LESSONS LEARNED AT THE INTERFACE OF MEDICINE AND PSYCHIATRY
The Psychiatric Consultation Service at Massachusetts General Hospital sees medical and surgical inpatients with comorbid psychiatric symptoms and conditions. During their twice-weekly rounds, Dr Stern and other members of the Consultation Service discuss diagnosis and management of hospitalized patients with complex medical or surgical problems who also demonstrate psychiatric symptoms or conditions. These discussions have given rise to rounds reports that will prove useful for clinicians practicing at the interface of medicine and psychiatry.
Prim Care Companion CNS Disord 2024;26(1):23f03602
Author affiliations are listed at the end of this article.
Have you ever wondered what is meant by the term hypoactive delirium? Have you considered what looks like hypoactive delirium but is not? Have you been uncertain about how to establish the diagnosis and how best to manage it effectively? If you have, the following case vignette and discussion should prove useful.
CASE VIGNETTE
Mr A, a 78-year-old man, was admitted to the cardiac care unit with a large anterior wall myocardial infarction, complicated by cardiogenic shock. He was afebrile, his heart rate was 122 beats/minute, his respirations were 20 breaths/minute, and his blood pressure was 94/50 mm Hg. He was disoriented and poorly attentive and had psychomotor slowing. To improve his mental status, Mr A received intravenous (IV) hydration, pressors, and an antipsychotic. Since he was already tachycardic, stimulants, while considered, were not administered.
What Is Hypoactive Delirium, and How Does It Differ From Agitated Delirium?
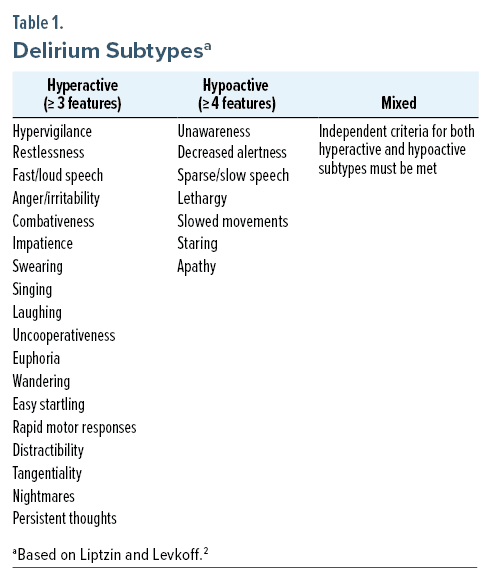
The classification of delirium subtypes characterized by phenotypic differences in motor activity was first suggested by Lipowski in 19831; this concept was later reviewed by Liptzin and Levkoff,2 in 1992, in their empirical study of delirium subtypes. They categorized delirium as “hyperactive” (with hypervigilance, restlessness, fast or loud speech, irritability, combativeness, impatience, swearing, singing, laughing, uncooperativeness, euphoria, anger, wandering, easy startling, fast motor responses, distractibility, tangentiality, nightmares, and persistent thoughts), “hypoactive” (with unawareness, decreased alertness, sparse or slow speech, lethargy, slowed movements, staring, and apathy), and “mixed” (when features of both hyperactive and hypoactive delirium fluctuate) (Table 1).2 Patients with ≥ 3 hyperactive symptoms were considered to have hyperactive delirium, while those with ≥ 4 hypoactive symptoms were considered to have hypoactive delirium; those with both types of symptoms were considered to have a mixed subtype of delirium. They also noted that patients with hyperactive delirium had a shorter length of stay in the hospital and a lower mortality rate (both during the hospitalization and at 6-month follow-up) than those with either a mixed or hypoactive subtype of delirium.2
How Can the Manifestations of Hypoactive Delirium Be Assessed?
Individuals with hypoactive delirium are drowsy, lethargic, or sluggish (in the absence of sedative-hypnotics, pain medications, or sedating psychotropic medications); they never fully awaken, repeatedly fall back to sleep midsentence, and need to be provided with frequent prompts by staff. They are typically psychomotorically slowed, withdrawn, and apathetic and have sparse speech (which is soft, slowed, and rarely spontaneous). On neurologic evaluation, tremor, myoclonus, reflex or muscle tone changes, and primitive reflexes (or frontal release signs, such as glabellar, rooting, snout, suck, grasp reflex) are often noted. Use of several validated delirium rating scales can help to identify and quantify delirium. Jones and colleagues3 conducted a systematic review of the quality and clinical outcomes of delirium rating scales. They noted that the Confusion Assessment Method (CAM)4 or CAM-S,5 the Delirium Rating Scale (DRS) or DRS-R-98,6 and the Memorial Delirium Assessment Scale (MDAS)7 were the most frequently used instruments for the assessment of delirium severity. These high-quality delirium scales were thought to be helpful to clinicians and for quality improvement efforts, while identifying patients who might benefit from interventions or follow-up monitoring. They excluded single-item ratings of global severity, including the Richmond Agitation Sedation Scale (RASS)8 and the Bush-Francis Catatonia Rating Scale (BFCRS).9 However, the RASS may help to categorize hyperactivity or hypoactivity, and the BFCRS may help identify those who have hypoactive features that may herald catatonia. Medication lists should be reviewed, as benzodiazepines, non-benzodiazepine sedative-hypnotics, opioids and sedating non-opioid analgesics, psychotropic agents, muscle relaxants, antihistamines, and anticholinergics can impair the mental status. Laboratory testing is often key in the identification of delirium regardless of its motoric subtype. Testing should include serum electrolytes, kidney function (including blood urea nitrogen and creatinine), liver function, complete blood count, blood glucose level, urinalysis and urine culture, urine toxicology, thyroid function, a chest x-ray, and an electrocardiogram. Changes on the electroencephalogram (EEG) typical of hypoactive delirium consist of generalized slowing; this finding would be uncommon in the setting of dementia or depression.
What Can Cause Hypoactive Delirium?
