Increased Self-Report of Obsessive-Compulsive Behaviors Among Hemodialysis Patients: A Case-Control Study
Background: Patients with end-stage renal insufficiency undergoing hemodialysis show important psychiatric morbidity, particularly increased depression and anxiety. Obsessive-compulsive symptoms, however, are much less frequently investigated. The purpose of the present study was thus to assess obsessive-compulsive symptoms in hemodialysis patients.
Method: Patients treated at an outpatient hospital hemodialysis unit (July 2007) were compared with controls on scores on the Maudsley Obsessional-Compulsive Inventory (MOCI) and its checking, cleaning, slowness, and doubting components as well as on measures of emotional (Beck Depression Inventory-Fast Screen), anxiety (Beck Anxiety Inventory), and cognitive (Trail Making Test) status. Student t tests, analyses of covariance, or nonparametric tests were used. Correlations were applied between behavioral outcomes and demographic, clinical, and laboratory data of patients.
Results: Patients showed more obsessive traits than controls on the MOCI total score (P < .001) and on the checking, cleaning, and doubting subscales. Significant differences between groups occurred also in Beck Depression and Anxiety Inventories (P ≤ .001). The MOCI total score did not correlate with marital status, education level, duration of hemodialysis, or the other psychological instrument scores in patients. By contrast, the MOCI total score was associated with the level of creatinine, and it showed an inverse correlation with the urea reduction ratio in patients (P < .05).
Conclusions: Obsessive-compulsive symptoms may constitute an important aspect of the psychiatric profile of patients undergoing hemodialysis. Potential interpretation involves disease- and treatment-associated factors or adaptive responses to emergence of otherwise uncontrollable stress.
Prim Care Companion J Clin Psychiatry
2010;12(3):e1-e6
© Copyright 2010 Physicians Postgraduate Press, Inc.
Submitted: May 24, 2009; accepted September 9, 2009.
Published online: May 6, 2010 (doi:10.4088/PCC.09m00841blu).
Corresponding author: Epameinondas Lyros, MD, PhD, Department of Neurology, Neuropsychology Section, University of Patras Medical School, 26500, Rion, Patras, Greece ([email protected]).
Obsessive-compulsive disorder (OCD) is characterized by recurrent, intrusive, and inappropriate thoughts or impulses (obsessions) as well as repetitive, ritualistic, or irrational behaviors (compulsions) aimed at reducing the distress and anxiety that are often caused by preceding obsessions.1 Although the etiology of the idiopathic syndrome remains obscure, interestingly, a number of situations with development of secondary obsessive-compulsive symptoms (OCS) have been reported in the literature,2 and thus biologic factors are increasingly believed to play important roles.
Patients with chronic renal disease undergoing hemodialysis treatment reportedly exhibit significant psychiatric morbidity, with depression and anxiety being the most common problems.3,4 Little is known, however, of the obsessive-compulsive effect on this patient population. Given that OCD is among the most common and debilitating diseases,5 it would be of interest to assess the presence of OCS in hemodialysis patients. A further motivation was provided by our observations of frequent vagrant and inflexible behaviors in patients, such as persisting movement patterns or, for example, refusal to change seating in the hemodialysis room, which we wanted to investigate systematically. Thus, in the present study we set out to assess the presence, type, and severity of OCS in patients treated with hemodialysis as compared to controls and to investigate possible clinical, psychological, and sociodemographic correlates of these symptoms.
METHOD
The study used a case-control design to assess obsessive-compulsive, depressive, and anxiety symptoms in hemodialysis patients by the use of self-report questionnaires. The study was approved by the local ethics committee, and all participants provided written informed consent for participation.
Clinical Points
- Obsessive-compulsive symptoms may constitute an important aspect of the psychiatric profile of patients undergoing hemodialysis treatment.
- The severity of obsessive-compulsive symptoms seems to be related to the degree of the metabolic abnormalities associated with kidney disease or to adequacy of dialysis.
