ABSTRACT
Objective: To assess the efficacy and safety of loxapine in acute agitation.
Data Sources: PubMed, Cochrane database, EMBASE, PsycINFO, and ClinicalTrials.gov were searched to identify relevant articles published in English or French from inception to March 15, 2022. The term “Loxap*” was searched in titles and abstracts.
Study Selection and Data Extraction: Interventional studies that compared the effectiveness of loxapine to any other intervention (including another administration route or dosage of loxapine, other drugs, and placebo) in acute agitation were included. From the 1,435 articles initially identified, and after the assessment of 73 full texts, 7 articles were selected, encompassing 1,276 participants. Two reviewers independently extracted data of interest using a predefined form.
Results: Among included studies, 5 were double-blind, 2 were open-label, and all were randomized. The risk of bias was low for 2 studies, involving 658 participants. Four articles compared loxapine to placebo, and 3 compared it with haloperidol, aripiprazole, and droperidol. Loxapine was found to be more effective and faster regarding acute agitation control. Also, across included studies, loxapine was well-tolerated, with mildly or moderately severe adverse effects.
Conclusions: Notwithstanding methodological limitations of the included studies, this systematic review provides reassuring results regarding the use of loxapine in acute agitation. However, further studies with methodological optimizations might be of interest.
Prim Care Companion CNS Disord 2023;25(6):23r03552
Author affiliations are listed at the end of this article.
Agitation is defined in the DSM-5 as an “excessive motor activity associated with a feeling of inner tension.”1 It can be related to various conditions including acute intoxication/withdrawal of a psychoactive substance, drug iatrogenesis, metabolic disturbances, infectious illnesses, neurologic disorders, and mental health disorders,2–5 which especially encompass anxiety, bipolar disorder (manic state), psychotic disorders, and personality disorders. As agitation can result in auto- or hetero-aggressive behaviors, it is necessary to intervene as quickly and effectively as possible to prevent self-harm or physical assault on other people including health care workers.6
The care of acute agitation includes nonpharmacologic approaches such as strategies aiming to appease patients and involving notably de-escalation.7,8 Nevertheless, in numerous cases there is a need for pharmacologic treatments, and despite that the oral route is preferred, there is currently no consensus regarding the pharmacologic class to use.7 Such consensual recommendations might be of interest considering the existence in countries such as France of laws governing the prescription of seclusion and restraint measures that can be considered as a last resort for severely agitated patients.9,10 Among the medications that can be used for acute agitation, the Société Française de Médecine d’Urgence (SFMU), in their 2003 consensus conference on agitation, mentioned loxapine, explaining that this medication seems unanimously accepted by medical professionals in France.11 More recently, in their 2021 Recommendations for Good Clinical Practice, they recognized loxapine as a standard for the treatment of patients with acute agitation.12 In France, loxapine is therefore one of the most frequently used pharmacologic agents for the control of agitated individuals.13 Loxapine is commercialized in Canada and was approved by the US Food and Drug Administration for the management of agitated patients.14,15
With available galenic forms for oral, nasal, and intramuscular administration, loxapine is a first-generation antipsychotic that belongs to the dibenzoxazepines and is known to have sedative as well as anxiolytic effects.16 Despite its widespread use for over 40 years in some settings, there is a scarcity of clinical trials on the efficacy and safety of this antipsychotic drug in acute agitation. In comparison, there is more research on other antipsychotic medications such as haloperidol or olanzapine, on benzodiazepines such as midazolam or lorazepam,17 and more recently on ketamine.18 Systematic reviews and meta-analyses have evaluated several psychotropic treatments in the control of acute agitation, but they did not include loxapine.19,20
Considering the high frequency of acute agitation notably in emergency and intensive care units, the frequent use of loxapine in some occidental countries, and the lack of dedicated summarizing data, we planned this systematic review. Indeed, an evaluation of loxapine effectiveness, through a systematic analysis of published literature, could contribute to good practice recommendations on agitation.
The objective of this systematic review was to evaluate the efficacy of loxapine in the care of patients with acute agitation. The PICOS approach was used to address this objective, with the following declinations: (1) Population: patients with acute agitation, regardless of the setting (emergency department, inpatient, outpatient); (2) Intervention: loxapine, regardless of route of administration (oral, intramuscular, inhaled); (3) Comparison: other loxapine dosage, other loxapine route, other medications or placebo; (4) Outcome: agitation control (in terms of time, intensity, duration); and (5) Study design: interventional studies.
METHODS
Our review’s sections are displayed in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) 2020 statement.21 The review was registered in the International Prospective Register of Systematic Reviews (registration no. CRD42022330846).
Eligibility Criteria
Inclusion criteria were based on the PICOS approach items detailed previously. We excluded studies performed on animal models, case reports, letters to the editor, comments, duplicated papers, articles for which the full text was unavailable even after request, the least recent studies if several studies were based on the same population, and studies written in a language other than English or French.