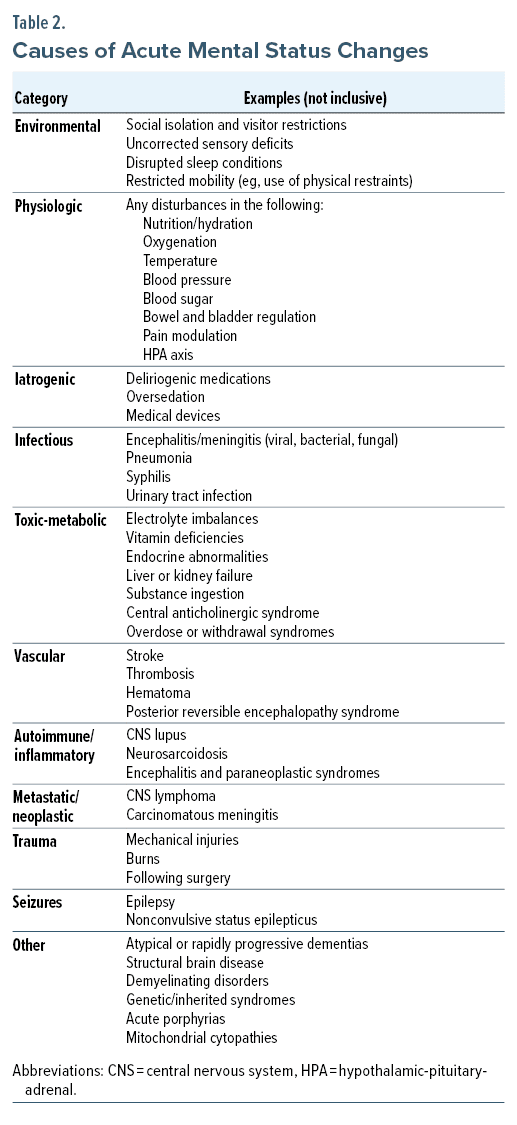
Acute alterations of mental status (involving disturbances in affect, behavior, and cognition) include environmental (eg, level of social isolation, uncorrected sensory deficits, restricted mobility), physiologic (eg, nutrition, oxygenation, blood pressure regulation), iatrogenic (eg, presence of deliriogenic medications, oversedation), infectious (eg, encephalitis, meningitis, pneumonia), toxic-metabolic (eg, electrolyte disturbances, liver failure, vitamin deficiencies), vascular (eg, stroke, thrombosis, hematoma), autoimmune (eg, central nervous system [CNS] lupus, neurosarcoidosis), metastatic/neoplastic (eg, CNS lymphoma, carcinomatous meningitis), traumatic (eg, mechanical injuries) etiologies, and a consequence of seizures (eg, epilepsy, nonconvulsive status epilepticus) (Table 2). The etiology of mental status abnormalities can also be identified by considering conditions associated with each organ system (eg, CNS, cardiac, pulmonary, gastrointestinal, hematologic, immune, urologic, musculoskeletal, endocrine, dermatologic).
The recognition of hypoactive delirium should prompt the search for its underlying etiologies, with initial attention paid to life-threatening or emergent causes. Unfortunately, the hypoactive phenotype of delirium is mistaken far too often for depression, which leads some clinicians to overlook serious medical and neurologic conditions.10,11 A historical study by Armstrong and colleagues12 examined diagnoses by nonpsychiatric providers on consult services over a 5-year period and noted 46% were misdiagnosed. Of those, 31% were given an incorrect diagnosis of depressive disorders. A prospective study on motoric subtypes of postoperative delirium in individuals aged ≥ 50 years revealed that the most common subtype was hypoactive (68%) and that the patients were older (71 ± 9 vs 65 ± 9, P = .002), more anemic (P = .002), and more likely to have decubitus ulcers.13 In a large, double-blind, randomized clinical trial, Girard and colleagues14 studied the use of haloperidol, ziprasidone, or placebo for the treatment of delirium in individuals in critical care settings. They noted that delirium developed in 48%, whereas 89% had hypoactive delirium.14 Particular etiologies that appear strongly correlated with the hypoactive phenotype in the critical care setting include prolonged sedation, hypoxia, and sepsis (infectious).14–16
To facilitate rapid recognition and timely treatment of delirium, a mnemonic to recall the life-threatening causes of delirium was created by Small (“WWHHHHIMPS”).17 Clinicians should consider these causes (Table 3) whenever mental status abnormalities are identified.18
What Looks Like Hypoactive Delirium But Isn’t?
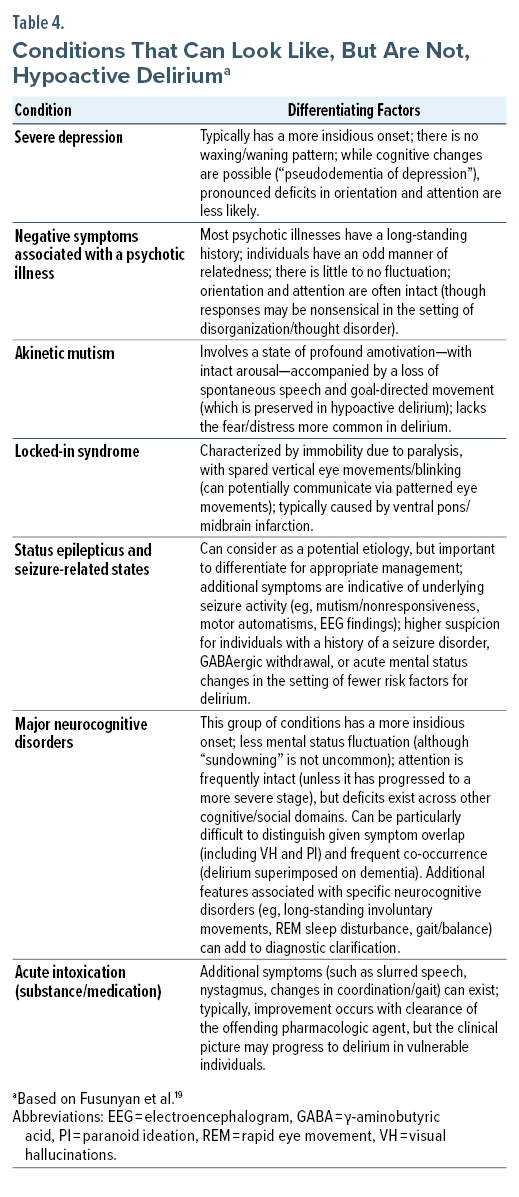
When differentiating hypoactive delirium from other conditions that are accompanied by disturbances of affect, behavior, cognition, and motor functions, it is helpful to consider the time course of symptoms and their onset, the presence of focal neurologic deficits, abnormal findings on brain imaging, and underlying risk factors and environmental exposures (Table 4).19 Atypical and rapidly progressive dementias, structural brain diseases, demyelinating disorders, and genetic/inherited syndromes (eg, acute porphyria, mitochondrial cytopathy) should also be considered when mental status changes are encountered.20
Since delirium involves an inability to sustain attention, downstream cognitive functions will undoubtedly be affected. Delirium tends to evolve over hours to days, and it waxes and wanes over the course of the day. EEG findings often show nonspecific background slowing. Acute changes on brain imaging and sustained focal deficits suggest an alternative diagnosis.21
How Can the Etiology of Hypoactive Delirium Be Investigated?
