LESSONS LEARNED AT THE INTERFACE OF MEDICINE AND PSYCHIATRY
The Psychiatric Consultation Service at Massachusetts General Hospital sees medical and surgical inpatients with comorbid psychiatric symptoms and conditions. During their twice-weekly rounds, Dr Stern and other members of the Consultation Service discuss diagnosis and management of hospitalized patients with complex medical or surgical problems who also demonstrate psychiatric symptoms or conditions. These discussions have given rise to rounds reports that will prove useful for clinicians practicing at the interface of medicine and psychiatry.
Prim Care Companion CNS Disord. 2023;25(4):22f03438
Author affiliations are listed at the end of this article.
Have you ever wondered which of your patients meets the criteria for treatment-resistant depression (TRD)? Have you been uncertain about the best treatment options for your patients with persistent depression? Have you struggled to decide when to refer your patients for specialty consultation or collaborative care? If you have, the following case vignette and discussion should prove useful.
CASE VIGNETTE
Ms A, a 79-year-old woman, was seen for management of depression. Her symptoms included low mood, crying spells, anhedonia, difficulty with the initiation and maintenance of sleep, low energy levels, reduced appetite, decreased attention and concentration, and hopelessness (with no active or passive suicidal ideation) in the context of multiple familial stressors. She lacked a history of mania, psychosis, or substance use issues. Her review of systems was notable for significant anxiety surrounding ongoing stressors. She had experienced multiple failed trials of selective serotonin reuptake inhibitors (SSRIs), including fluoxetine, escitalopram, and sertraline, due to medication-induced side effects (including upper extremity tremors), and eventually she could not tolerate these medication trials. She acknowledged having had 3 episodes of major depressive disorder (MDD) with no history of self-harm or psychiatric hospitalizations.
Her family history was remarkable for the completed suicide of her grandfather, while her daughter had MDD. Her medical history was significant for hypertension and type II diabetes mellitus.
DISCUSSION
MDD is a prevalent and complex psychiatric disorder and a growing public health issue,1 with an estimated lifetime risk of major depressive episodes approaching 30% in the US.2 MDD has been associated with substantial morbidity, health care costs, and mortality3 and is one of the leading causes of disability worldwide. In the US, primary care providers play a key role in recognizing and managing depression; moreover, roughly 60% of cases involving MDD are managed in primary care settings,4 and more than three-fourths of antidepressant prescriptions for MDD are written by nonpsychiatrists.5 Regardless of the clinical setting in which patients with depressive disorders are treated, failure to respond or partial response to an antidepressant therapy of an adequate dose and duration is a common challenge for clinicians who treat depression.6 However, due to the scarcity of mental health providers available to patients in primary care settings, the management of depressed patients with poor outcomes, despite antidepressant treatments, remains problematic.
What Is TRD?
Approximately 30%–60% of patients with MDD who are receiving antidepressant treatments have a less-than-complete clinical response. However, not all these patients should be labeled as having TRD, since many of them have pseudoresistance due to a suboptimal treatment approach.7 Pseudoresistance is a multifactorial phenomenon that can be attributed to both clinician-related factors (such as inappropriate prescribing behavior, misdiagnosis, or incomplete diagnosis) and patient-related factors (such as poor medication adherence). Pseudoresistance can be addressed using comprehensive intervention strategies that target clinicians (eg, enhancing knowledge of clinical guidelines, providing simplified dosage schedules, facilitating provision of personalized psychoeducation, invigorating social support, and improving care management).7
Unfortunately, a universally accepted definition of TRD8 is lacking, and TRD has not been identified as a discrete biological entity. Nevertheless, TRD is often defined as a failure to respond to trials of 2 mechanistically different antidepressant medications after adequate dosage, duration, and compliance.9 Most definitions of adequate treatment length for antidepressant medications are derived from the conventions of industry-sponsored trials that were designed to establish statistically significant differences between drugs and placebo for the treatment of MDD.10 Moreover, varying definitions of treatment resistance have been used in systematic TRD research.11 As a result, to advance TRD research it is crucial to develop a consensus definition of TRD that specifies the number of treatment failures and the adequacy of dose and duration while identifying a core package of standardized outcome measures.11 Additionally, the goal of treatment, whether a response, remission, or resumption of function, needs to be a component of defining TRD.
How Common Is TRD?
Based on the findings of the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial, approximately one-third of patients with MDD achieve remission with the first antidepressant trial. The likelihood of remission diminishes with each successive monotherapy,3 such that approximately one-third of patients with an episode of MDD are characterized as having TRD. A recent study12 of claims data for adults covered by Medicare, Medicaid, commercial plans, and the US Veterans Health Administration estimated that the 12-month prevalence of TRD was 30.9%. The prevalence of TRD, to our knowledge, has not been studied systematically in primary care settings across the US. However, Rizvi and colleagues13 reported that the prevalence of TRD, characterized by failure to respond to at least 2 antidepressants, was 21.7% among patients with MDD (n = 1,212), underscoring the substantial burden of TRD in primary care settings across Canada.13
Risk factors for TRD include an early age at onset of depression, a higher number of lifetime depressive episodes, a longer duration of depressive episodes, the presence of psychotic symptoms, and a history of psychiatric hospitalization.14 Nonresponse to the first antidepressant received in the patient’s lifetime increased the risk of resistance by 3.3-fold during the last episode. The presence of comorbid anxiety disorders (especially panic disorder and social phobia) has been associated with a 4.2-fold increased risk of treatment resistance. An increased risk of suicide (defined by the presence of at least 1 of the following items: having thoughts in the past month that it would be better being dead, the wish to die or to harm oneself, having thoughts about suicide, having a suicide plan, making an attempt at suicide, and having attempted suicide at least once in their lifetime) has been associated with a 2.6-fold increased risk of treatment resistance.14 Additionally, psychiatric comorbidities (such as anxiety disorders, posttraumatic traumatic stress disorder [PTSD], personality disorders, and substance use disorders) and a range of comorbid medical conditions (including pain syndromes, endocrinopathies, cardiovascular disease, neurologic diseases, and vitamin deficiencies), along with sleep disorders (including obstructive sleep apnea, insomnia disorder, restless leg syndrome) may also be associated with TRD. It is important to note that reassessment of diagnosis to rule out bipolar spectrum disorders and concurrent treatment of psychiatric, medical, and sleep comorbidity are crucial to successful outcomes for TRD.
Fortunately, advances have been made in identifying underlying neurobiological risk factors for TRD. These risk factors include polymorphisms in receptor and transporter genes (including serotonin transporter SLC6A4, presynaptic serotonin autoreceptor 5-HTR1A, catechol-O-methyltransferase, brain-derived neurotrophic factor, and the transcription factor CREB1).14 Decreased γ-aminobutyric acid levels in both the occipital cortex and the anterior cingulate cortex have been reported in patients with TRD (identified by an insufficient response to at least 3 adequate antidepressant trials in the current episode) as compared to healthy volunteers and patients with MDD but with no history of treatment resistance.15 From a neuroimaging perspective, TRD has been thought to be a distinct subtype of MDD due to alterations in the default mode network that appears to differentiate it from treatment-responsive MDD.16
What Are the Characteristics and Complications of TRD?