Information Sources and Search Strategy
We systematically searched for relevant articles in PubMed/MEDLINE, EMBASE, PsycINFO (Ovid), Cochrane database, and ClinicalTrials.gov from inception to March 15, 2022. To retrieve relevant articles, the term “Loxap*” was searched in titles and abstracts with no particular filters such as time limits.
Selection Process
The results were exported to the Rayyan platform (https://www.rayyan.ai/), which is a tool dedicated to the management of articles during the systematic review process. After duplicate removal, the remaining articles were evaluated on their title and/or abstract according to our eligibility criteria. This evaluation was independently performed by 2 reviewers (C.L. and F.T.E.), with the resolution of potential discrepancies by consensus or the intervention of a third assessor (G.C.). After this first selection step, the full texts of the retained articles were searched and scrutinized, also on the basis of our inclusion criteria, independently by 2 reviewers (C.L. and F.T.E.) with the same process of discrepancy resolution. Authors of articles for which we did not have access to the full text were contacted when possible, and lack of response within 1 month led to the exclusion of the article. The reasons for exclusion were reported.
Data Collection Process
After meticulous examinations of the final selected articles, we collected our data of interest using a predefined and pretested Google form. Further, using a blind process, 2 reviewers (C.L. and F.T.E.) independently extracted data, with resolution of divergences through consensual decisions after discussions or the intervention of a third author (G.C.).
Data Items
Data collected for each article were as follows:
(1) Bibliometric data with the name of the first author and the year of publication.
(2) General characteristics of the studies with the phase and the registration number of the clinical trial, the country and period of the study, the study design, the setting of the study (emergency unit, psychiatric unit, intensive care unit, etc), the randomization method and tool, the study hypothesis, the statistical analysis, and the experimental design.
(3) General characteristics of study participants encompassing the main selection criteria, the sample sizes in each arm, the mean age, the sex ratio, the underlying conditions with assessment/screening tools and cutoff used, the type of agitation, the tool used to assess acute agitation, the data collection method, and the follow-up duration.
(4) Information pertaining to interventions, especially drug classes, administration routes, protocols, daily doses, and timing of drug administrations.
(5) The outcomes regarding efficacy of the interventions (time to control agitation, proportions of successfully controlled agitations, sedation scale scores, agitation scale scores, need of additional treatments for sedation, use of seclusion and/or restraint, occurrences of self-harm, frequency and severity of side effects) and safety.
Risk of Bias Assessment