The assessment of hypoactive delirium is guided by the detection and appreciation of signs and symptoms. This is a dynamic process that depends on the patient’s evolving clinical picture. The differential diagnosis is refined by obtaining a thorough history of the present illness (aided by information from collateral sources), having knowledge about their comorbid conditions and medication regimen, as well as by their pattern of substance use.22
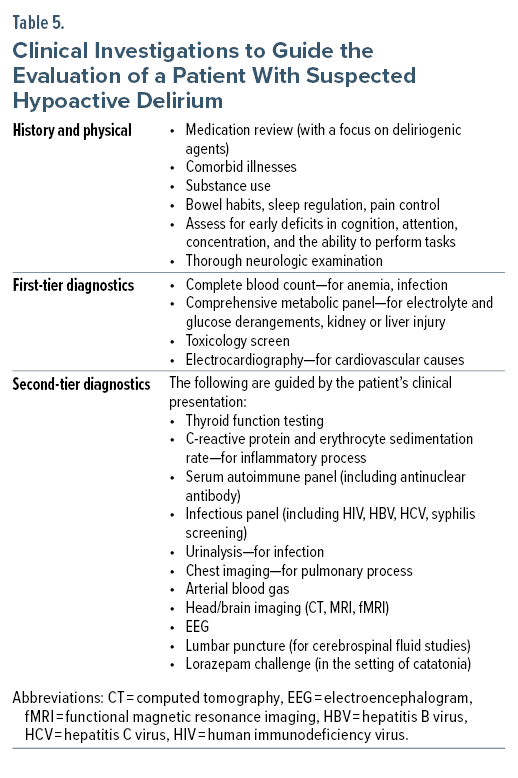
The clinical examination begins with a review of vital signs and a mental status examination. Assessment of hemodynamic support, such as the use of vasopressors and supplemental oxygen (or ventilation), alerts the clinician to the severity of active illness and contributing factors to the patient’s delirium. The mental status examination should evaluate attention, concentration, level of consciousness, and cognition. The physical examination includes a thorough neurologic evaluation, with a focus on the function of cranial nerves and cerebellar function, muscle tone (rigidity), reflexes (including the presence of clonus), asterixis, gait and balance, and the ability to perform complex tasks. Table 5 presents a list of general clinical investigations to consider when evaluating a patient with all motoric phenotypes of delirium, including the hypoactive subtype.
Regardless of its underlying etiology, delirium is hypothesized as a syndrome of impaired neuronal network connectivity and disintegration.23 Particularly, disruptions between the ascending reticular activating system (ARAS), a set of connected nuclei responsible for the regulation of wakefulness and alertness, have been implicated.24 Neuroimaging and neurophysiologic recordings across time series offer evidence to support this theory and serve as diagnostic tools to unearth the etiology of delirium. The use of the EEG, when available, often shows generalized slowing to the theta-delta range in the delirious patient and may also clarify the etiology (eg, alcohol and sedative withdrawal, hepatic encephalopathy) based on its specific findings and waveforms.25 Although not always clinically practical or feasible, functional magnetic resonance imaging using blood-oxygenation-level dependent signaling also reveals global brain network disintegration over time and space in the delirious state.26 Among patients with hypoactive delirium and nondelirious controls, those with delirium can be distinguished from controls with a high degree of accuracy based on the EEG’s topology (the presence of a less integrated network in the α band).27
Should Hypoactive Delirium and Its Components Be Treated (if so, how)?
Although the most effective treatment for delirium rests on the identification and the reversal of its underlying cause (eg, oxygen for a hypoxemic patient, glucose for a patient with hypoglycemia), management of delirium usually targets its problematic symptoms (eg, an antipsychotic to reduce agitation) rather than its cause. Since hypoactive delirium rarely jeopardizes the safety of afflicted patients (eg, via removal of indwelling catheters or ventilators), or the staff who cares for them, hypoactive delirium frequently goes undetected. Withdrawn and quietly confused patients tend not to generate staff concern or to precipitate emergency interventions. As a result, hypoactive and confused individuals tend to have their evaluation and treatment delayed, which may lead to persistence or exacerbation of an underlying medical condition that is responsible for acute brain failure and dysfunction.28 Systematic screening (ie, detection protocols) for delirium that use evidence-based tools (eg, the Confusion Assessment Method4 and its intensive care unit (ICU) counterpart [CAM, CAM-ICU], the Intensive Care Delirium Screening Checklist [ICDSC], or the Memorial Delirium Assessment Scale MDAS])7 can improve the recognition of delirium and serve to guide the initiation of timely treatment.29 When these tests are used routinely in the ICU, systematic detection contributes to improved outcomes (eg, decreased time on a ventilator, length of stay, and mortality rates).30
Multicomponent nonpharmacologic strategies that focus on enhancing cognition (eg, frequent reorientation, cognitive stimulation, music therapy, use of clocks, access to natural daylight), minimizing oversedation and sleep disruption (eg, reducing use of sedating agents, minimizing exposure to bright lights and loud noises, allowing periods of undisrupted sleep), increasing mobility (eg, encouraging early rehabilitation/mobilization), and reducing hearing and visual impairment (eg, wearing hearing aids and glasses) have consistently been shown to reduce the development and duration of delirium, as well as in-hospital mortality.31,32
Recently updated guidelines (eg, on the management of pain, agitation/sedation, delirium, immobility, and sleep disruption) do not recommend the routine use of pharmacologic treatments for motoric subtypes of delirium. In fact, they specifically recommend against the routine use of antipsychotics, statins, or bright-light therapies for delirium, although they do note that antipsychotics (eg, haloperidol, olanzapine) may reduce distress (eg, anxiety, fear, hallucinations, delusions) or agitation that may lead to self-harm or injuries to others, secondary to symptoms of delirium.29 Dexmedetomidine has also been suggested for the prevention or management of agitation.33,34 However, benzodiazepines (eg, lorazepam) have been shown to be an independent risk factor for delirium in ICUs, with a harm ratio of 1.2 for each 1 mg of lorazepam administered.35 As such, they are typically avoided, unless they are used to treat an underlying condition (eg, alcohol or benzodiazepine withdrawal, seizures, or catatonia) that is the source of delirium.
What Role Can Antipsychotics Play in Hypoactive Delirium?
Antipsychotic agents have been studied widely for their efficacy in the management of delirium. Recently, 2 large, randomized placebo-controlled trials in critically ill inpatients were undertaken to assess the effects of antipsychotics on specific outcomes (eg, aberrant behaviors, scores on the CAM-ICU, post-ventilation course, mortality rates, and adverse events) related to intensive care.14,36 Unfortunately, these trials failed to demonstrate significant differences between placebo and the antipsychotics trialed. Further, both studies did not differ in utilization of antipsychotics between motor subtypes, and each had populations with a high prevalence of hypoactive delirium, adding further randomized controlled data to the generally accepted position that antipsychotics do not have a standard role in the management of hypoactive delirium.
