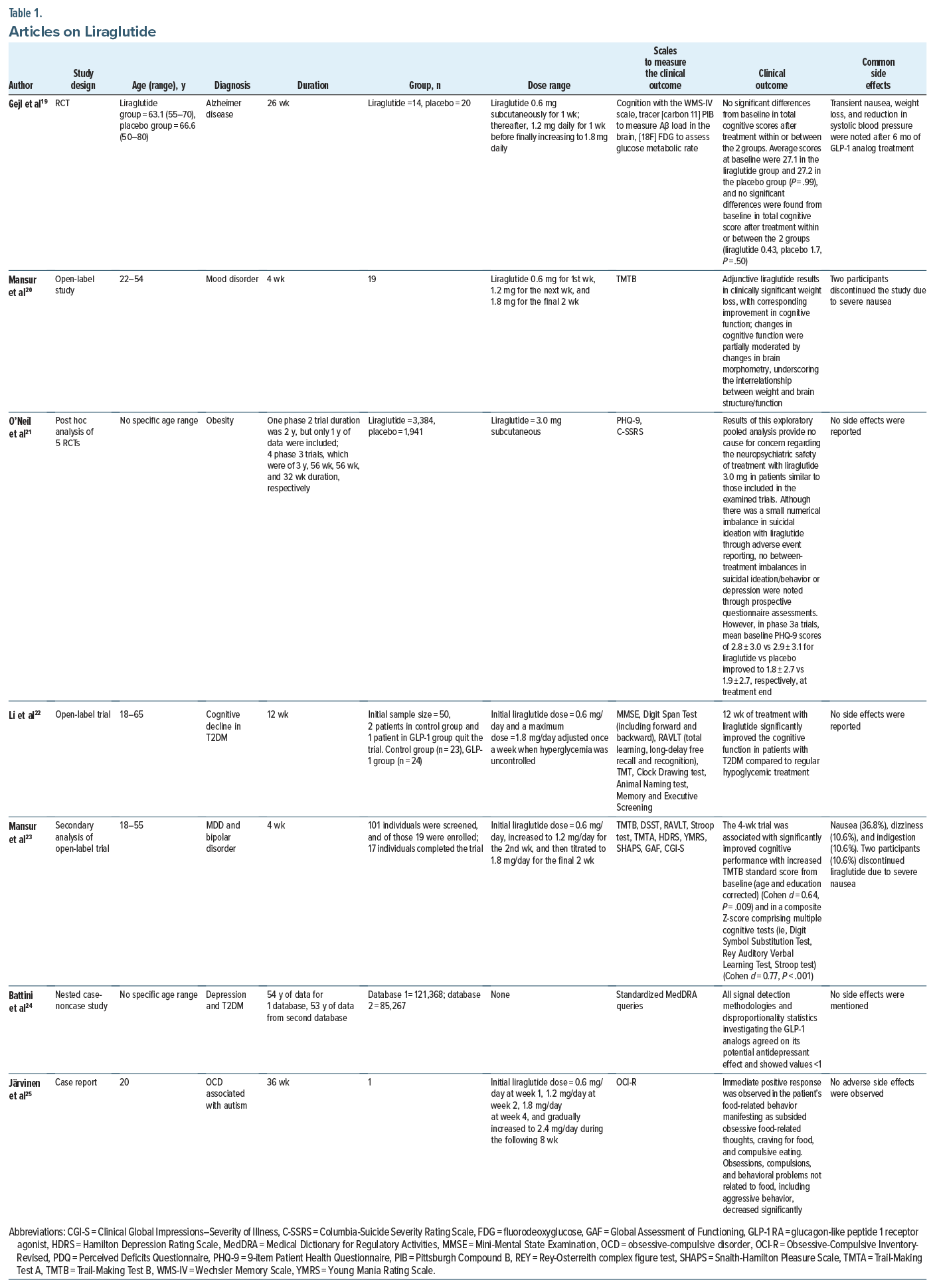
There were 2 cohort studies, 2 post hoc analyses of RCTs, 1 case-noncase study, 1 case-control, and 1 cross sectional study that studied the effects of GLP-1 agonists on depressive symptoms. The doses of GLP-1 agonist used in these studies ranged from 0.01 mg twice daily up to 3 mg daily. The highest dose was used by O’Neil et al21 and corresponded to 3 mg. Results included studies that discussed the positive/beneficial role of GLP-1 agonists on psychiatric illnesses, while others demonstrated no efficacy or detrimental effects of GLP-1 drugs on mood symptoms.