The risk of bias assessment was performed using the Cochrane Risk of Bias (RoB) 2.0 tool.22 This tool was developed in 2016 and released in August 2019 and consists of 5 domains identified as potential sources of bias. These domains include risk of bias resulting from the randomization process, risk of bias resulting from deviations from planned interventions, missing data on the outcome, risk of bias due to measurement of the outcome, and risk of bias in the selection of reported results. Two investigators performed this step (C.L. and F.T.E.) with a blind and independent evaluation, and disagreements were resolved by consensus.
From the assessment of each domain, we were able to identify the overall risk of bias for each study. A low overall risk of bias was retained when all domains had a low risk. An overall risk of bias with “some concern” was retained when there was a bias rated as “some concern” for 1 of the domains. A high risk of overall bias was retained if 1 of the domains had a high risk or if more than 1 domain had “some concerns.”
Synthesis Methods
We defined the following plan to present our findings: (1) graphical illustration of the selection process with a PRISMA-based flowchart, (2) tabular report of individual characteristics of the selected studies, (3) tabular presentation of results related to risk of bias assessment, and (4) tabular and text syntheses of results regarding efficacy and safety outcomes.
RESULTS
Study Selection
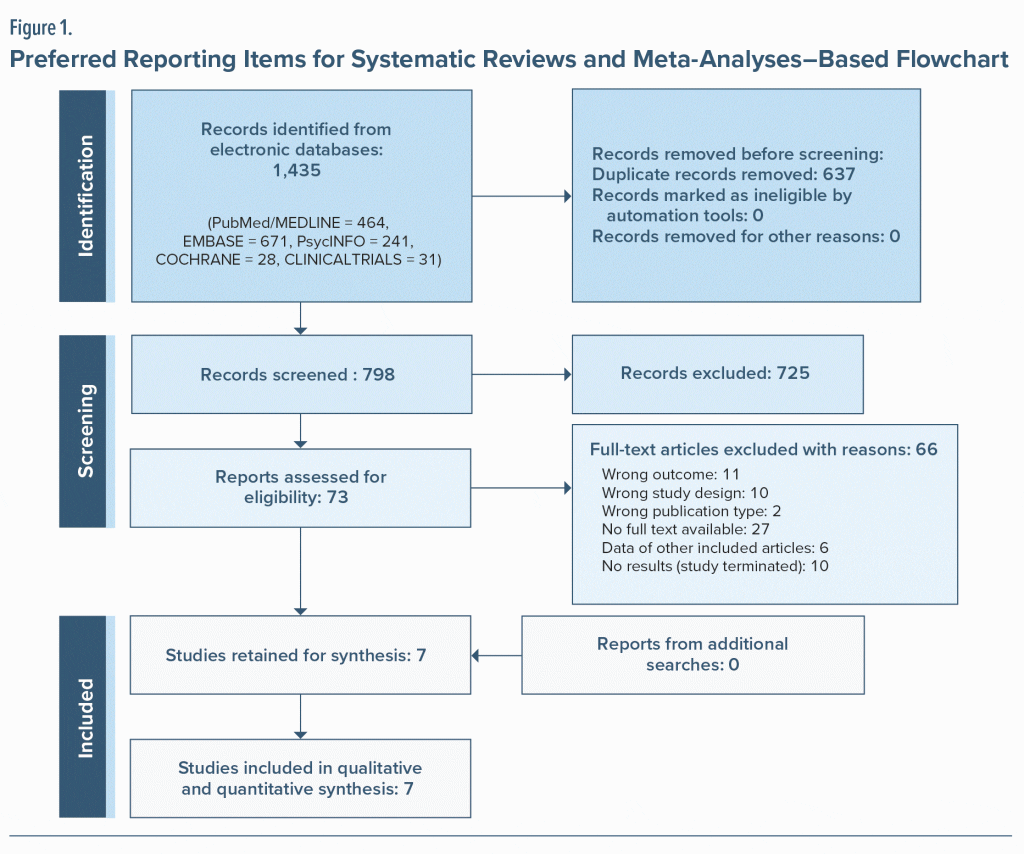
From the 1,435 articles initially identified, 637 duplicates were excluded, and of the 798 remaining articles, 73 were selected based on titles and abstracts. After searching and analyzing full texts (when it was possible), 7 studies were retained for our narrative synthesis.23–29 Of note, we requested full texts for 6 articles of interest regarding our eligibility criteria, received 4 of them, and finally included 2 in our review. The different stages of the selection process are illustrated in Figure 1.
Study Characteristics
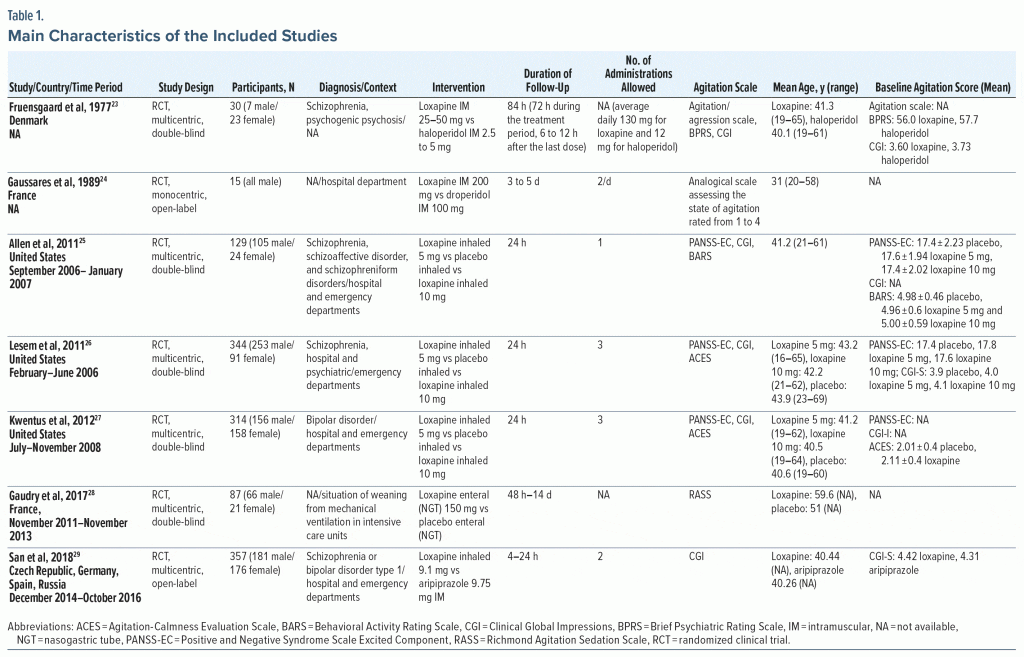
Of the 7 enrolled studies, 3 took place in the United States, 2 in France, 1 in Denmark, and 1 involved the Czech Republic, Germany, Spain, and Russia (Table 1). The studies were published between 1977 and 2018 and were all randomized controlled trials. More precisely, 5 were double-blind and 2 were open label. Only 1 of the trials was a single center study (see Table 1).
The total number of participants was 1,276, with sample sizes ranging from 15 to 357. The mean age of participants varied between 31 and 59.6 years old, with a minimum of 16 years and a maximum of 69 years, and the male to female ratio was 1.58 (783/493). Only 1 article24 reported the mean weight of the participants, and none reported the minimum and maximum weights. Three studies23,25,26 targeted patients with psychotic disorder, 127 focused on patients with bipolar disorder, and 129 selected patients with psychotic disorder or type 1 bipolar disorder. Also, 1 study28 selected participants whose agitation resulted from weaning from mechanical ventilation in an intensive care unit. The other trials were conducted in hospital or emergency departments (see Table 1).