Subjects
We recruited a convenience sample of 36 end-stage renal disease (ESRD) patients enrolled during July 2007 in hemodialysis therapy at the outpatient Hemodialysis Unit of the General Hospital of Amfissa, Amfissa, Greece, situated in an urban area. Eligible participants had no reported history of any psychiatric or neurologic disease and had not received treatment for any psychiatric or neurologic disease. To exclude the possibility of clinically important premorbid psychiatric status in patients, we specifically inquired for prolonged periods of sadness, thoughts of self-guilt or self-harm, exaggerated unexplained fear, dangerous behavior, and isolation that may have been experienced not only following but also prior to the onset of renal disease and/or hemodialysis treatment. The corroboration of an informant was obtained if possible. We tried to establish whether patients had ever been evaluated or treated by a psychiatrist, psychologist, or social worker; had been prescribed "psychiatric medications"; or had psychotherapy. Furthermore, eligible patients did not suffer from severe vision problems. Twenty-nine out of 36 patients returned the questionnaires, a response rate of 81%. Respondents did not differ from nonrespondents with respect to duration of hemodialysis treatment and main laboratory measurements. Four individuals’ responses were excluded due to incompleteness or because their cognitive performance fell below the set cutoff score for severe impairment (see below); thus, a final total of 25 patients was included in the analysis.
The study also included 20 healthy volunteers from the community matched by age, sex, and educational level to the group of 25 patients. Healthy volunteers showed values of routine blood laboratory measurements and urine analyses within the normal range. Further, they did not have any major medical condition as judged by history, clinical interview, and basic clinical examination. In order to exclude individuals with major psychiatric conditions, the psychiatric status of controls was reviewed as described above for patients.
Clinical and Laboratory Data of Subjects
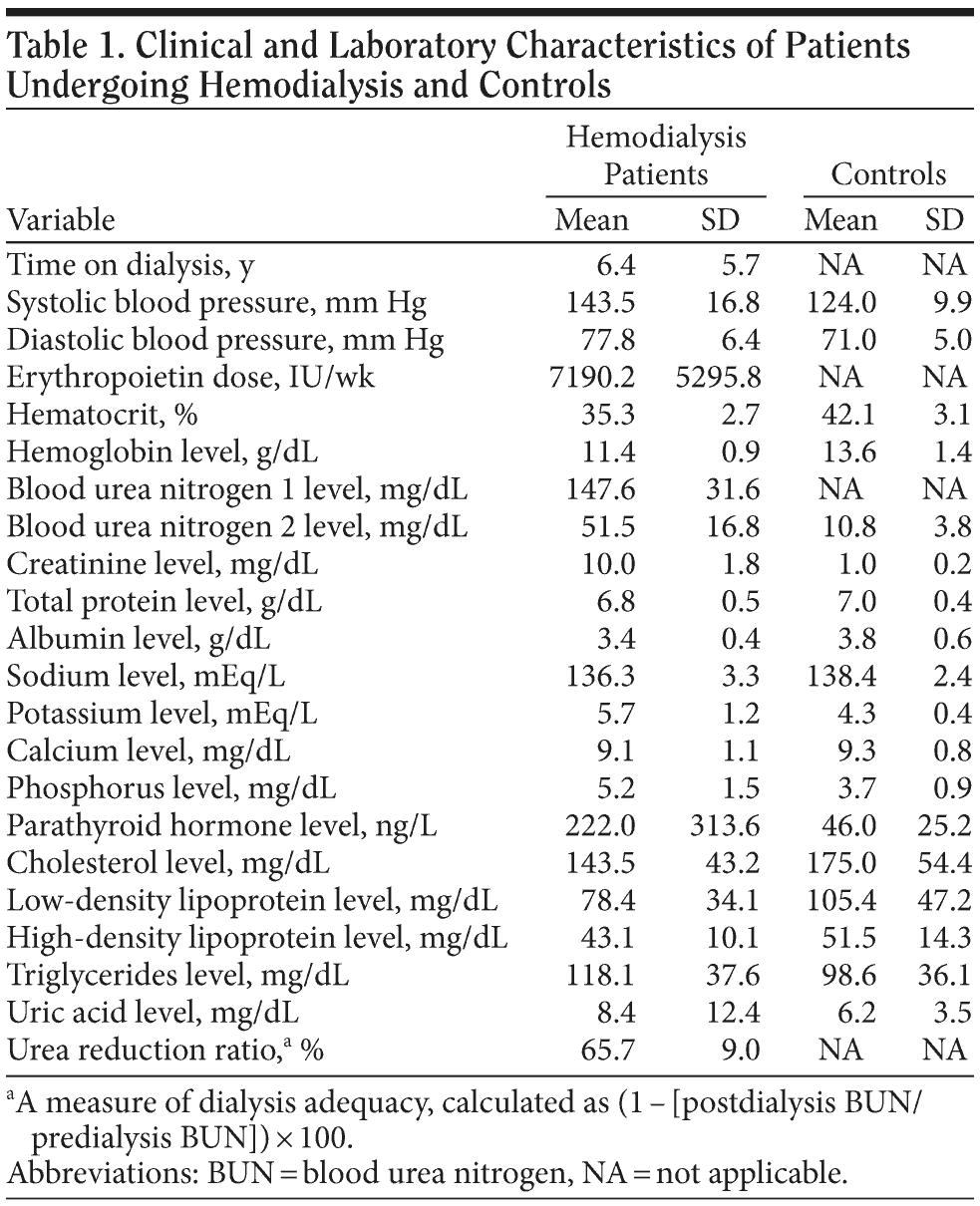
Renal failure in patients was attributed to arterial hypertension in 6 cases, diabetes mellitus in 2 cases, glomerulonephritis in 2 cases, polycystic kidney disease in 1 case, and Alport syndrome in 2 cases, whereas in the rest of the patients, the origin remained unknown. Patients received 3 hemodialysis sessions per week. Medication use was recorded for all study subjects. For patients, laboratory values closest in time to psychological assessment were also recorded. Important comorbidity in patients included coronary artery disease (7 cases), congestive heart failure (5 cases), and diabetes mellitus (8 cases), while no patients had experienced clinically overt stroke. Selected laboratory measurements in patients and controls are shown in Table 1.
Instruments