In the first of these trials,14 566 delirious patients were randomized to standing doses of IV haloperidol (up to 20 mg per day) and IV ziprasidone (up to 40 mg per day); 89% of them had hypoactive delirium. Haloperidol and ziprasidone failed to separate from placebo for the primary end point (ie, the number of days alive without delirium or coma during the 14-day intervention period). Both agents also failed to separate from placebo with respect to secondary end points (eg, 30-day and 90-day survival, time to freedom from mechanical ventilation, and time to ICU and hospital discharge). In addition, both agents failed to separate from placebo for the development of extrapyramidal symptoms (EPS); while ziprasidone showed a statistically significant increase in QT prolongation as compared to placebo, haloperidol did not.14
In the second of these trials,36 1,000 patients with delirium were randomized to receive standing doses of IV haloperidol (up to 20 mg per day) and placebo; 45% of them had hypoactive delirium. Haloperidol failed to separate from placebo in their primary outcome measures (ie, number of days alive and number out of the hospital at 90 days after randomization). Similarly, it failed to separate from placebo in secondary outcomes (eg, number of days alive without delirium or coma in the ICU at 90 days, number of days without mechanical ventilation at 90 days, number of patients receiving rescue medication, number of days receiving rescue medication, or number of patients with ≥ 1 serious adverse reactions.37
These studies seem to suggest that antipsychotics may not reduce the frequency, severity, or duration of delirium in critically ill patients. Further, they did not delineate the impact on specific symptoms of delirium that were distressing to patients, nor did they look at mental health outcomes. Moreover, they failed to comment on the etiology of delirium in patients or on the use of antipsychotics in those who were hypoactive or comatose (ie, not agitated), as antipsychotics are neither routinely used nor indicated in patients with these clinical states.
Several randomized, placebo-controlled trials have demonstrated that antipsychotics are efficacious in the management of heterogeneous motor types of delirium. Hu and colleauges37 found that both olanzapine and haloperidol showed a faster and more robust response than placebo in 175 delirious geriatric patients. Quetiapine has been shown to improve delirium more rapidly than placebo in 2 trials, with 36 patients in the ICU and 42 patients on acute care floors.38,39 In each of these studies, improvements were found in rating scale scores that addressed specific symptoms of delirium, as opposed to the binary studies described previously. In the palliative care setting, Agar et al40 studied 247 patients randomized to receive haloperidol, risperidone, or placebo; they identified that worse outcomes were associated with both antipsychotics.
Multiple nonplacebo prospective trials of antipsychotics (eg, haloperidol, chlorpromazine, olanzapine, risperidone, quetiapine, and aripiprazole) have shown improvements in delirium severity when validated scales were used on patients with all motoric subtypes of delirium. Comparison trials have generally shown no significant difference in efficacy among antipsychotics; 1 trial showed superior efficacy in those with hypoactive delirium when treated with aripiprazole as compared to haloperidol and suggested worse outcomes when comparing hypoactive and hyperactive subtypes in general.41,42 Few of these comparison trials comment on or differentiate outcomes with regard to motoric subtypes. Further, these comparison trials must be read cautiously given the lack of a placebo arm and the likelihood of delirium improving over time with appropriate medical intervention. The literature’s identification of mixed efficacy appears to depend on which outcomes were viewed; however, antipsychotics are usually not recommended routinely for those who are hypoactively delirious.29 However, they appear to be well-tolerated in delirium with no consistent evidence of worsened outcomes. Given this and the evidence of efficacy for specific symptoms in studies that looked at all motor subtypes of delirium, it is reasonable to consider antipsychotics in hypoactive patients when psychotic symptoms, significant emotional distress, or dysregulated sleep-wake cycles arise and also interfere with care, cause undue suffering, or are unresponsive to more conservative, evidence-based treatments including nonpharmacologic interventions.
Can Psychostimulants Be Useful in Hypoactive Delirium?
Arousal and attention are directly and indirectly modulated by several neurotransmitters (eg, dopamine, norepinephrine, acetylcholine, and serotonin).43 Of these agents, acetylcholine plays a crucial role in memory, attention, and alertness; dopamine plays a role in the regulation of attention; norepinephrine is involved in modulating the sleep-wake cycle and maintaining alertness and attention, and serotonin influences attention and cognitive function.
Psychostimulants (eg, amphetamine, methylphenidate, and modafinil) increase alertness and improve attention through optimizing levels of these centrally acting neurotransmitters, including within the ARAS. Both methylphenidate and amphetamine increase levels of synaptic dopamine, norepinephrine, and, to a lesser extent, serotonin through reuptake inhibition. In addition, amphetamine enhances the release of dopamine and norepinephrine from presynaptic neurons.44 Modafinil, a weak dopamine reuptake inhibitor, also affects norepinephrine and serotonin, and it is thought to stimulate the release of orexin, a neuropeptide that regulates wakefulness and arousal.45 This increase of neurotransmitters in the synaptic cleft facilitates neuronal communication and enhances overall brain function. Therefore, in theory, psychostimulants could alleviate disturbances of arousal and attention that are associated with hypoactive delirium; however, clinicians are often concerned that such an intervention might increase agitation.
Psychostimulants, predominantly studied and used for the treatment of attention-deficit/hyperactivity disorder and narcolepsy, have also been used successfully in the treatment of depression in medically ill elderly patients, during palliative care to offset opioid-induced drowsiness, and to enhance memory and attention in patients with a myriad of conditions (eg, brain tumors, traumatic brain injuries, and HIV-related cognitive impairment), despite a lack of large or systematic studies to support these findings.39 Psychopharmacologic studies on hypoactive delirium are few and far between; the literature is largely comprised of case reports. Gagnon and colleagues’46 prospective study described improved alertness, partial-to-complete resolution of psychomotor retardation, and normalization of slurred speech with use of methylphenidate in 14 patients with hypoactive delirium in the context of advanced cancer, but studies replicating these findings are lacking.
It is worth mentioning that attempts to mitigate delirium through the regulation of neurotransmitters have been attempted with the use of cholinesterase inhibitors, such as rivastigmine and donepezil. Donepezil has not been associated with a significant reduction of delirium prevalence, except in a recent study of patients who had been prescribed this agent as part of dementia treatment some time prior to hospital admission.47 Additionally, a randomized trial of rivastigmine for treatment of delirium in the ICU was terminated early due to an excess of deaths in the active treatment arm.48 Although cholinergic deficiency is one of the leading pathophysiologic hypotheses of delirium, there is currently no evidence to support use of cholinesterase inhibitors in its management or prevention.48
What Are the Side Effects and Potential Complications of Antipsychotics and Psychostimulants in Patients With Hypoactive Delirium?
Antipsychotic agents and psychostimulants have a bevy of side effects that warrant careful consideration before they are used in patients with hypoactive delirium. Since the cholinergic system is an important modulator of awareness and attention, antipsychotics with anticholinergic properties may exacerbate impairments in awareness and attention.24 Similarly, while sedative effects of certain antipsychotics can promote sleep, they can exacerbate lethargy and hypoarousal in patients with hypoactive delirium. Sedative effects also increase the risk of falls and injuries. Antipsychotics may prolong the QTc interval and increase risk of dangerous arrhythmias (eg, torsades de pointes), particularly in those who are predisposed to QTc widening or who are receiving other QTc-prolonging medications.49
EPS (eg, dystonia, akathisia, and parkinsonism) may occur within hours to days of initiation of an antipsychotic medication. These symptoms are often uncomfortable and distressing and can be frightening; moreover, they may be misinterpreted by health care providers as manifestations of a mixed or hyperactive delirium, which can erroneously lead to administration of additional antipsychotics.