Four studies25–28 compared loxapine to placebo, and the others compared loxapine to haloperidol, aripiprazole, or droperidol. Regarding the routes of administration, 4 trials25–27,29 studied the inhaled route, 223,24 the intramuscular route, and 128 the enteral route via a nasogastric tube. The frequency of administration varied from 1 to 3 times per day, and 1 study23 reported the mean daily amount of loxapine administered, which was 130 mg. The follow-up durations ranged from 24 hours (in 4 of the studies) to 14 days, and in 6 studies benzodiazepine was planned as a rescue medication.
The agitation scales used in the included studies22–28 were the Positive and Negative Syndrome Scale–Excited Component (PANSS-EC), the Clinical Global Impressions (CGI), the Brief Psychiatric Rating Scale, the Behavioral Activity Rating Scale, the Agitation-Calmness Evaluation Scale, the Richmond Agitation Sedation Scale, an analog agitation/aggression scale, and an analog scale assessing the state of agitation and rated from 1 to 4. The CGI was the most commonly used scale (5 of the included articles23,25–27,29), and the mean agitation score at baseline as measured by the CGI scale ranged from 3.60 to 4.42 (see Table 1).
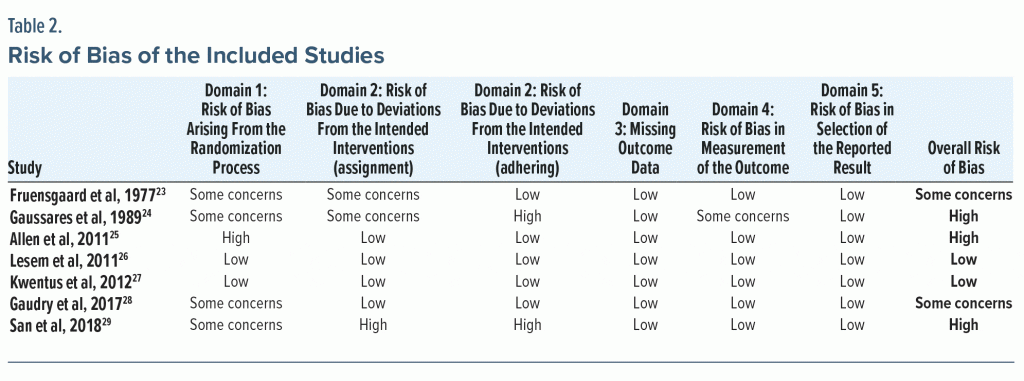
Risk of Bias
Our risk of bias appraisal revealed a good quality for 2 studies,26,27 with a low risk of overall bias (Table 2). The analysis classified 2 articles as “some concern” and 3 articles24,25,29 as high risk of overall bias. Although all studies were described as randomized, many did not provide details regarding the randomization method used. In addition, for 2 articles28,29 there were some significant differences between the intervention groups at baseline, suggesting an issue during the randomization process. Two studies24,29 were conducted in an open-label design. The risk of bias due to deviations from the intended interventions (effects of assignment and adherence to the intervention) was consequently high.
Results of Syntheses
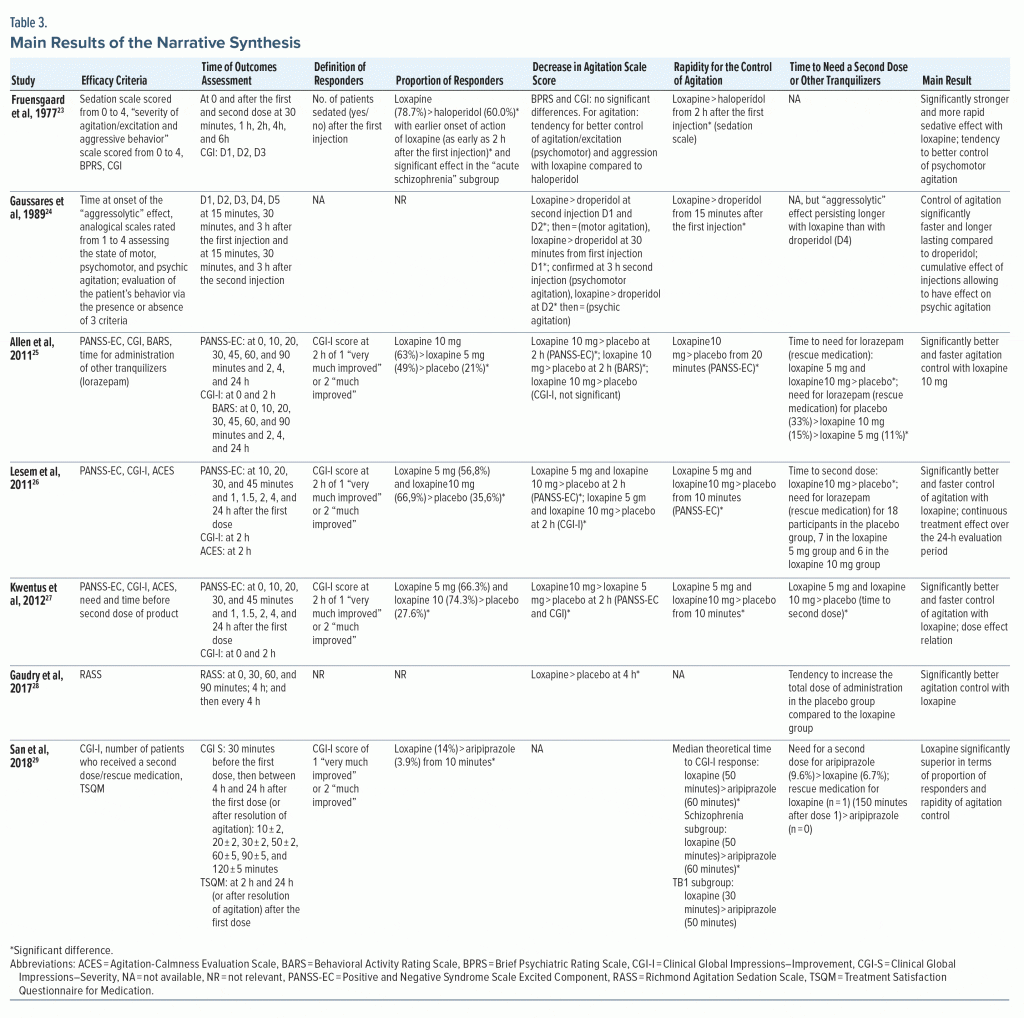
Five studies23,25–27,29 assessed the proportion of treatment responders, and for 4 of them, the definition of a responder was a 2-hour CGI-I score of 1 (“very much improved”) or 2 (“much improved”) (Table 3). The fifth23 defined a responder as a sedated patient (yes/no) after the first injection. It should be noted that for the oldest article,23 the efficacy outcome was sedation and not reduction in agitation. All the studies that assessed treatment responders showed a significantly higher proportion in the loxapine group. This superiority of loxapine was significant as early as 2 hours versus haloperidol, and as early as 10 minutes versus aripiprazole. Two studies23,29 performed subgroup analyses, and the effect of loxapine was significantly greater in patients with a diagnosis of schizophrenia, but no significant difference was found in bipolar disorder patients.