Characteristics of TRD, as described by Mrazek and colleagues,17 include having 3.8 ± 2.1 prior depressive episodes with an illness duration of 4.4 ± 3.3 years and completion of 4.7 ± 2.7 unsuccessful medication trials involving 2.1 ± 0.3 drug classes. Approximately 17% ± 6% of patients with TRD have made suicide attempts (1.1 ± 0.2 attempts per patient), and treatment response rates have been reported as 36% ± 1% for this population. Additionally, patients with TRD report having a significantly lower quality of life compared to those who experienced remission or a response in terms of depression.17 The burden of TRD increases as the duration of depressive episodes increases, and it is associated with a higher risk of impaired personal and social functioning, diminished quality of life, and increased risk of somatic morbidity and suicidality.18
In terms of complications, evidence suggests that TRD represents the highest direct and indirect medical costs among patients with MDD.19 Patients with TRD are twice as likely to be hospitalized, and the cost of their hospitalization is more than 6 times that of the mean total cost for those without TRD.20 Significantly higher health care utilization (in terms of hospitalization, emergency department [ED] visits, and outpatient visits) as well as higher annual costs of care have been reported in those with TRD compared to those with treatment-responsive depression.21,22 According to a systematic review that examined the burden of TRD on quality of life and cost of care, medical costs increased, while the patient’s health-related quality of life and health status decreased as TRD became more severe.23 At a prevalence rate of 12%–20% among all depressed patients, TRD may lead to an annual added societal cost of $29–$48 billion, thus escalating the total societal costs of MDD by as much as $106–$118 billion.17
What Are Obstacles to TRD Management?
For patients with TRD, numerous obstacles to treatment are apparent, including provider, patient, and systemic factors. Across health care, depression is detected at a low rate,24 which leads to a high percentage of untreated patients. Even when TRD is identified, many affected patients fail to receive treatment according to best-practice guidelines.25 Moreover, a retrospective study of patients with TRD showed that patients experienced significant delays in initiating treatment.24 Patients also received less psychotherapy than was recommended and did not receive a first-line agent of drug therapy or an adequate course of pharmacotherapy. Further, a large portion of patients with TRD are treated in primary care settings (where there may be more education gaps among providers), rather than in the care of mental health specialists.24 For instance, in a study13 in primary care practices, only 25% of clinicians used an empirically validated scale to evaluate depression symptom severity, and the decision to change medications was informed by patient self-reports, rather than by a more empirically informed approach. Overall, a variety of provider factors pose obstacles to the “gold-standard” treatment of TRD, including the adequate identification and treatment of TRD.
Patient and systemic hurdles also make receiving care more challenging. Patient nonadherence to treatment is an obstacle to receiving adequate treatment.26 Rizvi and colleagues13 found that patients with TRD tended to report more side effects than did their counterparts, potentially leading to premature medication discontinuation. Reasons for poor treatment adherence also include higher levels of pessimism about treatment efficacy (given past treatment failures) and challenges with social support, marital problems, and personality traits.26 Treatment nonadherence occurs for a variety of reasons, including the patient’s lack of understanding of the disease, patient attitudes toward pharmacologic treatment, lack of discussion and agreement regarding chosen treatment, medication side effects, and challenges with social and economic support.
Relatedly, several systemic factors impede access to care for those with TRD. Disabilities, medical-related absentee days, and work-loss days are higher in those with TRD, suggesting that there is a significant economic burden for these individuals.13,27 Another study,28 which asked primary care providers about their perceptions of barriers to quality depression care, suggested that patients often had difficulties with following a treatment plan, likely due to psychosocial obstacles. Given that individuals with TRD are likely to have more economic and social challenges than their counterparts, larger systemic structures should be considered in their contributions to TRD treatment obstacles.29
How Can TRD Be Treated?
Fortunately, TRD can be successfully managed with a variety of treatments. These treatments include talking therapies (eg, psychotherapy), psychopharmacologic agents (eg, SSRIs, serotonin-norepinephrine reuptake inhibitors, atypical antipsychotics, lithium, ketamine), augmentation with triiodothyronine, and neuromodulation interventions (eg, electroconvulsive therapy [ECT], transcranial magnetic stimulation [TMS], vagal nerve stimulation [VNS], and deep brain stimulation [DBS]).
Psychotherapy. Cognitive-behavioral therapy (CBT) focuses on the relationship between maladaptive thoughts, behaviors, and feelings. In TRD, maladaptive thoughts may take the shape of “I am worthless” or “I will never get better.” These thoughts may then catalyze behavioral changes (such as social and/or professional withdrawal, creating a self-reinforcing cycle) that increase depression.
Evidence for the effectiveness of CBT for refractory depression is solid, particularly when it is used as an augmentation to pharmacotherapy in a stepped-care approach.30,31 A meta-analysis32 of randomized trials demonstrated that pharmacotherapy augmented by CBT reduced depressive symptoms and had higher response and remission rates compared to individuals who did not receive CBT augmentation. Since these results were held at 6- and 12-month follow-ups, CBT augmentation seems to be an effective augmentation choice for patients with TRD.
The efficacies of other psychotherapies have also been studied. For depression more broadly, interpersonal psychotherapy (IPT) has a sizeable literature base. IPT focuses on increasing social functioning and solving problems in patients’ interpersonal relationships. Evidence suggests that IPT is efficacious in treating depression both as a monotherapy and when augmenting pharmacotherapy.33 A randomized controlled trial of TRD suggested that no additional clinical benefit occurred when IPT was added to pharmacotherapy,34 while another study35 found that cognitive therapy augmentation was equally efficacious to further pharmacotherapy augmentation, except that it had a slower onset of action but greater tolerance. Finally, brief supportive psychotherapy and a cognitive-behavioral analysis system of psychotherapy added no additional benefit when compared to pharmacotherapy alone for patients with TRD.36 Overall, although many psychotherapies can effectively treat depression, CBT, and potentially cognitive therapy, has the strongest support for treating TRD. However, more research on the efficacy of psychotherapies for TRD is required, as the number of high-quality trials on psychotherapy for TRD is limited.
Physical activity has been consistently shown to be associated with improved physical health, life satisfaction, cognitive functioning, and psychological well-being. Evidence suggests that exercise compares favorably to antidepressant medications as a first-line treatment for mild to moderate depression, and it has been shown to improve depressive symptoms when used as an adjunct to medications.37 The efficacy of exercise in the management of TRD remains to be established; however, it can be considered as one of the strategies in addition to behavioral activation techniques in primary care settings for the management of patients with difficult-to-treat depression.
How Can Primary Care Providers Optimize Depression Treatment?