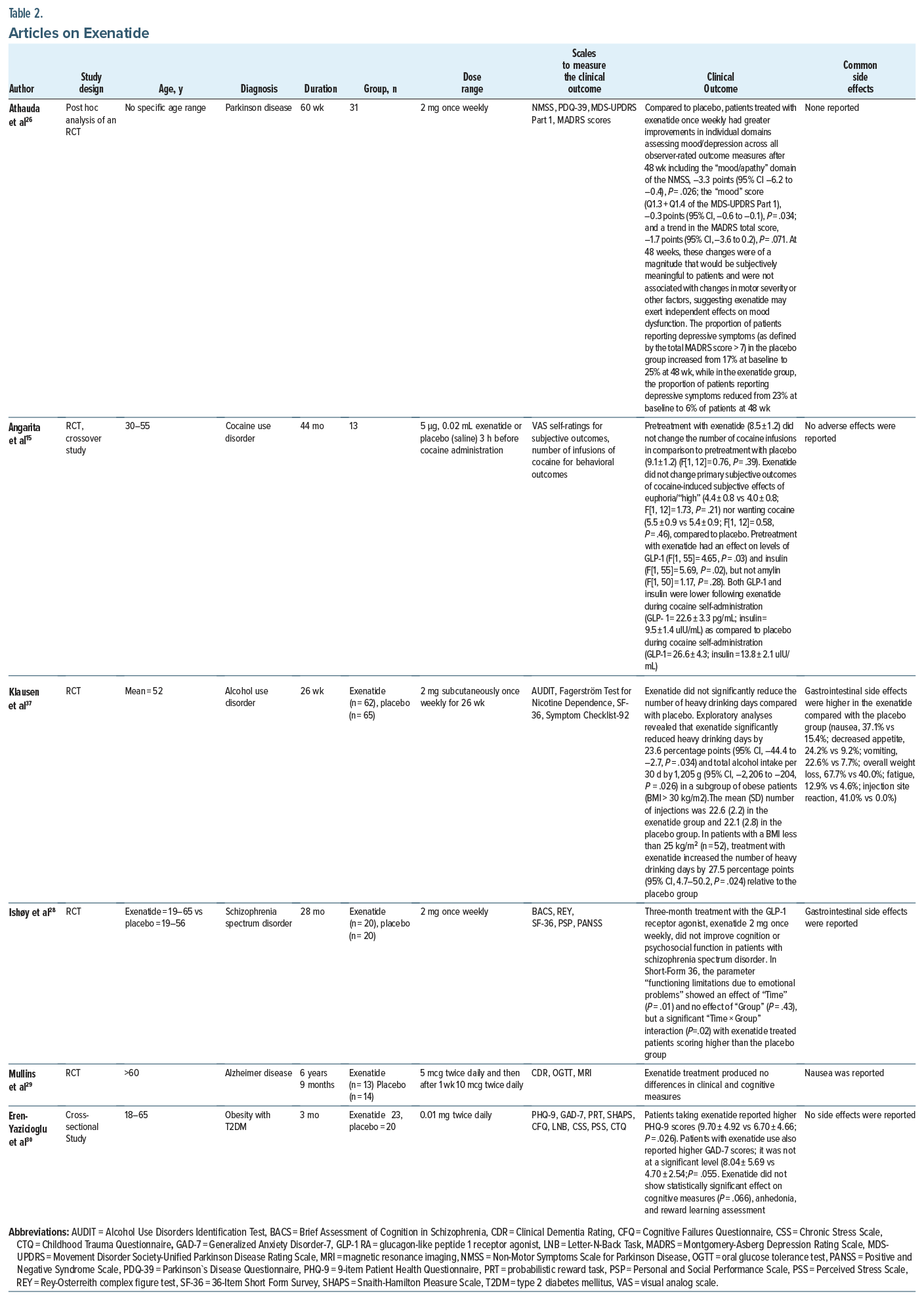
A post hoc analysis focused on the effects of exenatide compared to placebo on non-motor symptoms in Parkinson disease compared to placebo. Athauda and colleagues26 reported that exenatide 2 mg once weekly had numerically greater improvements in individual domains assessing mood/depression across all observer rated outcome measures including the “mood/apathy” domain of the Non-Motor Symptoms Scale in Parkinson Disease.
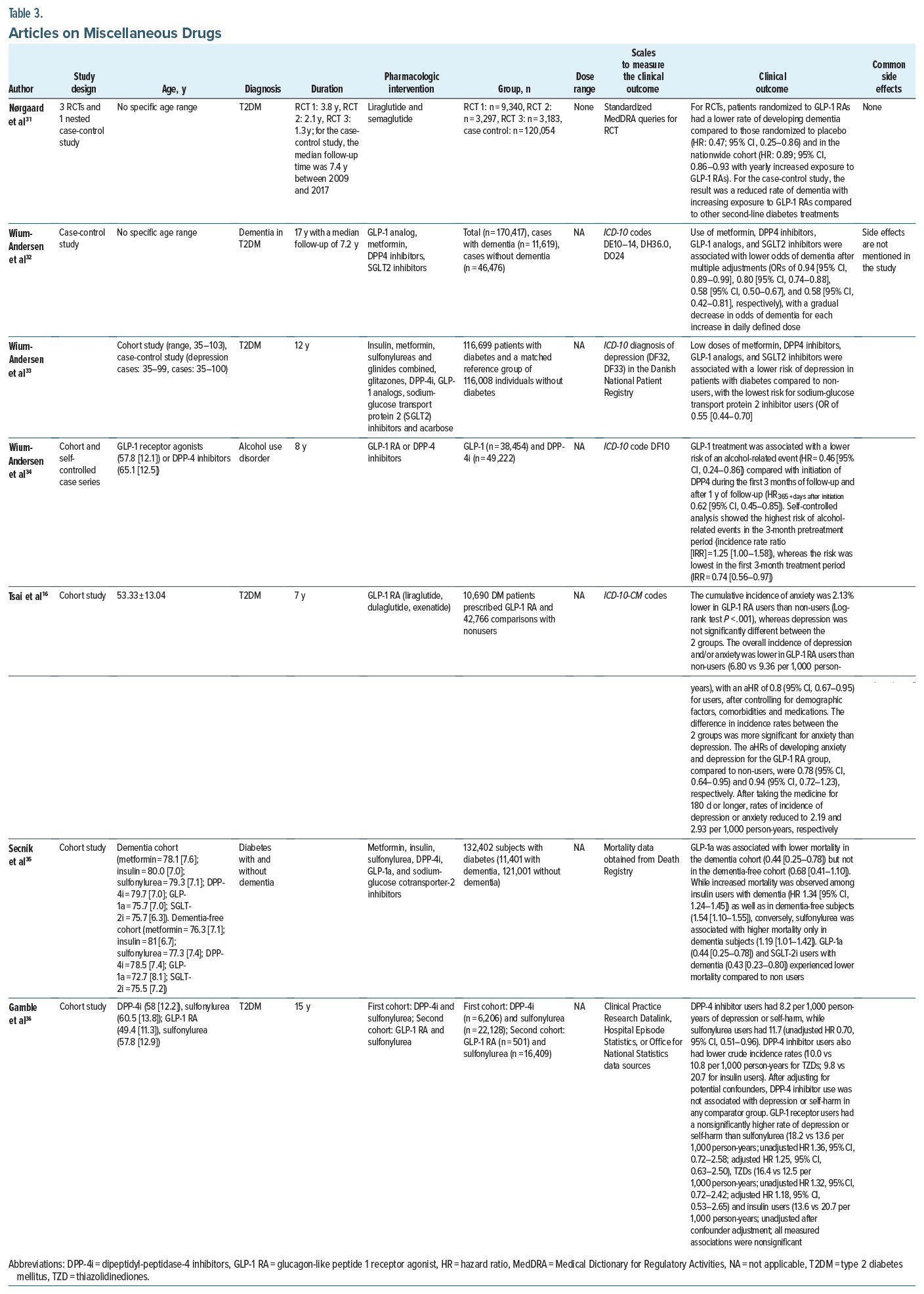
In a nested case-noncase study by Battini et al,24 all signal detection methodologies and disproportionality statistics investigating the GLP-1 analogs, in particular, liraglutide and glicazide, agreed on its statistically significant potential antidepressant effect. Similarly, in the combined case-control and cohort study by Wium-Andersen and colleagues,33 low doses of metformin, DPP-4 inhibitors, GLP-1 analogs, and SGLT2 inhibitors were associated with a lower risk of depression in patients with diabetes compared to non users, with the lowest risk for sodium-glucose transport protein 2 inhibitor users.
In another nationwide cohort study assessing the risk of depression in diabetic patients receiving GLP-1 agonists, Tsai et al16 demonstrated evidence of a lower combined incidence of anxiety and/or depression in GLP-1 RA users than in nonusers. The study further indicated that depression or anxiety decreased with increasing duration of treatment after the initiation of GLP-1 RA medication. The reduction trends were significant after controlling for all covariates.16
O’Neil and colleagues21 studied the neuropsychiatric safety of liraglutide for weight management. They concluded in the pooled analysis of 5,325 randomized and exposed individuals that rates of depression (2.1 vs 2.1 events/100 person-years) through adverse event reporting were similarly low in liraglutide and placebo groups. However, in phase 3a trials, mean baseline Patient Health Questionnaire-9 (PHQ-9) scores for liraglutide vs placebo improved at the treatment end.21