Loxapine significantly decreased the agitation scale score in 5 of the 6 articles23–28 that appraised this outcome, and while focusing on the time needed to reach efficacy, loxapine was significantly faster (see Table 3). The times at which the agitation scale score was significantly lower in the loxapine group were 10 minutes (for inhaled loxapine compared with placebo), 15 minutes, 20 minutes, and 2 hours (for loxapine compared with haloperidol). The delay between the first administration and a second dose or rescue medication (lorazepam) was significantly longer in the inhaled loxapine group compared with placebo. The 7 included articles hence concluded that loxapine is significantly more effective and faster in controlling acute agitation.
Two studies26,28 comparing loxapine to placebo reported no significant differences in the frequency of adverse events, and the other articles reported no statistical analyses on this outcome. The most commonly reported side effects were extrapyramidal syndromes, dizziness, and symptoms reflecting anticholinergic effects. In the articles that studied the inhaled route of administration,25–27,29 dysgeusia was also reported as a frequent side effect. Overall, the adverse events were considered by the authors to be minor or of moderate severity, with a resolution using dedicated treatment (benztropine for extrapyramidal syndromes). Of the 703 participants who received inhaled loxapine, 3 experienced bronchospasms, with 1 requiring intervention with albuterol. Loxapine does not appear to induce more important sedation levels compared to haloperidol or aripiprazole. Although the sedation rating scales were disparate, the included articles noted moderate sedation levels. Nevertheless, 2 participants experienced severe sedation after administration of inhaled loxapine 10 mg, and 1 participant died within 6 days after inhaled loxapine 10 mg. The cause of death was not reported and was considered by the investigators to be unrelated to treatment. No articles specified that QT interval measurements were performed, and no cardiac adverse events were reported.
The satisfaction of patients toward pharmacologic treatments was assessed in some studies, and we found that loxapine was significantly superior to droperidol (“feeling relaxed”) and to aripiprazole (“very” or “extremely satisfied”).
DISCUSSION
General Interpretation of the Results in the Context of Other Evidence
In this systematic review, we included 7 articles that were all randomized trials and encompassed a total of 1,276 participants. Our syntheses revealed that loxapine, while administrated for acute agitation and compared with other drugs, was associated with a greater proportion of responders, as well as a greater and faster decrease in agitation intensity. Also, loxapine was not associated with a greater frequency of adverse events and resulted in patient satisfaction with improvement.