Neuroleptic malignant syndrome, a rare but potentially fatal medical emergency, can be induced by administration of dopamine-blocking agents (eg, antipsychotics). While the syndrome is most often associated with use of high-potency first-generation antipsychotics, it has also been described with use of second-generation agents.50 Extreme caution should also be taken with patients experiencing catatonia, as antipsychotics can exacerbate this condition.
Psychostimulants are commonly associated with the development of insomnia, which can potentiate the sleep-wake disturbances that are commonly seen in delirium. These agents can also diminish appetite (when used in high doses), which can be particularly problematic in those who may already have diminished oral intake in the setting of a hypoactive delirium. Increases in blood pressure and heart rate are also common. Although these vital sign changes are typically small, they may be significant enough to exacerbate preexisting cardiovascular conditions, such as arrhythmias or congestive heart failure.51 Those with compromised cardiovascular function may be more susceptible to the impact of psychostimulants on autonomic regulation.51
Other side effects of psychostimulants include anxiety, irritability, emotional lability, and agitation. Since psychostimulants increase serum levels of dopamine, they may trigger or induce delusions or hallucinations during delirium.
What Is the Role of NMDA Receptor Agents in Hypoactive Delirium?
Responses to acute medical illness include neuroinflammation and oxidative stress, which result in neurotransmitter dysregulation and network dysconnectivity. However, the degree of neurotransmitter dysfunction varies depending upon delirium’s etiology.52 The oxidative stress hypothesis posits that tissue damage, infections, hypoxia, or another severe illness leads to increased oxygen consumption and/or to decreased availability of oxygen that disrupts oxidative metabolism and metabolic failure via ATPase pump dysfunction.53,54 Consequences of this dysfunction include an inability to maintain ionic gradients (with large influxes of calcium that are excitotoxic), as well as activation of phospholipases and proteases that break down cell membranes. Influx of calcium causes a dramatic extracellular release of glutamate that potentiates its own release through further activation of N-methyl-d-aspartate (NMDA)/α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors as well as further calcium influx.55 Given that glutamate excitotoxicity and free radical production play key roles in acute medical illness, NMDA receptor (NMDAr) antagonists may serve a unique role in blocking the NMDAr to prevent further calcium influx and its subsequent cellular consequences.
How Do NMDAr Antagonists Work?
Two NMDAr antagonists, memantine and amantadine, bind to voltage dependent–NMDArs that are located throughout the central nervous system (CNS), particularly on glutamatergic neurons. They are low-affinity antagonists with a higher affinity for these receptors than magnesium ions, which inhibit the intracellular influx of calcium ions to reduce neuronal excitotoxicity.56 Both agents have additional pharmacodynamic properties. Amantadine increases striatal dopamine release, blocks dopamine reuptake, and increases norepinephrine release. Memantine acts as a noncompetitive antagonist at the serotonin 5-HT3 receptor. Both are antagonists of the A7 nicotinic receptor. It is a noncompetitive antagonist at the A7 nicotinic receptor, and when antagonized, this upregulation of the A7 nicotinic receptors may explain their cognitive-enhancing effects.57
Have NMDAr Antagonists Been Efficacious for Hypoactive Delirium?
Several studies have evaluated the efficacy of NMDAr antagonists for the treatment of hypoactive delirium.58 However, many more trials have demonstrated the efficacy of NMDAr antagonists for the management of severe traumatic brain injury (TBI).59
Amantadine’s peak effect and half-life are 2–4 hours and 12–18 hours, respectively, while the peak effect and half-life of memantine are 3–7 hours and 60–80 hours, respectively. Dosing can begin at either 50 mg of amantadine or 5 mg of memantine each morning (eg, at 6 am), with reevaluation of the patient at the time of the drug’s peak effect. If there has been a minimal response (and activation has not occurred), then an additional dose can be administered, with reevaluation at the time of the next peak effect. Both medications can be titrated in their dose intervals (listed previously) until midday (ie, noon) at which point the trial should be paused until the following morning to prevent nocturnal insomnia. The total dose administered in a 24-hour period should be consolidated as the starting dose the following morning (at 6 am) with further dose titration as needed. The maximum doses to be trialed are 200–400 mg of amantadine and 20–30 mg of memantine. The duration of these drug trials should continue until the time it takes to achieve steady state levels, which is 3.5–5 half-lives.
What Are the Side Effects of NMDAr Antagonists?
In general, both NMDAr antagonists (mentioned previously) are well tolerated. Common side effects for memantine include dizziness, drowsiness, headache, agitation, hallucinations, vomiting, hypertonia, and cystitis. Amantadine’s side effects are like those of memantine, and its CNS side effects tend to occur at doses > 200 mg/day; at very high plasma concentrations, patients can develop hallucinosis, seizures, and cardiac arrhythmias. Amantadine may result in increased agitation for a delirious patient given the dopamine agonist properties of the medication. Co-ingestion with antihistamines or anticholinergic medications can worsen its gastrointestinal side effects and exacerbate symptoms of benign prostatic hypertrophy and glaucoma. Amantadine is contraindicated in patients with end-stage kidney disease because it is renally excreted. If the dose is not adjusted for the creatinine clearance, it can precipitate seizures.56 Additionally, amantadine is not dialyzable. Although it needs to be renally dosed, it typically is not able to be used in patients who require dialysis.
Monitoring for serious reactions to memantine or amantadine should include irritability or agitation, hallucinations, depression or suicidality, and worsened confusion.
Should Melatonin Agonists Be Used in Hypoactive Delirium?
Multiple reviews60–62 have examined the effects of melatonin agonists, including both melatonin and ramelteon, in the prevention or treatment of delirium. Regarding prevention, trials have shown conflicting results across both medical and surgical patients. While small trials of ramelteon and melatonin showed decreased incidence, the largest randomized controlled trial showed no benefit.63–65 There is not clear evidence that these interventions separate from placebo for prevention of delirium.60,61 Similarly, there are limited randomized controlled trials in support of melatonin agonism in the treatment of delirium. A recent review66 identified 3 randomized controlled trials that led to a meta-analysis showing a positive statistical difference between melatonin and placebo (−1.72 days; 95% CI, −2.66 to −0.77, P = .0004) when examining the duration of delirium. Smaller trials and observational studies also suggest a potential benefit, though the current data are limited in number and quality.
Which Other Somatic Interventions Can Mitigate the Symptoms of Hypoactive Delirium?
In addition to creating a general environment that is conducive to restoring cerebral homeostasis (eg, regulating the day/night cycle), the central tenet of managing delirium is reversing its underlying medical etiology. Within this framework, the choice of more specific somatic therapies is based on the presumed etiology, which is frequently multifactorial and at times difficult to identify definitively.