In a cohort study, Gamble et al36 examined the risk of depression and self-harm with incretin-based therapies in patients with type 2 diabetes. The incidence of depression or self-harm was 8.2 vs 11.7 events/1,000 person-years in the DPP-4i-cohort and 18.2 vs 13.6 events/1,000 person-years in the GLP-1 RA cohort for incretin-based therapies vs sulfonylureas, respectively. Incretin-based therapies were not associated with an increased or decreased incidence of depression or self-harm compared with sulfonylureas.36 Finally, a cross-sectional study also demonstrated adverse effects of GLP-1 agonists, with patients on exenatide reporting higher PHQ-9 scores (9.70 ± 4.92 vs 6.70 ± 4.66; P = .026). However, the high depressive scores were indirectly due to high perceived stress with exenatide use.30 No significant side effects were reported in any of these studies.
Cognitive Functioning/Dementia
At least 5 studies (1 case-control, 1 cohort, 3 RCTs) discussed the role of GLP-1 agonists and their action in AD and dementia. An open-label trial by Mansur et al23 reported that addition of liraglutide 1.8 mg daily to the existing medication regimen led to significant increases from baseline to week 4 in the Trail-Making Test-B standard score and in a composite Z-score comprising multiple cognitive tests. In a secondary analysis of this study, adjunctive liraglutide resulted in clinically significant weight loss and improvement in cognitive function. These changes were partially moderated by changes in brain morphometry, underscoring the interrelationship between weight and brain structure/ function.20 In another open-label trial, Li and colleagues22 assessed the effects of liraglutide on cognitive functioning. The study provided evidence at 12 weeks that patients in the GLP-1 group acquired better scores in all cognitive tests and showed remarkable improvement in memory and attention (P = .040) tests compared with the control group after multivariable adjustment.22 Compared with the control group, liraglutide also significantly increased activation of the dorsolateral prefrontal cortex and orbitofrontal cortex brain regions (P = .0038). After liraglutide treatment, cognitive scores were significantly correlated with changes in these activating brain regions (P < .05), but no correlation was observed between the changes in cognitive function and changes in body mass index, blood pressure, and glycemic levels, leading to the conclusion that this beneficial effect was independent of liraglutide hypoglycemic effect and weight loss.