In terms of efficacy and safety, our findings are respectively different and similar to that of Popovic and colleagues.30 Those authors performed a comprehensive and systematic literature review with the aim to examine the efficacy and tolerability of the various formulations of loxapine. They concluded that available data suggest that the antipsychotic efficacy of loxapine is similar to the efficacy of other typical or atypical antipsychotics, with an adverse effects profile comparable to that of the typical antipsychotics at high doses for chronic treatment.30 Nevertheless, their review differs from our study in that they focused their searches in PubMed/MEDLINE; targeted agitation and aggression in patients affected with schizophrenia, bipolar disorder, and other psychiatric conditions; and did not exclusively consider the “acute” feature of agitation. The findings pertaining to our narrative syntheses seem to confirm results of observational studies comparing loxapine to other antipsychotic drugs for acute agitation, especially in emergency departments.30,31 For instance, Ruch et al31 compared 61 agitated or aggressive patients that received inhaled loxapine to 29 that received non-parenteral treatment as usual and found a 6-fold faster and more robust symptom control with loxapine. McDowell and colleagues,32 while comparing 54 agitated patients that received inhaled loxapine to 225 and 127 patients managed with ziprasidone and haloperidol, respectively, found that inhaled loxapine may be a more effective and rapid treatment option.
The place of loxapine in other diagnoses such as personality disorders, intoxication, or somatic diseases has not been evaluated mainly because these conditions were exclusion criteria in included trials. However, a recently published naturalistic, unicentric, prospective study of 30 personality disorder subjects with acute agitation attending emergency departments suggested that inhaled loxapine could be a safe and effective therapeutic option.33 Regarding agitation in intoxicated patients, there are observational studies reporting the effective and quick management of acute agitation with inhaled loxapine, with the possibility of appropriate subsequent clinical evaluation.34 Articles on other psychiatric diagnoses, such as case reports on children with autistic disorders, suggest that loxapine is effective in treating agitation but need to be confirmed in clinical trials.35
Since its introduction in the 1970s, loxapine has gradually become part of the prescribing habits of practitioners in France or North American countries, especially for the management of acute agitation.14,15 This prevalence might be explained by its relative ease of use in emergency situations. Indeed, the existence of only 1 dosage of intramuscular injection ampoule, as well as 1 dosage of some commercialized inhaled form, and 2 dosages for oral loxapine, allows a fast action and limits the risk of administration errors. This systematic review allowed us to determine the place of this treatment in the current scientific literature and to highlight the lack of evidence.
Limitations
The data suggest that loxapine is an effective, rapid, and well-tolerated treatment for the control of acute agitation. Although this is the first systematic review investigating the effect of loxapine in acute agitation, the interpretation of our findings should take into consideration some limitations. First, we had high heterogeneity in terms of treatment arms and outcome assessments. Indeed, across studies, there is wide variability in the agitation rating scales used, which for instance did not allow us to perform a meta-analysis. It must be noted that the CGI, which was the most commonly used assessment tool in the included studies, is not specific to agitation as is the PANSS-EC for example.36,37 Some of the authors justified this choice by the greater ease of use, that it was for all types of practitioners (no need of specialized knowledge), and the short time needed for scoring, making this scale more applicable in clinical practice.29 Other limitations are the lack of studies comparing each route of administration with others (especially inhaled versus oral or intramuscular route), as well as the relatively low number of available articles. Indeed, the choice to select only interventional studies highlighted the current lack of high-level evidence. Only 2 articles were of good quality with a low overall risk of bias but still involved 658 participants, representing more than half of the total population. The risk of bias analysis highlighted higher risks in the area of randomization. The generalization of our findings is also hampered by the lack of data on populations such as adolescents and the elderly and the predominance of published studies in the United States and Europe. In addition, it should be noted that almost all of the studies in this review have links with pharmaceutical companies manufacturing loxapine. More specifically, there were 3 studies sponsored by Alexza Pharmaceuticals, 2 linked to Lederlè Laboratories through supply or affiliation of 1 of the authors, and 1 sponsored by Ferrer Internacional.
Implications of the Results for Practice, Policy, and Future Research
Despite the mentioned limitations, this review provides reassuring evidence regarding continuation of loxapine use in clinical practice for the management of acute agitation. The use of a common scale, instead of a visual analog, could contribute to standardized practices and avoid some escalations in treatment. Indeed, such a scale could guide decisions with progressively increasing strategies (“calming room,” oral treatment, injectable treatment, seclusion and restraint) and adjustments depending on the patient’s agitation score. In terms of future research, it could be interesting to perform interventional studies extended to other age groups or other profiles of underlying mental health disorders and with methodological optimization notably regarding randomization processes.
Article Information
Published Online: December 19, 2023. https://doi.org/10.4088/PCC.23r03552
© 2023 Physicians Postgraduate Press, Inc.
Submitted: May 1, 2023; accepted July 10, 2023.
To Cite: Lebel C, Endomba T, Chabridon C, et al. Efficacy and safety of loxapine in acute agitation: a systematic review of interventional studies. Prim Care Companion CNS Disord. 2023;25(6):23r03552.