When a patient tries a new antidepressant and obtains only a partial benefit after a reasonable period (typically 6 or 8 weeks), the clinician and the patient arrive at a crossroads and must decide what to do next to optimize treatment benefit.
The usual next-step strategies are as follows:
(1) Increase the dose of the antidepressant to a level that is still safe and tolerable, in the hopes that this will maximize the therapeutic effect of the original drug.
(2) Add a second antidepressant to the original one, optimize its dose in the same way as suggested in option 1, and hope that the combination will have a synergistic effect. Typically, this involves adding an antidepressant that works by a different mechanism than the original one. For example, a patient who has not benefited after optimizing an SSRI might benefit from the addition of bupropion, which has more dopaminergic activity.38
(3) Augment with an agent that may not be an antidepressant per se but has been shown to provide antidepressant effects in combination with standard antidepressants. For example, atypical antipsychotics, different forms of folic acid (such as 5-methyl tetrahydrofolate), thyroid hormone (T3), and lithium have been shown to have beneficial effects as an augmentation therapy.39,40
(4) Rather than adding other agents, the clinician might decide to switch the patient to a new antidepressant altogether. In this case, a decision needs to be made about whether to use an antidepressant with the same mechanism of action as the original agent—for example, switching from fluoxetine to sertraline—or try one with a different mechanism.
These strategies can be implemented in the primary care setting. However, some primary care providers may be reluctant to manage depression on their own, at least beyond a first-line strategy.41 If the patient has already tried a few standard antidepressants with no success, a consultation with a psychopharmacologist may be needed. In such cases, the primary care provider can obtain recommendations about the next steps or ask the psychiatrist to manage the patient’s psychopharmacologic regimen going forward.
The literature has provided different treatment guidelines for managing resistant depression, but there are relatively few prospective studies seeking to determine the optimal approach. Deciding between the above 4 approaches may be difficult, as no clear strategy has been demonstrated to be significantly superior to another in meta-analyses.42–44
It is worth mentioning that in cases of resistant depression, tricyclic antidepressants (TCAs)45 and monoamine oxidase inhibitors (MAOIs)46 may prove successful where other agents have failed. However, their bothersome side effects, risk of interactions, and (for MAOIs) requirement of a special diet and avoidance of many other drugs make them less desirable and may be best prescribed by psychiatrists who are comfortable with their use.
Given the limitations of the current literature, it may be best for clinicians to use their own judgment and take patient preference into account when deciding how to proceed in cases of TRD. For example, some patients prefer to keep their medication regimen to a minimum and may therefore be reluctant about combination therapies. With such a patient, optimization or switching might be approached earlier in treatment as opposed to combination or augmentation strategies. Optimization of the dose of the original antidepressant is the most conservative step and often the most acceptable to patients, as it does not involve adding or changing antidepressants. With many of the antidepressants commonly used in clinical practice, particularly SSRIs, very high doses are often achievable and prescribed, though the advantages are unclear in the literature.47–49 In our practice, we have used doses of fluoxetine ≥ 100 mg or sertraline ≥ 500 mg in some cases of refractory depression, as well as in obsessive-compulsive disorder (OCD), which may require higher antidepressant doses to control symptoms effectively, though again, the literature is unclear on the benefits of higher doses.50
If dose optimization does not provide the desired benefits, and/or produces bothersome side effects, the conservative patient may favor a switch to keep the number of medications to a minimum. This strategy, which reduces the chances of interactions and side effects, nonetheless carries a risk that whatever benefit was obtained from the original antidepressant could be lost during the transition and result in significant depressive worsening, particularly if the new antidepressant proves ineffective or causes troublesome side effects. If the depression is more moderate to severe, adding a second agent may be safer, as it avoids sacrificing the modest benefit of the original antidepressant while the new agent is given a chance to work. If the patient remains reluctant to take too many medications, they might be advised to take both medications just until the second one is optimized, and if a benefit is obtained, then attempt to taper the original antidepressant with a goal of eventual discontinuation in the hope that the new antidepressant is effective enough to sustain the response or remission. In such a case, the patient would end up on one single antidepressant, which is always the most desirable scenario.
When exploring combination and augmentation strategies, the pros and cons of adding a second antidepressant versus other agents, such as atypical antipsychotics, should be considered. Drugs that are used for augmentation, such as atypical antipsychotics and lithium, may be more prone to causing bothersome side effects.40,51 These side effects may include weight gain with both, potential imbalances of blood glucose with the atypical antipsychotics, and various other known side effects of lithium (such as thirst, hair loss, and increased urination). Lithium will also require periodic blood monitoring to ensure adequate drug levels, kidney and thyroid function, and a normal blood count.52 Adding US Food and Drug Administration (FDA)–approved antidepressants may therefore be simpler from a monitoring standpoint as well as from a safety and tolerability standpoint. In all cases, there should be a careful discussion between the doctor and the patient about the rationale for adding a particular augmenting agent and the relevant risks and benefits.
Regardless of whether the treater is a primary care provider or a psychiatrist, if a response or remission remains elusive after many cycles of treatment, it may become clear that antidepressants alone may not be enough to fully control the patient’s depression. In those cases, other strategies should be considered. For example, if the patient is not receiving psychotherapy, a discussion might be had about the pros and cons of different types of psychotherapy, which are generally supported by the literature as an effective approach to depression, sometimes on their own and sometimes in combination with antidepressants.53
When working with a patient with TRD, clinicians will often have to manage the patient’s feelings of disappointment and disillusionment when a new treatment is tried and fails to work. For many, seeking relief from depression may prove a Sisyphean task, with a cycle of elicited hopes, new treatments tried, and then disappointment due to lack of benefit. It is therefore important to emphasize to patients that the treatment of depression is largely a trial-and-error process. There are many treatments available, including dozens of antidepressants as well as the other therapies reviewed here. Except for ECT, no one treatment has proved to be significantly superior to others. Consequently, the choice must be made based on known efficacy, tolerability and safety, and personal concerns that the patient may have. For example, patients might be willing to tolerate certain side effects but not others, and a careful discussion about them may help the patient and clinician to arrive at a reasonable choice.
While the goal of depression treatment remains remission, some refractory patients may benefit from more realistic expectations about what they can expect. Research has shown that individuals with more resistant depression are less likely to obtain benefits from any treatment, particularly if they have already failed to respond to many antidepressants from different categories.3 In those cases, amelioration of symptoms and maximization of day-to-day function rather than full remission may be more realistic goals that, if attained, can have a significant positive impact on the patient’s life.
What Is the Role of Somatic Treatments in TRD?