Nørgaard et al31 assessed exposure to GLP-1 RAs in patients with type 2 diabetes and subsequent diagnosis of dementia in 2 large data sources with long-term follow-up: pooled data from 3 randomized double-blind placebo-controlled cardiovascular outcome trials (15,820 patients) and a nationwide Danish registry based cohort (120,054 patients). The study found that the dementia rate was lower both in patients randomized to GLP-1 RAs vs placebo and in the nationwide cohort.31 Another cohort study by Secnik and colleagues35 investigated the effectiveness of the glucose-lowering drug (GLD) among dementia patients, in particular, analyzing the all-cause mortality among users of 6 GLDs in dementia and dementia-free subjects. GLP-1a and SGLT-2i users with dementia experienced lower mortality compared to non-users.35 Another case control study found favorable evidence for metformin, DPP-4 inhibitors, GLP-1 analogs, and SGLT2 inhibitors in reducing odds of dementia in patients with diabetes.32
Other evidence that was reviewed revealed no convincing data about the role of GLP agonists playing a pivotal role in cognitive function. In an RCT by Ishøy et al,28 a 3-month treatment with the GLP-1 RA exenatide 2 mg once weekly did not improve cognition in schizophrenia spectrum patients. Another study by Eren-Yazicioglu et al30 assessed the effects of GLP analogs on cognitive measures, and while patients on exenatide reported higher Cognitive Failures Questionnaire scores, this effect was statistically insignificant (29.52 ± 12.06 vs 22.85 ± 13.74; P = .066). Mullins and colleagues’29 double-blind 18-month RCT to assess the safety and tolerability of exenatide also demonstrated no differences or trends compared to placebo for clinical and cognitive measures. Finally, Gejl et al,19 in their RCT consisting of individuals with AD receiving liraglutide or placebo, found no significant differences from baseline in total cognitive score after treatment within or between the 2 groups.19
In terms of side effects, Mansur et al23 reported nausea, dizziness, and indigestion with GLP-1 agonist use. Two participants discontinued liraglutide due to severe nausea. Gejl et al19 also reported transient nausea, weight loss, and reduction in systolic blood pressure after 6 months of GLP-1 analog treatment. Nausea was a common side effect reported in some other studies as well.
Autism/Behavioral Problems
Only 1 case report was identified during this review that highlighted the beneficial effects of GLP-1 agonists in a male with compulsive food-related behaviors associated with autism.25 Treatment with liraglutide was initiated with a dose of 0.6 mg/day and gradually increased to 2.4 mg/day during the following 8 weeks. An immediate positive response was observed in the patient’s food-related behavior, manifesting as drastically subsiding obsessive food-related thoughts, craving for food, and compulsive eating. After the first week of treatment, a clear reduction in the patient’s body weight was seen. Also, obsessions, compulsions, and behavioral problems not related to food, including aggressive behavior, decreased in a significant way at home. The treatment was continued for 36 weeks with a dose of 2.4 mg/day. At the time point of 8 weeks, the weight was already reduced by 6%. No adverse side effects of liraglutide were observed in this case.25
Psychosocial Function in Schizophrenia
One RCT (40 patients) revealed no significant effect of GLP-1 RAs on neurocognition and psychosocial outcomes in schizophrenia.28 Exenatide 2 mg once weekly (Bydureon) was administered subcutaneously in the treatment group for 3 months. The self-reported relief of emotional problems in the schizophrenia population was the only significant treatment outcome of this trial, which the authors indicated might be a chance finding. For the Personal and Social Performance Scale and the Positive and Negative Syndrome Scale only a significant effect of “time” was found. Post hoc analyses did not alter the significance level of the results. Level 2 evidence was found in this RCT (OCEBM). Intolerable gastrointestinal (GI) side effects were observed in the exenatide treatment group.28
Anxiety
This review includes 1 cohort and 1 cross-sectional study for anxiety.16,30 Tsai and colleagues16 revealed in the population-based cohort study that dulaglutide could significantly reduce the risk of anxiety and depression, while liraglutide and exenatide showed no significant reductions in risks of either anxiety or depression. The cumulative incidence of anxiety was 2.13% lower in GLP-1 RA users than in nonusers. The overall incidence of depression and/or anxiety was lower in GLP-1 RA users than in nonusers. This treatment’s effectiveness on anxiety was observed in female users but not in male users. The beneficial effect on anxiety was specific to patients between 40 and 60 years old.16
The effectiveness increased with the duration of medication, and significant risk reduction was observed after 6 months or longer of therapy. GLP-1 RA users taking metformin or sulfonylurea at the same time had a decreased risk of anxiety, which was also noted in patients with hypertension. Eren-Yazicioglu et al30 found no significant difference in Generalized Anxiety Disorder-7 scores between the type 2 diabetes mellitus patients treated with and without exenatide. No adverse effects were reported in these studies.
Substance Use Disorders
One RCT (127 patients) and 1 cohort study (patients on GLP-1 RA= 38,454 and patients on DPP-4= 49,222) highlighted alcohol use disorder (AUD), and 1 RCT (13 patients) was on cocaine use disorder.15,34,37
In 1 RCT, Klausen and colleagues37 found that exenatide 2 mg once weekly significantly reduced the heavy drinking days and total alcohol intake in a subgroup of obese patients (body mass index [BMI] > 30 kg/m2) compared to placebo after 26 weeks of trial participation. In patients with a BMI less than 25 kg/m2, treatment with exenatide increased the number of heavy drinking days by 27.5 percentage points relative to the placebo group. However, in this subgroup (BMI < 25 kg/m2), the total alcohol intake did not differ between treatment groups.37
In a cohort study, Wium-Andersen and colleagues34 found that GLP-1 RA use was associated with a lower risk of a subsequent alcohol-related event compared with DPP-4 inhibitor use both in the time period closest to initiation and after 1 year of follow-up. Self-controlled analysis showed the highest risk of alcohol-related events in the 3-month pretreatment period, whereas the risk was lowest in the first 3-month treatment period.34
Angarita et al15 found that pretreatment with a clinically relevant (antidiabetic) dose of exenatide (5 μg, 0.02 mL) did not alter the cocaine-related behaviors or subjective effects such as euphoria and wanting cocaine in people with cocaine use disorder compared to placebo (saline 0.02 mL). Pretreatment with exenatide had an effect on levels of GLP-1 and insulin but not amylin. Both GLP-1 and insulin were lower following exenatide during cocaine self-administration compared to placebo during cocaine self-administration. The main effects of time indicated overall decreases related to cocaine administration for GLP-1, insulin, and amylin levels. No exenatide-by-time interactions were observed.15
There was no significant adverse effect of exenatide such as hypoglycemia noted in any subject during the cocaine sessions. Klausen et al37 reported adverse effects related to GI symptoms, body weight loss, fatigue, and injection site reactions, and the incidence was higher in the exenatide group compared with the placebo group.