Author Affiliations: Psychiatry Internship Program, University of Burgundy, Dijon, France (Lebel, Endomba); Service de Psychiatrie Adultes, Centre Hospitalier Universitaire, Dijon, France (Chabridon, Chauvet-Gélinier); INSERM, LNC-UMR 1231, University of Burgundy, Dijon, France (Chauvet-Gélinier).
Corresponding Author: Camille Lebel, MD, Psychiatry Internship Program, University of Burgundy, 21000 Dijon, France ([email protected]).
Relevant Financial Relationships: None.
Funding/Support: None.
Clinical Points
- Compared to other interventions (including placebo, haloperidol, aripiprazole, or droperidol), loxapine was well-tolerated and more effective regarding acute agitation control, and its action was faster than comparators.
- Loxapine use for acutely agitated patients, in combination with nonpharmacologic techniques and depending on the agitation severity including the risk of aggressive behaviors, may lower the probability of seclusion and restraint use.
- Since there is only one dosage of intramuscular injection as well as the inhaled form and considering the cardiovascular safety compared to other antipsychotics, loxapine is relatively easy to use in clinical practice. In case of insufficient efficacy, a benzodiazepine can be added.
References (37)

- Crocq MA, Guelfi JD. DSM-5: Manuel Diagnostique et Statistique des Troubles Mentaux. Fifth Edition. Issy-les-Moulineaux: Elsevier Masson; 2015.
- Hall RCW, Gardner ER, Stickney SK, et al. Physical illness manifesting as psychiatric disease, II: analysis of a state hospital inpatient population. Arch Gen Psychiatry. 1980;37(9):989–995. PubMed CrossRef
- Gregory RJ, Nihalani ND, Rodriguez E. Medical screening in the emergency department for psychiatric admissions: a procedural analysis. Gen Hosp Psychiatry. 2004;26(5):405–410. PubMed CrossRef
- Koran LM, Sheline Y, Imai K, et al. Medical disorders among patients admitted to a public-sector psychiatric inpatient unit. Psychiatr Serv. 2002;53(12):1623–1625. PubMed CrossRef
- Nordstrom K, Zun LS, Wilson MP, et al. Medical evaluation and triage of the agitated patient: consensus statement of the american association for emergency psychiatry project Beta medical evaluation workgroup. West J Emerg Med. 2012;13(1):3–10. PubMed CrossRef
- Roppolo LP, Morris DW, Khan F, et al. Improving the management of acutely agitated patients in the emergency department through implementation of Project BETA (Best Practices in the Evaluation and Treatment of Agitation). J Am Coll Emerg Physicians Open. 2020;1(5):898–907. PubMed CrossRef
- Haute Autorité de Santé. Guide méthodologique : Mieux prévenir et prendre en charge les moments de violence dans l’évolution clinique des patients adultes lors des hospitalisations en service de psychiatrie [Internet]. 2016. Accessed April 8, 2023. https://www.has-sante.fr/upload/docs/application/pdf/2016-10/guide_methodo_violence_hospi_psy.pdf
- Gerson R, Malas N, Feuer V, et al. Best practices for evaluation and treatment of agitated children and adolescents (BETA) in the emergency department: consensus statement of the american association for emergency psychiatry. West J Emerg Med. 2019;20(2):409–418. PubMed CrossRef
- Légifrance. Article 84 - LOI n° 2020-1576 du 14 décembre 2020 de financement de la sécurité sociale pour [Internet]. Accessed April 8, 2023. https://www.legifrance.gouv.fr/jorf/article_jo/JORFARTI000042665379#:~:text=%2DLa%20mesure%20d%27isolement%20est,totale%20de%20quarante%2Dhuit%20heures
- Légifrance. Article L3222-5-1 - Code de la santé publique [Internet]. Accessed April 8, 2023. https://www.legifrance.gouv.fr/codes/article_lc/LEGIARTI000031918948
- SFMU. (Société Française de Médecine d’Urgence). Conférence de Consensus : “ L’agitation en urgence (petit enfant excepté) ” [Internet]. 2003. Accessed April 8, 2023. https://www.sfmu.org/upload/consensus/cc_agitation-court.pdf
- SFMU. (Société Française de Médecine d’Urgence). Recommandations de Bonne Pratique Clinique. Prise en charge du patient adulte à présentation psychiatrique dans les structures d’urgences [Internet]. 2021. https://www.sfmu.org/upload/consensus/rbpc-psychiatrie_sfmu2021.pdf
- Bourdinaud V, Pochard F. Survey of management methods for patients in a state of agitation at admission and emergency departments in France. Encephale. 2003;29(2):89–98. PubMed
- Chakrabarti A, Bagnall A, Chue P, et al. Loxapine for schizophrenia. Cochrane Database Syst Rev. 2007;2007(4):CD001943. PubMed
- Drug Approval Package. Adasuve (loxapine) NDA #022549 [Internet]. Accessed April 8, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/022549_adasuve_toc.cfm
- de Berardis D, Fornaro M, Orsolini L, et al. The role of inhaled loxapine in the treatment of acute agitation in patients with psychiatric disorders: a clinical review. Int J Mol Sci. 2017;18(2):349. PubMed CrossRef
- Buchanan RW, Kreyenbuhl J, Kelly DL, et al; Schizophrenia Patient Outcomes Research Team (PORT). The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull. 2010;36(1):71–93. PubMed CrossRef
- Barbic D, Andolfatto G, Grunau B, et al. Rapid agitation control with ketamine in the emergency department: a blinded, randomized controlled trial. Ann Emerg Med. 2021;78(6):788–795. PubMed CrossRef
- Muir-Cochrane E, Oster C, Gerace A, et al. The effectiveness of chemical restraint in managing acute agitation and aggression: a systematic review of randomized controlled trials. Int J Ment Health Nurs. 2020;29(2):110–126. PubMed CrossRef
- deSouza IS, Thode HC Jr, Shrestha P, et al. Rapid tranquilization of the agitated patient in the emergency department: a systematic review and network meta-analysis. Am J Emerg Med. 2022;51:363–373. PubMed CrossRef
- Page MJ, McKenzie JE, Bossuyt PM, et al. Declaracion PRISMA 2020: Una Guia Actualizada Para La Publicacion De Revisiones Sistematicas. (The PRISMA 2020 statement: an updated guideline for reporting systematic reviews). Rev Esp Cardiol (Engl Ed). 2021;74(9):790–799.