ECT. This treatment involves the administration of an electric current through scalp electrodes, which results in a generalized seizure typically lasting 20–60 seconds.54 ECT has been in use since 1938, preceding the first effective pharmacotherapy for depression. While the mechanism of ECT remains unknown, it is believed to act both on neurohormones and on underlying brain structures, with studies indicating increased gray-matter volume in frontolimbic areas of treated individuals.55 ECT is delivered under general anesthesia, and typical practice in the US is for treatments 3 times per week. Multiple different ECT techniques can be utilized, including unilateral or bilateral stimulation and various electrical pulse widths, which may affect the efficacy and tolerability of response. The precise duration of treatment is individualized for patients, but it typically consists of 6–20 treatments. Efficacy for ECT is high, with 60%–80% of patients showing clinical response and 50%–60% remitting from depression.54 ECT is effective for patients across the age span, from adolescence through geriatrics.56
As of 2018, FDA devices are classified as Class II (moderate risk) for the treatment of unipolar and bipolar depression in patients aged ≥ 13 years whose disorder is “treatment resistant or who require a rapid response due to the severity of their psychiatric or medical condition.” Correspondingly, ECT is indicated in patients with TRD that is refractory to multiple medication trials, or alternatively, those who are so ill (due to catatonia, suicidality, or physical effects of depression, such as immobility or weight loss) that rapid response is required. While there are no absolute contraindications to ECT, patients with unstable cardiac disease, space-occupying brain lesions, recent stroke, and severe pulmonary disease are at increased risk of medical complications from ECT.57
ECT is associated with cognitive adverse effects, particularly subjective memory loss, which was reported by 29%–55% of patients following ECT in one systematic review.58 Reassuringly, objective neuropsychiatric testing does not show evidence of worsened cognitive functioning more than a few days following ECT,59 and a large-scale registry study60 did not link ECT to the development of dementia. Serious physical complications from ECT are rare, with an estimated mortality rate of 2 per 100,000 treatments, which is comparable to the risks of anesthesia alone.61
Repetitive TMS (rTMS). This treatment involves the application of a rapidly alternating magnetic field (comparable in strength to that of a typical magnetic resonance imaging [MRI] scanner) to induce an electrical stimulation in a focal region of the brain.62 While the mechanism of action of rTMS is unclear, it is hypothesized to modulate individual brain circuits associated with depression.63 rTMS is generally directed toward the left dorsal lateral prefrontal cortex and is administered as a series of daily treatments over 4 to 6 weeks, with each treatment lasting between 3 and 40 minutes depending on the type of rTMS stimulus. Treatments are given while awake, with no need for anesthesia. Numerous types of rTMS exist, which differ in the type of magnetic field induced, the frequency of stimulation, and the anatomic target. Efficacy for rTMS in the treatment of MDD has been demonstrated in multiple clinical trials, generally compared to sham rTMS, with an odds ratio for response with active treatment generally > 3.0 relative to sham rTMS.64 In a network meta-analysis, rTMS was associated with higher response rates than sham treatment, but lower response rates than ECT.65
The first rTMS device was FDA classified as Class II (moderate risk) for the treatment of MDD in 2008. TMS is indicated in the treatment of MDD that has not responded to at least 1 antidepressant trial. rTMS is contraindicated in patients at high risk for seizures and in those with implanted cranial metal hardware (including cochlear implants) or implanted electrical devices (including cardiac defibrillators or pacemakers).62
rTMS is well tolerated with few significant side effects. Seizures may be induced by rTMS, with reported rates of ~1 per 1,000 patients.62,66 Other side effects include headache, scalp pain at the administration site, and vasovagal syncope. The machine also produces a loud clicking noise during treatment, so hearing protection is generally worn during treatments to prevent hearing loss.
Ketamine. Originally developed as an anesthetic agent, ketamine has been used for decades for the induction of general anesthesia. It consists of 2 enantiomers, R-ketamine and S-ketamine, and can be administered as the racemic mixture of both enantiomers or as pure S-ketamine (esketamine). Pharmacologically, ketamine binds to multiple receptors in the brain, including opioid receptors and N-methyl-d-aspartate receptors.67 Ketamine can be administered in multiple ways (including intravenously, intramuscularly, orally, or intranasally). Esketamine is self-administered intranasally followed by 2 hours of observation in the office setting. Rigorous trials comparing these various routes of administration have not been conducted.68 Ketamine is associated with a large improvement in depressive symptoms and suicidal ideations over a matter of hours.69 While single-treatment efficacy wanes quickly following a single dose, repeated administration of ketamine has shown efficacy for several weeks following the final dose.70
Ketamine was FDA approved as an anesthetic in 1970, and the intranasal esketamine formulation was approved for the treatment of TRD in 2019, with the indication expanded to adults with depression and suicidal ideation or suicidal behavior in 2020. Ketamine is contraindicated in patients with aneurysmal vascular disease, arteriovenous malformation, or intracerebral hemorrhage. Ketamine should be used with caution in individuals with active substance use (ie, a substance use disorder) or a history of psychosis, although these are not absolute contraindications.
Ketamine treatment is generally well tolerated.71 Side effects include an increase in heart rate and blood pressure, dissociation, psychosis, dizziness, sedation, and nausea. Longer-term side effects observed in recreational ketamine users include addiction, neurotoxicity, and bladder toxicity, but these longer-term toxicities have not been clearly demonstrated in patients receiving therapeutic ketamine for depression.
What Are the Invasive Neuromodulation Treatments for TRD?
The use of invasive neuromodulation interventions, including VNS and DBS, is generally a “last-resort” step for highly treatment-resistant patients who have failed multiple trials of FDA-approved medications and somatic treatments for the management of depression.
VNS is an invasive neuromodulation treatment that involves the implantation of a pulse generator connected to bipolar electrodes that are placed around the left vagus nerve.72 The pulse generator is implanted under the skin of the left chest. Intermittent electrical currents are sent from the pulse generator to the left vagus nerve and via the nucleus tractus solitarius to various regions of the brain. VNS stimulation parameters include current (mA), frequency (Hz), pulse width (μs), and duty cycle (the duration that stimulation is on or off).72 Evidence from brain-imaging studies suggests that VNS is associated with metabolic changes in the prefrontal cortex and in limbic structures relevant to mood regulation, possibly through the modulation of monoaminergic neurotransmission.73 VNS was utilized initially for intractable epilepsy; however, when researchers noted improvements in mood in patients with epilepsy who were receiving VNS treatment, its uses expanded.74 VNS was approved by the FDA as an adjunctive treatment for severe (nonpsychotic), recurrent unipolar, and bipolar depression in 200572 based on the interpretation of the long-term efficacy data with VNS, despite the failed short-term sham-controlled trial.75 Eligibility criteria for VNS include adults aged ≥ 18 years with a history of depression treatment failure after at least 4 antidepressant interventions (patients were not required to have failed ECT to be eligible for VNS).72
DBS is a neurosurgical technique that aims to deliver continuous electrical stimulation of deep neural targets using electrodes that are connected subcutaneously to a pulse generator placed below the clavicle.76 DBS stimulation parameters include amplitude (volts/mA), pulse width (μs), and frequency (Hz). The amplitude is defined as the intensity of DBS stimulation, pulse width as the duration of each stimulus, and frequency as the number of pulses per second. Preclinical evidence suggests that the antidepressant effects of DBS may be related to stimulation parameters, neuroanatomical targets, and its modulatory effects on neurotransmitters in the target regions or interconnected brain networks.77 DBS is currently FDA approved for the treatment of medication-refractory Parkinson disease (PD), essential tremor, dystonia, and epilepsy. In terms of psychiatric indications, DBS has been approved for the treatment of OCD for humanitarian device exemption use only. In 2005, Mayberg and colleagues78 reported the first use of DBS that targeted the subgenual anterior cingulate cortex for the management of TRD. DBS has shown preliminary evidence for antidepressant effects primarily in open-label studies, and it remains an experimental treatment for the management of TRD.76
How Can TRD Be Managed in Primary Care Settings?