DISCUSSION
This review aimed to comprehensively examine the potential impact of GLP-1 RAs on various psychiatric disorders. Exenatide has demonstrated potential in ameliorating mood and depressive symptoms, particularly in nonmotor symptoms among Parkinson patients. Chronic exendin-4 treatment has shown anxiolytic and antidepressant-like effects in a Parkinson disease model induced by lipopolysaccharide and accompanied by alterations in dopaminergic signaling.38,39 Evidence suggests that GLP-1 receptor analogs may possess neuroprotective, anxiolytic, and antidepressant effects, promoting neurogenesis and enhancing synaptic plasticity in the brain.22,40–42
The efficacy of GLP-1 RAs in depressive symptoms primarily derives from animal studies, making it challenging to draw definitive conclusions. Variations in the study outcomes can be attributed to differences in study designs and patient populations. Though some studies suggest benefits, conflicting findings highlight the need for more research, especially in humans, to understand their impact on mood disorders.
Studies on GLP-1 agonists like liraglutide and their effects on cognitive performance in people with bipolar disorder, major depressive disorder, and AD have produced mixed results. The studies conducted by Mansur et al23 and Li et al22 and their respective findings on liraglutide’s effect on cognitive function are promising, as they observed improvements in cognitive scores and memory, along with enhanced brain activation, indicating the potential neurocognitive benefits of GLP-1 agonists. These positive effects of GLP-1 RAs on cognitive function can be attributed to neuroprotective and neurotrophic mechanisms. GLP-1 RAs facilitate glucose and insulin signaling, which is crucial for brain health and function. Additionally, these agonists have been shown to suppress oxidative stress and inflammation, which are known contributors to cognitive decline.43
The review of studies included in this analysis also reveals a potential link between the use of GLP-1 RAs and a reduced risk of dementia in AD patients, as well as a lower risk of mortality in dementia patients.31,35 The associated risk of developing dementia in diabetic patients who use GLP-1 RAs appears to be relatively low and is likely attributed to their effects on cerebral glucose metabolism. The decline in cerebral glucose metabolism has been consistently associated with the pathological progression of AD and subsequent cognitive impairment.44,45
Animal studies have shown significant reductions in Aβ load in the early stages of AD following treatment with liraglutide, indicating a potential role in mitigating disease progression.46 However, the effects may vary at different stages of the disease, as evidenced by the study showing that the reduction was most pronounced in the early stages but less so in more advanced stages with behavioral symptoms.46
In contrast to the studies supporting the positive impact of GLP-1 RAs, some findings challenge the notion that these agonists consistently enhance cognitive function in various populations.19,28,30 Further research is needed to clarify the role of GLP-1 agonists in cognitive disorders due to variability in study designs, patient populations, and treatment regimens that may contribute to differing outcomes.
In essence, GLP-1 RAs like liraglutide may improve cognitive function and reduce dementia risk in some patients, but more research is needed to confirm these findings due to variability in study outcomes. Further investigations are required to elucidate the precise mechanisms by which GLP-1 agonists influence cognitive function and to identify the specific patient populations that may benefit most from such treatment.
Further, the role of GLP-1 RAs in the treatment of AUD is a promising area of research. The review shows that exenatide seemed to decrease the heavy drinking days in obese people through its effect on the brain areas controlling drug reward and addiction (Klausen et al37). The GLP-1 analog semaglutide showed similar results in mice by a dose-dependent reduction in binge drinking via the probable mechanism of modulating GABA neurotransmission centrally.47,48 GLP-1 agonists impact the dopamine signaling in the mesolimbic pathway and regulate the reward-motivated behavior in alcohol consumption, thus showing potential as a pharmacotherapeutic intervention.49
Our review includes a study by Wium-Andersen et al34 proposing the association of GLP-1 agonist treatment with a reduced risk of alcohol-related events which include alcohol withdrawal syndrome and alcohol dependence. Liraglutide has shown the potential to help with alcohol withdrawal symptoms and reduce alcohol intake in rodents and monkeys.50 Contrary to preclinical literature about the positive role of GLP-1 RAs on the rewarding and reinforcing behavior of drug abuse, the study in our review proposed that exenatide treatment did not help with euphoria and cocaine craving in the subjects.37
To summarize, the current evidence suggests that GLP-1 agonists may have a beneficial impact on AUD and related behaviors. However, more human studies are required for confirmation. Since AUD is a chronic and recurrent disorder, a comprehensive randomized clinical trial with ample sample size is necessary to determine the long-term efficacy of GLP-1 agonists as a treatment for this disorder. The study15 on cocaine use disorder only observed the effect of acute treatment with exenatide in a small set of patients, and thus more evidence is needed to understand the role of GLP-1 analogs in cocaine use disorder.