- Risk of Bias 2 (RoB 2) tool | Cochrane Methods [Internet]. Accessed September 15, 2022. https://methods.cochrane.org/risk-bias-2
- Fruensgaard K, Korsgaard S, Jorgensen H, et al. Loxapine versus haloperidol parenterally in acute psychosis with agitation: a double-blind study. Acta Psychiatr Scand. 1977;56(4):256–264. PubMed CrossRef
- Gaussares C, Gerard H, Bosc M. Interest in injectable loxapine in severe agitation states. (French) Inf Psychiatr. 1989;65(6):656–660.
- Allen MH, Feifel D, Lesem MD, et al. Efficacy and safety of loxapine for inhalation in the treatment of agitation in patients with schizophrenia: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2011;72(10):1313–1321. PubMed CrossRef
- Lesem MD, Tran-Johnson TK, Riesenberg RA, et al. Rapid acute treatment of agitation in individuals with schizophrenia: multicentre, randomised, placebo-controlled study of inhaled loxapine. Br J Psychiatry. 2011;198(1):51–58. PubMed CrossRef
- Kwentus J, Riesenberg RA, Marandi M, et al. Rapid acute treatment of agitation in patients with bipolar I disorder: a multicenter, randomized, placebo-controlled clinical trial with inhaled loxapine. Bipolar Disord. 2012;14(1):31–40. PubMed CrossRef
- Gaudry S, Sztrymf B, Sonneville R, et al. Loxapine to control agitation during weaning from mechanical ventilation. Crit Care. 2017;21(1):235. PubMed CrossRef
- San L, Estrada G, Oudovenko N, et al. PLACID study: a randomized trial comparing the efficacy and safety of inhaled loxapine versus intramuscular aripiprazole in acutely agitated patients with schizophrenia or bipolar disorder. Eur Neuropsychopharmacol. 2018;28(6):710–718. PubMed CrossRef
- Popovic D, Nuss P, Vieta E. Revisiting loxapine: a systematic review. Ann Gen Psychiatry. 2015;14(1):15. PubMed CrossRef
- Ruch T, Nuss S, Yeruva RR, et al. Inhaled loxapine for acute agitation in a psychiatric emergency service. Ann Clin Psychiatry. 2021;33(3):162–167. PubMed CrossRef
- McDowell M, Nitti K, Kulstad E, et al. Clinical outcomes in patients taking inhaled loxapine, haloperidol, or ziprasidone in the emergency department. Clin Neuropharmacol. 2019;42(2):23–26. PubMed CrossRef
- Ferrer M, Soto-Angona Ó, Soler-Artigas M, Ibáñez P, Fadeuilhe C, Palma-Álvarez RF, et al. Inhaled loxapine as a rapid treatment for agitation in patients with personality disorder: a prospective study on the effects of time. 2022;20(3):482–490.
- Roncero C, Ros-Cucurull E, Palma-Álvarez RF, et al. Inhaled loxapine for agitation in intoxicated patients: A case series. Clin Neuropharmacol. 2017;40(6):281–285. PubMed CrossRef
- Reinblatt SP, Abanilla PK, Jummani R, et al. Loxapine treatment in an autistic child with aggressive behavior: therapeutic challenges. J Child Adolesc Psychopharmacol. 2006;16(5):639–643. PubMed CrossRef
- Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. PubMed CrossRef
- Montoya A, Valladares A, Lizán L, et al. Validation of the excited component of the Positive and Negative Syndrome Scale (PANSS-EC) in a naturalistic sample of 278 patients with acute psychosis and agitation in a psychiatric emergency room. Health Qual Life Outcomes. 2011;9(1):18. PubMed CrossRef
Enjoy this premium PDF as part of your membership benefits!