Nonresponse to antidepressant medications is common in patients seeking depression treatment in primary care settings. Unfortunately, little is known about how primary care providers manage patients with depression who tend not to respond to antidepressant medication trials. According to a UK study, 235 patients with TRD were randomized to continue with usual primary care and were followed up at 3-month intervals for 1 year.25 The authors25 reported that most patients were maintained on the same dose of a single antidepressant between their initial treatment and 3 months later during follow-up visits. Medication changes (such as increasing the dose, switching to a different antidepressant, or adding a second antidepressant) were uncommon. Participants described usual care as predominantly taking antidepressants, with consultations focused on other physical health concerns. Few of the participants accessed other treatments or were referred for specialty care.25
To our knowledge, there are no systematic estimates of the prevalence of TRD in US primary care settings. Nonetheless, it is quite plausible that TRD poses a major health concern in primary care settings across the US due to limited access to specialist care and to advanced treatments for depression. Collaborative care models provide a pragmatic and cost-efficient strategy to deliver integrated mental health and medical care for patients with mental health conditions who are treated in primary care settings.79 Collaborative care models are team-based interventions that enact system-level redesign by improving patient care, provider decision support, and clinical information systems, in addition to engaging patients in their care through self-management support and linkages to community resources.80
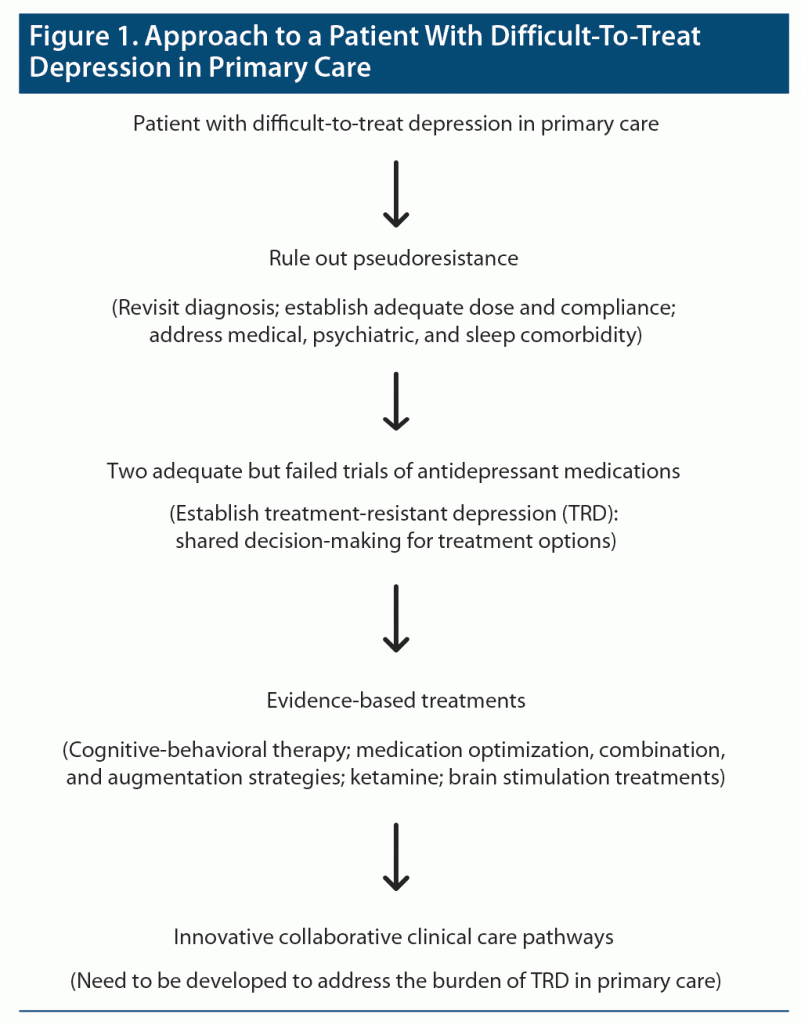
A multicomorbidity collaborative care approach using an intervention involving nurses who provided guideline-based, patient-centered management of depression and chronic diseases (diabetes and coronary artery disease) significantly improved control of medical diseases and depression in primary care settings.80 The impact of collaborative care models on addressing the management of TRD in primary care has not yet been systematically evaluated. We envision a care pathway that addresses the major impediments for patients with TRD in primary care (ie, with access to specialist care and advanced treatment options for TRD management). This care pathway aligns primary care specialists, collaborative care psychiatrists, and TRD specialist teams (psychiatrists and psychologists with expertise in complex mood disorders, social workers, and pharmacists) toward a common goal of identifying, treating, and preventing TRD in primary care settings.
Given the high prevalence of TRD, the risk for the development of TRD should be assessed at the onset of depression treatment in primary care. Novel strategies (such as the use of machine learning) can be implemented to generate personalized risk-prediction models of depression treatment outcomes that utilize demographic, clinical, genetic, and psychosocial factors associated with the development of TRD in primary care settings. Evidence suggests that machine learning could prove to be a valid approach to better classify and stratify TRD, thus helping clinicians in the assessment of MDD.81 After one antidepressant treatment failure, collaborative care teams should assist primary care providers to identify and mitigate risk factors that lead to pseudoresistance (eg, reassessment of diagnosis and comorbidity). Two or more antidepressant failures should prompt consultative assessment with TRD specialist teams to help develop a comprehensive individualized depression treatment plan based on the nature and severity of depressive symptoms, prognostic factors, and psychiatric or medical comorbidity.
The provision of evidence-based and personalized next-step interventions to address core depressive symptomatology and psychiatric comorbidity can improve TRD outcomes in this complex population. By virtue of expedited access to TRD specialist teams, patients can be referred for advanced FDA-approved interventions (such as ketamine, rTMS, ECT, and VNS) for the management of TRD. If depression symptoms remit or respond to suggested interventions, then it is vital to focus on relapse prevention of depressive symptoms in patients with TRD. In cases of nonresponse or relapse after suggested interventions, patients should be reevaluated with TRD specialist teams. Figure 1 summarizes the approach to patients with difficult-to-treat depression in primary care.
What Happened to Ms A?