Decreased cerebral perfusion/oxygenation that leads to ischemia is a contributing factor for altered mental status; this is often a complication of invasive procedures, neurologic emergencies, and critical illness. Cerebral perfusion is a complex process that depends on well-functioning cerebrovascular autoregulation and adequate mean arterial pressure, which in turn can be supported by use of fluid resuscitation and vasopressors, such as norepinephrine.67 Nonetheless, matching perfusion to cerebral metabolic demands is challenging (despite evolving monitoring techniques), as the blood-brain barrier can be compromised by injury and systemic inflammatory processes. This may help to explain why vasoactive agents appear to have a more direct effect on cerebral vasculature.68 In addition, anemia (eg, with a hemoglobin [Hgb] level ≤ 6.0) is another risk factor for delirium, presumably due to decreased cerebral oxygenation; in this case, transfusions can be protective.69 However, protocols that have used more liberal thresholds for transfusions (eg, a Hgb ≤ 10.0) have no benefit over more restrictive strategies.70,71 At the same time, other studies have noted that receiving a transfusion of red blood cells increases the risk of postoperative delirium, possibly due to neuroinflammation.
The role of temperature management in critically ill patients is complex.72 In theory, mild therapeutic hypothermia may be neuroprotective (by reducing inflammation, preventing neuronal cell death, and decreasing brain metabolism/oxygen consumption). In practice, some results show improved neurologic outcomes for survivors of cardiac arrest (complicated by coma) managed with mild hypothermia, while others showed no clear difference between use of mild hypothermia and targeted normothermia. In cases of neurologic emergencies (eg, TBI, ischemic and hemorrhagic strokes), fever is associated with a worse neurologic outcome, but the role for induced hypothermia is similarly unclear—unless it is used to reduce intracranial pressure unresponsive to other interventions. Unlike in neurologic injury, with sepsis, fever is associated with improved outcomes, and while patients frequently receive antipyretics or are warmed to normothermia, there is no strong evidence for benefit. Interestingly, some researchers have found that temperature variability is more common in those with delirium, presumably as a marker of underlying encephalopathy and hypothalamic dysfunction.73
Lastly, as catatonia can both mimic and co-present with delirium (potentially co-occurring in > 40% of critically ill delirious patients),74 electroconvulsive therapy (ECT) can be helpful in benzodiazepine-resistant or life-threatening cases, with impressive response rates.75,76 More broadly, promptly addressing the underlying medical etiologies and risk factors is essential, as longer durations of delirium—almost regardless of likely mechanism—are associated with persistent cognitive impairment.77
What Happened to Mr A?
Over the next several days, Mr A’s cardiac function (and vital signs) returned to normal, and his mental status improved, enabling him to be transferred to a regular medical service before he returned home. He was thankful for the care he received and was neither depressed nor cognitively impaired.
CONCLUSION
While delirium can present with hyperactivity, hypoactivity, or a mixture of features, hypoactive delirium is frequently underdiagnosed and misdiagnosed. It is more likely to be overlooked than hyperactive or “mixed” delirium phenotypes, and it can contribute to subacute and chronic conditions. Timely recognition and treatment (with pharmacologic and nonpharmacologic interventions) that address its underlying etiology can facilitate its resolution with a corresponding improvement in the clinical picture.
Article Information
Published Online: February 8, 2024. https://doi.org/10.4088/PCC.23f03602
© 2024 Physicians Postgraduate Press, Inc.
Submitted: July 10, 2023; accepted October 19, 2023.
To Cite: Rosen JH, Bieber E, Matta SE, et al. Hypoactive delirium: differential diagnosis, evaluation, and treatment. Prim Care Companion CNS Disord. 2024;26(1):23f03602.
Author Affiliations: Department of Psychiatry and Neurobehavioral Sciences, University of Virginia, Charlottesville, Virginia (Rosen); Ann & Robert H. Lurie Children’s Hospital of Chicago, Illinois (Bieber); Department of Psychiatry and Behavioral Sciences, Northwestern University Feinberg School of Medicine, Chicago, Illinois (Bieber); Department of Psychiatry, Massachusetts General Hospital, Massachusetts (Matta, Stern); Harvard Medical School, Boston, Massachusetts (Matta, Fedotova, Stern); Division of Consultation-Liaison Psychiatry, Department of Psychiatry, Columbia University Irving Medical Center, New York, New York (Sayde); Department of Psychiatry, Brigham and Women’s Hospital, Boston, Massachusetts (Fedotova); Department of Psychiatry, University of Texas, Southwestern, Dallas, Texas (deVries, Rafferty).
Additional Information: Drs Rosen, Bieber, Matta, Sayde, Fedotova, deVries, and Rafferty are co-first authors. Dr Stern is the senior author.
Corresponding Author: Theodore Stern, MD, Harvard Medical School, Massachusetts General Hospital, 55 Fruit St, WRN 606, Boston, MA 02114 ([email protected]).
Relevant Financial Relationships: None.
Funding/Support: None.
Clinical Points
- Hypoactive delirium is typically manifest by unawareness, decreased alertness, sparse or slow speech, lethargy, slowed movements, staring, and apathy.
- Recognition of hypoactive delirium should prompt the search for its underlying etiologies, with initial attention paid to life-threatening or emergent causes. The hypoactive phenotype of delirium is often mistaken for depression, which leads some clinicians to overlook serious medical and neurologic conditions.
- Optimal management of hypoactive delirium involves a timely and targeted multipronged approach (involving pharmacologic and nonpharmacologic interventions) that addresses the underlying etiologies and precipitating factors.
References (77)

- Lipowski ZJ. Transient cognitive disorders (delirium, acute confusional states) in the elderly. Am J Psychiatry. 1983;140(11):1426–1436. PubMed CrossRef
- Liptzin B, Levkoff SE. An empirical study of delirium subtypes. Br J Psychiatry. 1992;161(6):843–845. PubMed CrossRef
- Jones RN, Cizginer S, Pavlech L, et al; Better Assessment of Illness (BASIL) Study Group. Assessment of instruments for measurement of delirium severity: a systematic review. JAMA Intern Med. 2019;179(2):231–239. PubMed CrossRef
- Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method: a new method for detection of delirium. Ann Intern Med. 1990;113(12):941–948. PubMed CrossRef
- Inouye SK, Kosar CM, Tommet D, et al. The CAM-S: development and validation of a new scoring system for delirium severity in 2 cohorts. Ann Intern Med. 2014;160(8):526–533. PubMed CrossRef
- Trzepacz PT, Mittal D, Torres R, et al. Validation of the Delirium Rating Scale-revised-98: comparison with the delirium rating scale and the cognitive test for delirium. J Neuropsychiatry Clin Neurosci. 2001;12(2):229–242. PubMed CrossRef
- Breitbart W, Rosenfeld B, Roth A, et al. The Memorial Delirium Assessment Scale. J Pain Symptom Manage. 1997;13(3):128–137. PubMed CrossRef
- Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338–1344. PubMed CrossRef
- Bush G, Fink M, Petrides G, et al. Catatonia, I: rating scale and standardized examination. Acta Psychiatr Scand. 1996;93(2):129–136. PubMed CrossRef
- O’Sullivan R, Inouye SK, Meagher D. Delirium and depression: inter-relationship and clinical overlap in elderly people. Lancet Psychiatry. 2014;1(4):303–311. PubMed CrossRef
- Swigart SE, Kishi Y, Thurber S, et al. Misdiagnosed delirium in patient referrals to a university-based hospital psychiatry department. Psychosomatics. 2008;49(2):104–108. PubMed CrossRef
- Armstrong SC, Cozza KL, Watanabe KS. The misdiagnosis of delirium. Psychosomatics. 1997;38(5):433–439. PubMed CrossRef
- Robinson TN, Raeburn CD, Tran ZV, et al. Motor subtypes of postoperative delirium in older adults. Arch Surg. 2011;146(3):295–300. PubMed CrossRef
- Girard TD, Exline MC, Carson SS, et al; MIND-USA Investigators. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379(26):2506–2516. PubMed CrossRef
- Krewulak KD, Stelfox HT, Leigh JP, et al. Incidence and prevalence of delirium subtypes in an adult ICU: a systematic review and meta-analysis. Crit Care Med. 2018;46(12):2029–2035. PubMed CrossRef
- Bowman EML, Cunningham EL, Page VJ, et al. Phenotypes and subphenotypes of delirium: a review of current categorisations and suggestions for progression. Crit Care. 2021;25(1):334. PubMed CrossRef
- Caplan JP, Stern TA. Mnemonics in a nutshell: 32 aids to psychiatric diagnosis. Curr Psychiatr. 2008;7(10):27–33.