Two studies in this review investigated GLP-1 agonists in anxiety disorders. Dulaglutide was anxiolytic, while liraglutide and exenatide were not. The latter evidence was reinforced by Eren-Yazicioglu et al,30 as they found no significant difference in Generalized Anxiety Disorder-7 scores of the exenatide and placebo groups. The association of GLP 1 RAs with reduced risk of anxiety in patients with diabetes was consistent with previous studies.16,51 The anxiolytic effect of GLP-1 agonists has been hypothesized due to the preservation of brain-derived neurotrophic factor/nuclear factor erythroid 2–related factor 2 levels, decreased oxidative stress, and lipocalin 2 levels in the hippocampus.52 The evidence showing the potential benefit of GLP-1 RAs for combating anxiety disorders seems sparse and is an area requiring future research.
Despite the valuable insights gained from this scoping review, several limitations should be acknowledged. The studies included in the analysis demonstrated variability in terms of their study design, the GLP-1 agonists investigated, the dosages administered, and the outcome measures assessed. This diversity poses a challenge in reaching conclusive findings. Furthermore, the lack of studies conducted in specific fields, such as autism and substance use disorders, highlights the necessity for additional rigorous investigations in these domains to establish a thorough understanding.
Additionally, it is important to consider the potential presence of publication bias, when studies that have good results are more likely to be published, so distorting the overall understanding of the existing body of research. The potential limitations of certain studies may include the period of the research, as it may not adequately capture the long-term effects and potential negative effects of GLP-1 agonists.
Hence, by addressing these methodological limitations, such as adequacy of follow-up, generalizability, outcome measures, and small sample sizes, future research can provide more robust evidence of the potential benefits and risks of GLP-1 RAs in psychiatric disorders. Furthermore, diverse patient populations, encompassing individuals with various psychiatric and medical comorbidities, introduce complexities in the extrapolation of results. Variability in outcome measurements may arise due to the absence of standardized evaluation tools and diagnostic criteria employed across different research studies.
Finally, we would like to mention the following studies that have been published after this review’s search date but could not be included:
- Psychiatric Safety of Semaglutide for Weight Management in People Without Known Major Psychopathology.53
- Ecological Momentary Assessment and Cue-Elicited Drug Craving as Primary End Points: Study Protocol for a Randomized, Double-Blind, Placebo-Controlled Clinical Trial Testing the Efficacy of a GLP-1 RA in Opioid Use Disorder.54
- Liraglutide in Obese or Overweight Individuals With Stable Bipolar Disorder.55
CONCLUSION
This scoping review provides valuable insights into the complex relationship between GLP-1 agonists and psychiatric comorbidities, and further well-designed, long-term, and standardized studies are needed to clarify the mechanisms, dosage-effect relationships, and potential clinical applications of GLP-1 agonists in the field of mental health.
Article Information
Published Online: June 10, 2025. https://doi.org/10.4088/PCC.24nr03828
© 2025 Physicians Postgraduate Press, Inc.
Submitted: August 11, 2024; accepted January 23, 2025.
To Cite: Ali M, Ahmed A, Khan BA, et al. Efficacy of GLP-1 agonists in psychiatric illnesses: a scoping review. Prim Care Companion CNS Disord 2025;27(3):24nr03828.
Author Affiliations: King Edward Medical University, Lahore, Pakistan (Ali, Ahmed); Department of Psychiatry and Human Behavior, University of Mississippi Medical Center, Jackson, Mississippi (Khan); Nishtar Medical University and Hospital, Multan, Pakistan (Shah); Eastern Connecticut Health Network, Manchester, Connecticut (Naveed); KCU-GME/Ozark Center Psychiatry Residency Program, Joplin, Missouri (Ashraf).
Corresponding Author: Mohsan Ali, MBBS, King Edward Medical University, St 12, Wapda Town, Phase #1, Lahore, Pakistan ([email protected]).
Relevant Financial Relationships: None.
Funding/Support: None.
Clinical Points
- GLP-1 receptor agonists (GLP-1 RAs), such as exenatide, demonstrated efficacy in reducing heavy drinking days and total alcohol intake in individuals with obesity-related alcohol use disorders, suggesting a potential role for GLP-1 RAs in targeted treatments for alcohol dependency, particularly in patients with specific metabolic profiles.
- GLP-1 RAs such as dulaglutide and liraglutide may lower the risk of depressive and anxiety symptoms in diabetic populations, particularly with prolonged use; however, gender- and age-specific responses, as well as concurrent medication use (eg, metformin), might influence outcomes.
- Despite promising results in depression and anxiety, GLP-1 RAs have not shown significant benefits in improving cognitive functioning or psychosocial outcomes in schizophrenia or dementia, highlighting the need for cautious optimism and further investigation into their broader neuropsychiatric applications.
References (55)

- Graaf CD, Donnelly D, Wootten D, et al. Glucagon-like peptide-1 and its class B G protein-coupled receptors: a long march to therapeutic successes. Pharmacol Rev. 2016;68(4):954–1013.
- Isaacs D, Prasad-Reddy L, Srivastava SB. Role of glucagon-like peptide 1 receptor agonists in management of obesity. Am J Health Syst Pharm. 2016;73(19):1493–1507.
- Grieco M, Giorgi A, Gentile MC, et al. Glucagon-like peptide-1: a focus on neurodegenerative diseases. Front Neurosci. 2019;13:1112.
- Nauck MA, Quast DR, Wefers J, et al. GLP-1 receptor agonists in the treatment of type 2 diabetes - state-of-the-art. Mol Metab. 2021;46:101102.
- Tramutola A, Arena A, Cini C, et al. Modulation of GLP-1 signaling as a novel therapeutic approach in the treatment of Alzheimer’s disease pathology. Expert Rev Neurother. 2017;17(1):59–75.