After psychiatric assessment, it was apparent that Ms A had some initial response to fluoxetine; however, she could not tolerate that medication or another SSRI medication trial due to adverse side effects. Given these concerns and a history of significant sleep disturbances that interfered with her functioning, a trial of low-dose (7.5–15 mg) mirtazapine at bedtime was initiated, since its mechanism of action was different from that of her SSRI trials. She responded well and had no side effects. Her mood improved, and she had full remission of symptoms over the next 3 months. The case of Ms A highlights the phenomenon of pseudoresistance in the treatment of depression that was precipitated by mechanistically similar medications (SSRIs) and poor tolerability due to antidepressant-induced side effects.
CONCLUSIONS
TRD is prevalent and is a significant issue in primary care settings, especially in the context of scarce mental health resources and limited access to specialist care with advanced depression treatment interventions. Fortunately, TRD can be successfully managed with a variety of evidence-based treatments; these include talking therapies (eg, CBT), the use of psychopharmacologic agents (eg, antidepressants, atypical antipsychotics, lithium, triiodothyronine, ketamine), and neuromodulation interventions (eg, ECT, TMS, VNS, DBS). Collaborative care models provide a pragmatic and cost-efficient strategy to deliver integrated mental health and medical care for patients with mental health conditions who are treated in primary care settings. Innovative care pathways need to be developed by incorporating a stepped-care approach in collaborative care models with expedited access to TRD specialist teams for optimal management of TRD patients in primary care.
Article Information
Published Online: July 25, 2023. https://doi.org/10.4088/PCC.22f03438
© 2023 Physicians Postgraduate Press, Inc.
Prim Care Companion CNS Disord. 2023;25(4):22f03438
Submitted: October 24, 2022; accepted January 18, 2023.
To Cite: Chopra A, Luccarelli J, Cohen JN, et al. Evaluation, treatment, and referral of treatment-resistant depression in primary care. Prim Care Companion CNS Disord. 2023;25(4):22f03438.
Author Affiliations: Department of Psychiatry, Massachusetts General Hospital, Harvard Medical School, Boston (Chopra, Luccarelli, Cohen, Mischoulon, Stern).
Corresponding Author: Amit Chopra, MBBS, Massachusetts General Hospital, 55 Fruit St, Boston, MA 02114 ([email protected]).
Relevant Financial Relationships: Dr Luccarelli has received equity from Revival Therapeutics and research support from National Institute of Mental Health (T32MH112485). Dr Mischoulon has received research support from Nordic Naturals and Heckel Medizintechnik GmbH and honoraria for speaking from the Massachusetts General Hospital Psychiatry Academy and works with the MGH Clinical Trials Network and Institute (CTNI), which has received research funding from multiple pharmaceutical companies and the National Institute of Mental Health. Drs Chopra, Cohen, and Stern report no relevant financial relationships.
Funding/Support: None.
Clinical Points
- Approximately 30%–60% of patients with depression receiving antidepressant treatment have an incomplete clinical response, but not all should be labeled as having treatment-resistant depression (TRD), as many have pseudoresistance due to suboptimal treatment.
- Risk factors for TRD include early age at onset of depression, higher number of lifetime depressive episodes, longer duration of depressive episodes, presence of psychotic symptoms, and history of psychiatric hospitalization.
- TRD can be successfully managed with a variety of evidence-based treatments, including cognitive-behavioral therapy, psychopharmacologic agents, and neuromodulation interventions.
- Collaborative care models provide a pragmatic and cost-efficient strategy to deliver integrated mental health and medical care for patients with mental health conditions treated in primary care settings. Novel and innovative clinical care pathways that integrate TRD specialist care need to be implemented to address the burden of TRD in primary care.
References (81)

- Park LT, Zarate CA Jr. Depression in the primary care setting. N Engl J Med. 2019;380(6):559–568. PubMed CrossRef
- Kessler RC, Petukhova M, Sampson NA, et al. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21(3):169–184. PubMed CrossRef
- Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905–1917. PubMed CrossRef
- Frank RG, Huskamp HA, Pincus HA. Aligning incentives in the treatment of depression in primary care with evidence-based practice. Psychiatr Serv. 2003;54(5):682–687. PubMed CrossRef
- Mark TL, Levit KR, Buck JA. Datapoints: psychotropic drug prescriptions by medical specialty. Psychiatr Serv. 2009;60(9):1167. PubMed CrossRef
- Dodd S, Bauer M, Carvalho AF, et al. A clinical approach to treatment resistance in depressed patients: what to do when the usual treatments don’t work well enough? World J Biol Psychiatry. 2021;22(7):483–494. PubMed CrossRef
- Steegen G, Catthoor KCEER, Sabbe BGC, et al. Between response and resistance: pseudo-resistance during treatment of major depressive disorder. Tijdschr Psychiatr. 2021;63(3):189–196 (Tussen respons en resistentie: pseudoresistentie bij de behandeling van een depressieve stoornis). PubMed
- Trevino K, McClintock SM, McDonald Fischer N, et al. Defining treatment-resistant depression: a comprehensive review of the literature. Ann Clin Psychiatry. 2014;26(3):222–232. PubMed
- Souery D, Amsterdam J, de Montigny C, et al. Treatment resistant depression: methodological overview and operational criteria. Eur Neuropsychopharmacol. 1999;9(1-2):83–91. PubMed CrossRef
- Fava M. Diagnosis and definition of treatment-resistant depression. Biol Psychiatry. 2003;53(8):649–659. PubMed CrossRef
- Gaynes BN, Lux L, Gartlehner G, et al. Defining treatment-resistant depression. Depress Anxiety. 2020;37(2):134–145. PubMed CrossRef
- Zhdanava M, Pilon D, Ghelerter I, et al. The prevalence and national burden of treatment-resistant depression and major depressive disorder in the United States. J Clin Psychiatry. 2021;82(2):20m13699. PubMed CrossRef
- Rizvi SJ, Grima E, Tan M, et al. Treatment-resistant depression in primary care across Canada. Can J Psychiatry. 2014;59(7):349–357. PubMed CrossRef
- Bennabi D, Aouizerate B, El-Hage W, et al. Risk factors for treatment resistance in unipolar depression: a systematic review. J Affect Disord. 2015;171:137–141. PubMed CrossRef
- Price RB, Shungu DC, Mao X, et al. Amino acid neurotransmitters assessed by proton magnetic resonance spectroscopy: relationship to treatment resistance in major depressive disorder. Biol Psychiatry. 2009;65(9):792–800. PubMed CrossRef
- Runia N, Yücel DE, Lok A, et al. The neurobiology of treatment-resistant depression: a systematic review of neuroimaging studies. Neurosci Biobehav Rev. 2022;132:433–448. PubMed CrossRef