- Caplan JP. Delirious Patients. In: Stern TA, Freudenreich O, Smith FA, et al, eds. Massachusetts General Hospital Handbook of General Hospital Psychiatry. 7th ed. Elsevier; 2018:83–93.
- Fusunyan M, Praschan N, Fricchione G, et al. Akinetic mutism and Coronavirus disease 2019: a narrative review. J Acad Consult Liaison Psychiatry. 2021;62(6):625–633. PubMed CrossRef
- Geschwind MD. Rapidly progressive dementia. Continuum (Minneap Minn). 2016;22(2 Dementia):510-537.
- Shah SB, Levy-Carrick NC, Steele I, et al. Delirium and Catatonia. In: Silbersweig DA, Safar LT, Daffner KR, eds. Neuropsychiatry and Behavioral Neurology: Principles and Practice. New York: McGraw-Hill; 2021:619–633.
- Hosker C, Ward D. Hypoactive delirium. BMJ. 2017;357:j2047. PubMed CrossRef
- Wilson JE, Mart MF, Cunningham C, et al. Delirium. Nat Rev Dis Primers. 2020;6(1):90. PubMed CrossRef
- Maldonado JR. Delirium pathophysiology: an updated hypothesis of the etiology of acute brain failure. Int J Geriatr Psychiatry. 2018;33(11):1428–1457. PubMed CrossRef
- Jacobson S, Jerrier H. EEG in delirium. Semin Clin Neuropsychiatry. 2000;5(2):86–92. PubMed
- van Montfort SJT, van Dellen E, van den Bosch AMR, et al. Resting-state fMRI reveals network disintegration during delirium. Neuroimage Clin. 2018;20:35–41. PubMed CrossRef
- Numan T, Slooter AJC, van der Kooi AW, et al. Functional connectivity and network analysis during hypoactive delirium and recovery from anesthesia. Clin Neurophysiol. 2017;128(6):914–924. PubMed CrossRef
- Ely EW, Siegel MD, Inouye SK. Delirium in the intensive care unit: an under-recognized syndrome of organ dysfunction. Semin Respir Crit Care Med. 2001;22(2):115–126. PubMed CrossRef
- Devlin JW, Skrobik Y, Gélinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46(9):e825–e873. PubMed CrossRef
- Barnes-Daly MA, Phillips G, Ely EW. Improving hospital survival and reducing brain dysfunction at seven California community hospitals: implementing PAD guidelines via the ABCDEF bundle in 6,064 patients. Crit Care Med. 2017;45(2):171–178. PubMed CrossRef
- Hanison J, Conway D. A multifaceted approach to prevention of delirium on intensive care. BMJ Qual Improv Rep. 2015;4(1):u209656.w4000. PubMed CrossRef
- Moon KJ, Lee SM. The effects of a tailored intensive care unit delirium prevention protocol: a randomized controlled trial. Int J Nurs Stud. 2015;52(9):1423–1432. PubMed CrossRef
- Larsen KA, Kelly SE, Stern TA, et al. Administration of olanzapine to prevent postoperative delirium in elderly joint-replacement patients: a randomized, controlled trial. Psychosomatics. 2010;51(5):409–418. PubMed CrossRef
- Skrobik Y, Duprey MS, Hill NS, et al. Low-dose nocturnal dexmedetomidine prevents ICU delirium. a randomized, placebo-controlled trial. Am J Respir Crit Care Med. 2018;197(9):1147–1156. PubMed CrossRef
- Pandharipande P, Shintani A, Peterson J, et al. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006;104(1):21–26. PubMed CrossRef
- Andersen-Ranberg NC, Poulsen LM, Perner A, et al; AID-ICU Trial Group. Haloperidol for the treatment of delirium in ICU patients. N Engl J Med. 2022;387(26):2425–2435. PubMed CrossRef
- Hu H, Deng W, Yang H, et al. Olanzapine and haloperidol for senile delirium: a randomized controlled observation. Zhongguo Linchuang Kangfu. 2006;10:188–190.