- Drucker DJ. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab. 2018;27(4):740–756.
- Turton MD, O’Shea D, Gunn I, et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379(6560):69–72.
- Mao D, Cao H, Shi M, et al. Increased co-expression of PSMA2 and GLP-1 receptor in cervical cancer models in type 2 diabetes attenuated by Exendin-4: a translational case-control study. EBioMedicine. 2021;65:103242.
- Hirsch IB. The future of the GLP-1 receptor agonists. JAMA. 2019;321(15):1457–1458.
- Erbil D, Eren CY, Demirel C, et al. GLP-1’s role in neuroprotection: a systematic review. Brain Inj. 2019;33(6):734–819.
- Bradbury J. Hope for AD with NGF gene-therapy trial. Lancet Neurol. 2005;4(6):335.
- Kuipers SD, Bramham CR. Brain-derived neurotrophic factor mechanisms and function in adult synaptic plasticity: new insights and implications for therapy. Curr Opin Drug Discov Devel. 2006;9(5):580–586.
- Chang YF, Zhang D, Hu WM, et al. Semaglutide-mediated protection against Aβ correlated with enhancement of autophagy and inhibition of apotosis. J Clin Neurosci. 2020;81:234–239.
- Zhang L, Zhang L, Li L, et al. Neuroprotective effects of the novel GLP-1 long acting analogue Semaglutide in the MPTP Parkinson’s disease mouse model. Neuropeptides. 2018;71:70–80.
- Angarita GA, Matuskey D, Pittman B, et al. Testing the effects of the GLP-1 receptor agonist Exenatide on cocaine self-administration and subjective responses in humans with cocaine use disorder. Drug Alcohol Depend. 2021;221:108614.
- Tsai WH, Sung FC, Chiu LT, et al. Decreased risk of anxiety in diabetic patients receiving glucagon-like peptide-1 receptor agonist: a nationwide, population based cohort study. Front Pharmacol. 2022;13:765446.
- Howick J, Chalmers I, Glasziou P, et al. The 2011 Oxford CEBM Levels of Evidence (Introductory Document). Oxford Centre for Evidence-Based Medicine. https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. PubMed CrossRef
- Gejl M, Gjedde A., Egefjord L., et al. In Alzheimer’s disease, 6-month treatment with GLP-1 analog prevents decline of brain glucose metabolism: randomized, placebo-controlled, double-blind clinical trial. Front Aging Neurosci. 2016;8:108.
- Mansur RB, Zugman A, Ahmed J, et al. Treatment with a GLP− 1R agonist over four weeks promotes weight loss-moderated changes in frontal-striatal brain structures in individuals with mood disorders. Eur Neuropsychopharmacol. 2017;27(11):1153–1162.
- O’Neil PM, Aroda VR, Astrup A, et al. Neuropsychiatric safety with liraglutide 3.0 mg for weight management: results from randomized controlled phase 2 and 3a trials. Diabetes Obes Metab. 2017;19(11):1529–1536.
- Li Q, Jia M, Yan Z, et al. Activation of glucagon-like peptide-1 receptor ameliorates cognitive decline in type 2 diabetes mellitus through a metabolism independent pathway. J Am Heart Assoc. 2021;10(14):e020734.
- Mansur RB, Ahmed J, Cha DS, et al. Liraglutide promotes improvements in objective measures of cognitive dysfunction in individuals with mood disorders: a pilot, open-label study. J Affect Disord. 2017;207:114–120.
- Battini V, Van Manen RP, Gringeri M, et al. The potential antidepressant effect of antidiabetic agents: new insights from a pharmacovigilance study based on data from the reporting system databases FAERS and VigiBase. Front Pharmacol. 2023;14:1128387.
- Järvinen A, Laine MK, Tikkanen R, et al. Beneficial effects of GLP-1 agonist in a male with compulsive food-related behavior associated with autism. Front Psychiatry. 2019;10:97.
- Athauda D, Maclagan K, Budnik N, et al. What effects might exenatide have on non-motor symptoms in Parkinson’s disease: a post hoc analysis. J Parkinsons Dis. 2018;8(2):247–258.
- Klausen MK, Thomsen M, Wortwein G, et al. The role of glucagon-like peptide 1 (GLP-1) in addictive disorders. Br J Pharmacol. 2022;179(4):625–641.
- Ishøy PL, Fagerlund B, Broberg BV, et al. No cognitive-enhancing effect of GLP-1 receptor agonism in antipsychotic-treated, obese patients with schizophrenia. Acta Psychiatr Scand. 2017;136(1):52–62.
- Mullins RJ, Mustapic M, Chia CW, et al. A pilot study of exenatide actions in Alzheimer’s disease. Curr Alzheimer Res. 2019;16(8):741–752.
- Eren-Yazicioglu CY, Kara B, Sancak S, et al. Effect of exenatide use on cognitive and affective functioning in obese patients with type 2 diabetes mellitus: exenatide use mediates depressive scores through increased perceived stress levels. J Clin Psychopharmacol. 2021;41(4):428–435.
- Nørgaard CH, Friedrich S, Hansen CT, et al. Treatment with glucagon-like peptide-1 receptor agonists and incidence of dementia: data from pooled double blind randomized controlled trials and nationwide disease and prescription registers. Alzheimers Dement. 2022;8(1):e12268.
- Wium-Andersen IK, Osler M, Jørgensen MB, et al. Antidiabetic medication and risk of dementia in patients with type 2 diabetes: a nested case–control study. Eur J Endocrinol. 2019;181(5):499–507.