- Mrazek DA, Hornberger JC, Altar CA, et al. A review of the clinical, economic, and societal burden of treatment-resistant depression: 1996-2013. Psychiatr Serv. 2014;65(8):977–987. PubMed CrossRef
- Halaris A, Sohl E, Whitham EA. Treatment-resistant depression revisited: a glimmer of hope. J Pers Med. 2021;11(2):155. PubMed CrossRef
- Gibson TB, Jing Y, Smith Carls G, et al. Cost burden of treatment resistance in patients with depression. Am J Manag Care. 2010;16(5):370–377. PubMed
- Crown WH, Finkelstein S, Berndt ER, et al. The impact of treatment-resistant depression on health care utilization and costs. J Clin Psychiatry. 2002;63(11):963–971. PubMed CrossRef
- Shrestha A, Roach M, Joshi K, et al. Incremental health care burden of treatment-resistant depression among commercial, Medicaid, and Medicare payers. Psychiatr Serv. 2020;71(6):593–601. PubMed CrossRef
- Sussman M, O’sullivan AK, Shah A, et al. Economic burden of treatment-resistant depression on the US health care system. J Manag Care Spec Pharm. 2019;25(7):823–835. PubMed CrossRef
- Johnston KM, Powell LC, Anderson IM, et al. The burden of treatment-resistant depression: a systematic review of the economic and quality of life literature. J Affect Disord. 2019;242:195–210. PubMed CrossRef
- Day E, Shah R, Taylor RW, et al. A retrospective examination of care pathways in individuals with treatment-resistant depression. BJPsych Open. 2021;7(3):e101. PubMed CrossRef
- Wiles N, Taylor A, Turner N, et al. Management of treatment-resistant depression in primary care: a mixed-methods study. Br J Gen Pract. 2018;68(675):e673–e681. PubMed CrossRef
- Thase ME, Friedman ES, Howland RH. Management of treatment-resistant depression: psychotherapeutic perspectives. J Clin Psychiatry. 2001;62(suppl 18):18–24. PubMed
- Ivanova JI, Birnbaum HG, Kidolezi Y, et al. Direct and indirect costs of employees with treatment-resistant and non-treatment-resistant major depressive disorder. Curr Med Res Opin. 2010;26(10):2475–2484. PubMed CrossRef
- Nutting PA, Rost K, Dickinson M, et al. Barriers to initiating depression treatment in primary care practice. J Gen Intern Med. 2002;17(2):103–111. PubMed CrossRef
- Schroder HS, Patterson EH, Hirshbein L. Treatment-resistant depression reconsidered. SSM-Mental Health. 2022;2:100081. CrossRef
- Fava GA, Savron G, Grandi S, et al. Cognitive-behavioral management of drug-resistant major depressive disorder. J Clin Psychiatry. 1997;58(6):278–282, quiz 283–284. PubMed CrossRef
- Wiles N, Thomas L, Abel A, et al. Cognitive behavioural therapy as an adjunct to pharmacotherapy for primary care based patients with treatment resistant depression: results of the CoBalT randomised controlled trial. Lancet. 2013;381(9864):375–384. PubMed CrossRef
- Li JM, Zhang Y, Su WJ, et al. Cognitive behavioral therapy for treatment-resistant depression: a systematic review and meta-analysis. Psychiatry Res. 2018;268:243–250. PubMed CrossRef
- Cuijpers P, Geraedts AS, van Oppen P, et al. Interpersonal psychotherapy for depression: a meta-analysis. Am J Psychiatry. 2011;168(6):581–592. PubMed CrossRef
- Souza LH, Salum GA, Mosqueiro BP, et al. Interpersonal psychotherapy as add-on for treatment-resistant depression: a pragmatic randomized controlled trial. J Affect Disord. 2016;193:373–380. PubMed CrossRef
- Thase ME, Friedman ES, Biggs MM, et al. Cognitive therapy versus medication in augmentation and switch strategies as second-step treatments: a STAR*D report. Am J Psychiatry. 2007;164(5):739–752. PubMed CrossRef
- Kocsis JH, Gelenberg AJ, Rothbaum BO, et al; REVAMP Investigators. Cognitive behavioral analysis system of psychotherapy and brief supportive psychotherapy for augmentation of antidepressant nonresponse in chronic depression: the REVAMP Trial. Arch Gen Psychiatry. 2009;66(11):1178–1188. PubMed CrossRef
- Carek PJ, Laibstain SE, Carek SM. Exercise for the treatment of depression and anxiety. Int J Psychiatry Med. 2011;41(1):15–28. PubMed CrossRef
- Carroll FI, Blough BE, Mascarella SW, et al. Bupropion and bupropion analogs as treatments for CNS disorders. Adv Pharmacol. 2014;69:177–216. PubMed CrossRef
- Bedson E, Bell D, Carr D, et al. Folate Augmentation of Treatment–Evaluation for Depression (FolATED): randomised trial and economic evaluation. Health Technol Assess. 2014;18(48):vii–viii, 1–159. PubMed CrossRef
- Rybakowski JK. Lithium - past, present, future. Int J Psychiatry Clin Pract. 2020;24(4):330–340. PubMed CrossRef
- Beardsley RS, Gardocki GJ, Larson DB, et al. Prescribing of psychotropic medication by primary care physicians and psychiatrists. Arch Gen Psychiatry. 1988;45(12):1117–1119. PubMed CrossRef
- Davies P, Ijaz S, Williams CJ, et al. Pharmacological interventions for treatment-resistant depression in adults. Cochrane Database Syst Rev. 2019;12(12):CD010557. PubMed CrossRef
- Epstein I, Szpindel I, Katzman MA. Pharmacological approaches to manage persistent symptoms of major depressive disorder: rationale and therapeutic strategies. Psychiatry Res. 2014;220(suppl 1):S15–S33. PubMed CrossRef
- Voineskos D, Daskalakis ZJ, Blumberger DM. Management of treatment-resistant depression: challenges and strategies. Neuropsychiatr Dis Treat. 2020;16:221–234. PubMed CrossRef
- Moraczewski J, Aedma KK. Tricyclic Antidepressants. Treasure Island, FL: StatPearls. StatPearls Publishing; 2022.
- Sub Laban T, Saadabadi A. Monoamine Oxidase Inhibitors (MAOIs). Treasure Island, FL: StatPearls. StatPearls Publishing; 2022.
- Baethge C, Braun C, Rink L, et al. Dose effects of tricyclic antidepressants in the treatment of acute depression: a systematic review and meta-analysis of randomized trials. J Affect Disord. 2022;307:191–198. PubMed CrossRef
- Braun C, Adams A, Rink L, et al. In search of a dose-response relationship in SSRIs-a systematic review, meta-analysis, and network meta-analysis. Acta Psychiatr Scand. 2020;142(6):430–442. PubMed CrossRef
- Rink L, Adams A, Braun C, et al. Dose-response relationship in selective serotonin and norepinephrine reuptake inhibitors in the treatment of major depressive disorder: a meta-analysis and network meta-analysis of randomized controlled trials. Psychother Psychosom. 2022;91(2):84–93. PubMed CrossRef
- Xu J, Hao Q, Qian R, et al. Optimal dose of serotonin reuptake inhibitors for obsessive-compulsive disorder in adults: a systematic review and dose-response meta-analysis. Front Psychiatry. 2021;12:717999. PubMed CrossRef
- Singh R, Bansal Y, Medhi B, et al. Antipsychotics-induced metabolic alterations: Recounting the mechanistic insights, therapeutic targets and pharmacological alternatives. Eur J Pharmacol. 2019;844:231–240. PubMed CrossRef
- Hedya SA, Avula A, Swoboda HD. Lithium Toxicity. Treasure Island, FL: StatPearls. StatPearls Publishing; 2022.