- Devlin JW, Roberts RJ, Fong JJ, et al. Efficacy and safety of quetiapine in critically ill patients with delirium: a prospective, multicenter, randomized, double-blind, placebo-controlled pilot study. Crit Care Med. 2010;38(2):419–427. PubMed CrossRef
- Tahir TA, Eeles E, Karapareddy V, et al. A randomized controlled trial of quetiapine versus placebo in the treatment of delirium. J Psychosom Res. 2010;69(5):485–490. PubMed CrossRef
- Agar MR, Lawlor PG, Quinn S, et al. Efficacy of oral risperidone, haloperidol, or placebo for symptoms of delirium among patients in palliative care: a randomized clinical trial. JAMA Intern Med. 2017;177(1):34–42. PubMed CrossRef
- Seitz DP, Gill SS, van Zyl LT. Antipsychotics in the treatment of delirium: a systematic review. J Clin Psychiatry. 2007;68(1):11–21. PubMed CrossRef
- Boettger S, Friedlander M, Breitbart W, et al. Aripiprazole and haloperidol in the treatment of delirium. Aust N Z J Psychiatry. 2011;45(6):477–482. PubMed CrossRef
- Maness EB, Burk JA, McKenna JT, et al. Role of the locus coeruleus and basal forebrain in arousal and attention. Brain Res Bull. 2022;188:47–58. PubMed CrossRef
- Challman TD, Lipsky JJ. Methylphenidate: its pharmacology and uses. Mayo Clin Proc. 2000;75(7):711–721. PubMed CrossRef
- Minzenberg MJ, Carter CS. Modafinil: a review of neurochemical actions and effects on cognition. Neuropsychopharmacology. 2008;33(7):1477–1502. PubMed CrossRef
- Gagnon B, Low G, Schreier G. Methylphenidate hydrochloride improves cognitive function in patients with advanced cancer and hypoactive delirium: a prospective clinical study. J Psychiatry Neurosci. 2005;30(2):100–107. PubMed
- Lieberman OJ, Lee S, Zabinski J. Donepezil treatment is associated with improved outcomes in critically ill dementia patients via a reduction in delirium. Alzheimers Dement. 2023;19(5):1742–1751. PubMed CrossRef
- Marcantonio ER, Palihnich K, Appleton P, et al. Pilot randomized trial of donepezil hydrochloride for delirium after hip fracture. J Am Geriatr Soc. 2011;59(suppl 2):S282–S288. PubMed CrossRef
- Beach SR, Celano CM, Noseworthy PA, et al. QTc prolongation, torsades de pointes, and psychotropic medications. Psychosomatics. 2013;54(1):1–13. PubMed CrossRef
- Belvederi Murri M, Guaglianone A, Bugliani M, et al. Second-generation antipsychotics and neuroleptic malignant syndrome: systematic review and case report analysis. Drugs R D. 2015;15(1):45–62. PubMed CrossRef
- Zorn SZ. The safety of stimulant medication use in cardiovascular and arrhythmia patients. expert analysis. Am Coll Cardiol. 2015. https://www.acc.org/latest-in-cardiology/articles/2015/04/28/10/06/the-safety-of-stimulant-medication-use-in-cardiovascular-and-arrhythmia-patients
- Maldonado JR. Pathoetiological model of delirium: a comprehensive understanding of the neurobiology of delirium and an evidence-based approach to prevention and treatment. Crit Care Clin. 2008;24(4):789–856, ix. PubMed CrossRef
- Aliev G, Obrenovich ME, Smith MA, et al. Hypoperfusion, mitochondria failure, oxidative stress, and alzheimer disease. J Biomed Biotechnol. 2003;(3):162–163. PubMed CrossRef
- Cotran RS, Robbins SL, Aster JC, et al, eds. Robbins & Cotran Pathologic Basis of Disease. 10th ed. Elsevier; 2021.
- Choi DW, Weiss JH, Koh JY, et al. Glutamate neurotoxicity, calcium, and zinc. Ann N Y Acad Sci. 1989;568(1):219–224. PubMed CrossRef
- Chabner B, Knollmann BC, Goodman LS, et al. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 12th ed. McGraw-Hill Medical; 2011.
- Aracava Y, Pereira EF, Maelicke A, et al. Memantine blocks alpha7* nicotinic acetylcholine receptors more potently than n-methyl-D-aspartate receptors in rat hippocampal neurons. J Pharmacol Exp Ther. 2005;312(3):1195–1205. PubMed CrossRef
- Rühl L, Kuramatsu JB, Sembill JA, et al. Amantadine treatment is associated with improved consciousness in patients with non-traumatic brain injury. J Neurol Neurosurg Psychiatry. 2022;93(6):582–587. PubMed CrossRef
- Giacino JT, Whyte J, Bagiella E, et al. Placebo-controlled trial of amantadine for severe traumatic brain injury. N Engl J Med. 2012;366(9):819–826. PubMed CrossRef
- Siddiqi N, Harrison JK, Clegg A, et al. Interventions for preventing delirium in hospitalised non-ICU patients. Cochrane Database Syst Rev. 2016;3:1465–1858. PubMed CrossRef
- Walker CK, Gales MA. Melatonin receptor agonists for delirium prevention. Ann Pharmacother. 2017;51(1):72–78. PubMed CrossRef
- Khaing K, Nair BR. Melatonin for delirium prevention in hospitalized patients: A systematic review and meta-analysis. J Psychiatr Res. 2021;133:181–190. Published online Dec 13, 2020. PubMed CrossRef
- Al-Aama T, Brymer C, Gutmanis I, et al. Melatonin decreases delirium in elderly patients: a randomized, placebo-controlled trial. Int J Geriatr Psychiatry. 2011;26(7):687–694. PubMed CrossRef
- Hatta K, Kishi Y, Wada K, et al; DELIRIA-J Group. Preventive effects of ramelteon on delirium: a randomized placebo-controlled trial. JAMA Psychiatry. 2014;71(4):397–403. PubMed CrossRef
- Jonghe A, Munster BC, Goslings JC, et al. A randomized, double‐blind controlled trial of melatonin versus placebo in delirium. European Geriatric Medicine 2013 Conference: 9th Congress of the European Union Geriatric Medicine Society. Venice, Italy, 2013:S175–S176.
- Beaucage-Charron J, Rinfret J, Coveney R, et al. Melatonin and Ramelteon for the treatment of delirium: a systematic review and meta-analysis. J Psychosom Res. 2023;170:111345. PubMed CrossRef
- Cecconi M, Evans L, Levy M, et al. Sepsis and septic shock. Lancet. 2018;392(10141):75–87. PubMed CrossRef
- Steiner LA, Siegemund M. Vasoactive agents to improve brain perfusion: pathophysiology and clinical utilization. Curr Opin Crit Care. 2019;25(2):110–116. PubMed CrossRef
- van der Zanden V, Beishuizen SJ, Scholtens RM, et al. The effects of blood transfusion on delirium incidence. J Am Med Dir Assoc. 2016;17(8):748–753. PubMed CrossRef
- Fan YX, Liu FF, Jia M, et al. Comparison of restrictive and liberal transfusion strategy on postoperative delirium in aged patients following total hip replacement: a preliminary study. Arch Gerontol Geriatr. 2014;59(1):181–185. PubMed CrossRef
- Gruber-Baldini AL, Marcantonio E, Orwig D, et al. Delirium outcomes in a randomized trial of blood transfusion thresholds in hospitalized older adults with hip fracture. J Am Geriatr Soc. 2013;61(8):1286–1295. PubMed CrossRef
- Drewry A, Mohr NM. Temperature management in the ICU. Crit Care Med. 2022;50(7):1138–1147. PubMed CrossRef
- van der Kooi AW, Kappen TH, Raijmakers RJ, et al. Temperature variability during delirium in ICU patients: an observational study. PLoS One. 2013;8(10):e78923. PubMed CrossRef
- Wilson JE, Carlson R, Duggan MC, et al; Delirium and Catatonia (DeCat) Prospective Cohort Investigation. Delirium and catatonia in critically ill patients: the delirium and catatonia prospective cohort investigation. Crit Care Med. 2017;45(11):1837–1844. PubMed CrossRef
- Espinoza RT, Kellner CH. Electroconvulsive therapy. N Engl J Med. 2022;386(7):667–672. PubMed CrossRef
- Lloyd JR, Silverman ER, Kugler JL, et al. Electroconvulsive therapy for patients with catatonia: current perspectives. Neuropsychiatr Dis Treat. 2020;16:2191–2208. PubMed CrossRef
- Girard TD, Thompson JL, Pandharipande PP, et al. Clinical phenotypes of delirium during critical illness and severity of subsequent long-term cognitive impairment: a prospective cohort study. Lancet Respir Med. 2018;6(3):213–222. PubMed CrossRef
This PDF is free for all visitors!