- Wium-Andersen IK, Osler M, Jørgensen MB, et al. Diabetes, antidiabetic medications and risk of depression - a population-based cohort and nested case-control study. Psychoneuroendocrinology. 2022;140:105715.
- Wium-Andersen IK, Wium-Andersen MK, Fink-Jensen A, et al. Use of GLP-1 receptor agonists and subsequent risk of alcohol-related events. A nationwide register-based cohort and self-controlled case series study. Basic Clin Pharmacol Toxicol. 2022;131(5):372–379.
- Secnik J, Xu H, Schwertner E, et al. Glucose-lowering medications and post dementia survival in patients with diabetes and dementia. J Alzheimers Dis. 2022;86(1):245–257.
- Gamble JM, Chibrikov E, Midodzi WK, et al. Examining the risk of depression or self-harm associated with incretin-based therapies used to manage hyperglycaemia in patients with type 2 diabetes: a cohort study using the UK Clinical Practice Research Datalink. BMJ Open. 2018;8(10):e023830.
- Klausen MK, Jensen ME, Møller M, et al. Exenatide once weekly for alcohol use disorder investigated in a randomized, placebo-controlled clinical trial. JCI insight. 2022;7(19):e159863.
- Anderberg RH, Richard JE, Hansson C, et al. GLP-1 is both anxiogenic and antidepressant; divergent effects of acute and chronic GLP-1 on emotionality. Psychoneuroendocrinology. 2016;65:54–66.
- Ventorp F, Bay-Richter C, Nagendra AS, et al. Exendin-4 treatment improves LPS induced depressive-like behavior without affecting pro-inflammatory cytokines. J Parkinsons Dis. 2017;7(2):263–273.
- Porter D, Faivre E, Flatt PR, et al. Actions of incretin metabolites on locomotor activity, cognitive function and in vivo hippocampal synaptic plasticity in high fat fed mice. Peptides. 2012;35:1–8.
- Gumuslu E, Mutlu O, Celikyurt IK, et al. Exenatide enhances cognitive performance and upregulates neurotrophic factor gene expression levels in diabetic mice. Fundam Clin Pharmacol. 2016;30(4):376–384.
- Hunter K, Hölscher C. Drugs developed to treat diabetes, liraglutide and lixisenatide, cross the blood-brain barrier and enhance neurogenesis. BMC Neurosci. 2012;13:33.
- Pipatpiboon N, Pintana H, Pratchayasakul W, et al. DPP4-inhibitor improves neuronal insulin receptor function, brain mitochondrial function and cognitive function in rats with insulin resistance induced by high-fat diet consumption. Eur J Neurosci. 2013;37(5):839–849.
- Engler H, Forsberg A, Almkvist O, et al. Two-year follow-up of amyloid deposition in patients with Alzheimer’s disease. Brain. 2006;129(Pt 11):2856–2866.
- Mosconi L. Brain glucose metabolism in the early and specific diagnosis of Alzheimer’s disease. FDG-PET studies in MCI and AD. Eur J Nucl Med Mol Imaging. 2005;32(4):486–510.
- McClean PL, Parthsarathy V, Faivre E, et al. The diabetes drug liraglutide prevents degenerative processes in a mouse model of Alzheimer’s disease. J Neurosci. 2011;31(17):6587–6594.
- Chuong V, Farokhnia M, Khom S, et al. The glucagon-like peptide-1 (GLP-1) analogue Semaglutide reduces alcohol drinking and modulates central GABA neurotransmission. JCI insight. 2023;8(12):e170671.
- Aranäs C, Edvardsson CE, Shevchouk OT, et al. Semaglutide reduces alcohol intake and relapse-like drinking in male and female rats. EBioMedicine. 2023;93:104642.
- Colvin KJ, Killen HS, Kanter MJ, et al. Brain site-specific inhibitory effects of the GLP-1 analogue exendin-4 on alcohol intake and operant responding for palatable food. Int J Mol Sci. 2020;21(24):9710.
- Jerlhag E. Alcohol-mediated behaviours and the gut-brain axis; with focus on glucagon-like peptide-1. Brain Res. 2020;1727:146562.
- de Wit HM, Vervoort GM, Jansen HJ, et al. Liraglutide reverses pronounced insulin-associated weight gain, improves glycaemic control and decreases insulin dose in patients with type 2 diabetes: a 26 week, randomised clinical trial (ELEGANT). Diabetologia. 2014;57(9):1812–1819.
- Sağlam C, Turan İ, Özaçmak HS. The effect of glucagon like peptide-1 receptor agonist on behavioral despair and anxiety-like behavior in ovariectomized rats: modulation of BDNF/CREB, Nrf2 and lipocalin 2. Behav Brain Res. 2022;435:114053.
- Wadden TA, Brown GK, Egebjerg C, et al. Psychiatric safety of semaglutide for weight management in people without known major psychopathology: post hoc analysis of the STEP 1, 2, 3, and 5 trials. JAMA Intern Med. 2024;184(11):1290–1300.
- Freet CS, Evans B, Brick TR, et al. Ecological momentary assessment and cue-elicited drug craving as primary endpoints: study protocol for arandomized, double-blind, placebo-controlled clinical trial testing the efficacy of a GLP-1 receptor agonist in opioid use disorder. Addict Sci Clin Pract. 2024;19(1):56.
- McElroy SL, Guerdjikova AI, Blom TJ, et al. Liraglutide in obese or overweight individuals with stable bipolar disorder. J Clin Psychopharmacol. 2024;44(2):89–95.
Enjoy this premium PDF as part of your membership benefits!