- McPherson S, Senra H. Psychological treatments for persistent depression: a systematic review and meta-analysis of quality of life and functioning outcomes. Psychotherapy (Chic). 2022;59(3):447–459. PubMed CrossRef
- Espinoza RT, Kellner CH. Electroconvulsive therapy. N Engl J Med. 2022;386(7):667–672. PubMed CrossRef
- Ousdal OT, Brancati GE, Kessler U, et al. The neurobiological effects of electroconvulsive therapy studied through magnetic resonance: what have we learned, and where do we go? Biol Psychiatry. 2022;91(6):540–549. PubMed CrossRef
- Luccarelli J, McCoy TH Jr, Seiner SJ, et al. Real-world evidence of age-independent electroconvulsive therapy efficacy: a retrospective cohort study. Acta Psychiatr Scand. 2022;145(1):100–108. PubMed CrossRef
- Committ APA, Weiner RD. Practice of Electroconvulsive Therapy: Recommendations for Treatment, training, and Privileging. 2nd ed. Washington, D.C: Amer Psychiatric Pub Inc; 2001.
- Rose D, Fleischmann P, Wykes T, et al. Patients’ perspectives on electroconvulsive therapy: systematic review. BMJ. 2003;326(7403):1363. PubMed CrossRef
- Semkovska M, McLoughlin DM. Objective cognitive performance associated with electroconvulsive therapy for depression: a systematic review and meta-analysis. Biol Psychiatry. 2010;68(6):568–577. PubMed CrossRef
- Osler M, Rozing MP, Christensen GT, et al. Electroconvulsive therapy and risk of dementia in patients with affective disorders: a cohort study. Lancet Psychiatry. 2018;5(4):348–356. PubMed CrossRef
- Blumberger DM, Seitz DP, Herrmann N, et al. Low medical morbidity and mortality after acute courses of electroconvulsive therapy in a population-based sample. Acta Psychiatr Scand. 2017;136(6):583–593. PubMed CrossRef
- Perera T, George MS, Grammer G, et al. The clinical TMS society consensus review and treatment recommendations for TMS therapy for major depressive disorder. Brain Stimul. 2016;9(3):336–346. PubMed CrossRef
- Baeken C, De Raedt R. Neurobiological mechanisms of repetitive transcranial magnetic stimulation on the underlying neurocircuitry in unipolar depression. Dialogues Clin Neurosci. 2011;13(1):139–145. PubMed CrossRef
- Brunoni AR, Chaimani A, Moffa AH, et al. Repetitive transcranial magnetic stimulation for the acute treatment of major depressive episodes: a systematic review with network meta-analysis. JAMA Psychiatry. 2017;74(2):143–152. PubMed CrossRef
- Mutz J, Vipulananthan V, Carter B, et al. Comparative efficacy and acceptability of non-surgical brain stimulation for the acute treatment of major depressive episodes in adults: systematic review and network meta-analysis. BMJ. 2019;364:l1079. PubMed CrossRef
- Rossi S, Hallett M, Rossini PM, et al; Safety of TMS Consensus Group. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120(12):2008–2039. PubMed CrossRef
- Williams NR, Schatzberg AF. NMDA antagonist treatment of depression. Curr Opin Neurobiol. 2016;36:112–117. PubMed CrossRef
- McIntyre RS, Rosenblat JD, Nemeroff CB, et al. Synthesizing the evidence for ketamine and esketamine in treatment-resistant depression: an international expert opinion on the available evidence and implementation. Am J Psychiatry. 2021;178(5):383–399. PubMed CrossRef
- Xiong J, Lipsitz O, Chen-Li D, et al. The acute antisuicidal effects of single-dose intravenous ketamine and intranasal esketamine in individuals with major depression and bipolar disorders: a systematic review and meta-analysis. J Psychiatr Res. 2021;134:57–68. PubMed CrossRef
- Phillips JL, Norris S, Talbot J, et al. Single and repeated ketamine infusions for reduction of suicidal ideation in treatment-resistant depression. Neuropsychopharmacology. 2020;45(4):606–612. PubMed CrossRef
- Short B, Fong J, Galvez V, et al. Side-effects associated with ketamine use in depression: a systematic review. Lancet Psychiatry. 2018;5(1):65–78. PubMed CrossRef
- O’Reardon JP, Cristancho P, Peshek AD. Vagus nerve stimulation (VNS) and treatment of depression: to the brainstem and beyond. Psychiatry (Edgmont). 2006;3(5):54–63. PubMed
- Grimonprez A, Raedt R, Baeken C, et al. The antidepressant mechanism of action of vagus nerve stimulation: Evidence from preclinical studies. Neurosci Biobehav Rev. 2015;56:26–34. PubMed CrossRef
- Harden CL, Pulver MC, Ravdin LD, et al. A pilot study of mood in epilepsy patients treated with vagus nerve stimulation. Epilepsy Behav. 2000;1(2):93–99. PubMed CrossRef
- Rush AJ, Marangell LB, Sackeim HA, et al. Vagus nerve stimulation for treatment-resistant depression: a randomized, controlled acute phase trial. Biol Psychiatry. 2005;58(5):347–354. PubMed CrossRef
- Davidson B, Gouveia FV, Rabin JS, et al. Deep brain stimulation for treatment-resistant depression: current status and future perspectives. Expert Rev Med Devices. 2020;17(5):371–373. PubMed CrossRef
- Dandekar MP, Fenoy AJ, Carvalho AF, et al. Deep brain stimulation for treatment-resistant depression: an integrative review of preclinical and clinical findings and translational implications. Mol Psychiatry. 2018;23(5):1094–1112. PubMed CrossRef
- Mayberg HS, Lozano AM, Voon V, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45(5):651–660. PubMed CrossRef
- Goodrich DE, Kilbourne AM, Nord KM, et al. Mental health collaborative care and its role in primary care settings. Curr Psychiatry Rep. 2013;15(8):383. PubMed CrossRef
- Katon WJ, Lin EH, Von Korff M, et al. Collaborative care for patients with depression and chronic illnesses. N Engl J Med. 2010;363(27):2611–2620. PubMed CrossRef
- Pigoni A, Delvecchio G, Madonna D, et al. Can machine learning help us in dealing with treatment resistant depression? a review. J Affect Disord. 2019;259:21–26. PubMed CrossRef
Enjoy this premium PDF as part of your membership benefits!