In order to assess OCS, we administered the Maudsley Obsessional-Compulsive Inventory (MOCI), a patient-completed questionnaire of most common obsessive-compulsive symptoms.6 The MOCI comprises 30 sentences that a principal component analysis has classified into 4 factors: checking (9 items), cleaning (11 items), doubting (7 items), and slowness (7 items). Subjects are instructed to respond with either true or false. The inventory includes both affirmative and negative sentences. Together with MOCI, other psychological instruments were also administered to provide parallel estimates of affective (Beck Depression Inventory-Fast Screen [BDI-FS]7), anxiety (Beck Anxiety Inventory [BAI]8), and cognitive (Clock Drawing Test9 and Trail Making Test [TMT]10) status, since hemodialysis patients are known to present high rates of depression, anxiety, and cognitive dysfunction.3,4,11,12 The employed instruments have been adapted to the Greek language according to standard procedures, including independent forward translation from English into Greek by 2 health professionals, followed by independent backward translation of the Greek version into English. Final versions have been assessed for semantic equivalence to the original, and issues of clarity have been addressed. During an initial validation phase, the MOCI self-administered instrument score correlated well with the clinician-rated Yale-Brown Obsessive-Compulsive Scale (YBOCS)13 in subjects of the target hemodialysis population and in nonhemodialysis subjects with normal renal function. We used the BDI-FS instead of the full scale to focus only on cognitive and affective symptoms of depression and thereby avoid overlap with somatic symptoms caused by renal disease. The Clock Drawing Test served as a gross assessment of cognitive status, and it was scored according to the method proposed by Shua-Haim et al,9 awarding a maximum of 6 points. We defined a cutoff score of 4 as determining cognitively intact participants.
Statistical Analysis
Internal consistency of the 4 subscales of MOCI and the entire test were found to be satisfactory in both the control group and the patient group yielding Cronbach α coefficients between 0.63 and 0.85. We also conducted a confirmatory factorial analysis on the responses of our patients and controls, the results of which corresponded well to the 4-component solution of the original Hodgson and Rachman study.6
Continuous variables were compared across groups with Student t tests or, in the case of nonnormally distributed variables, with nonparametric tests. Analysis of covariance was used to simultaneously adjust for confounding variables. Categorical variables were compared using the χ2 test. Correlations between psychological measures and demographic and clinical variables, including laboratory measurements, were performed with Spearman rank correlations or Pearson correlation coefficients, as appropriate. Partial correlations were run to control for potentially secondary effects. Two-sided P values less than .05 were considered to be statistically significant. Analyses were conducted using the SPSS version 12 software (SPSS Inc, Chicago, Illinois). Statistical power was calculated by the online application (http://www.dssresearch.com/toolkit/spcalc/power_p1.asp).
RESULTS
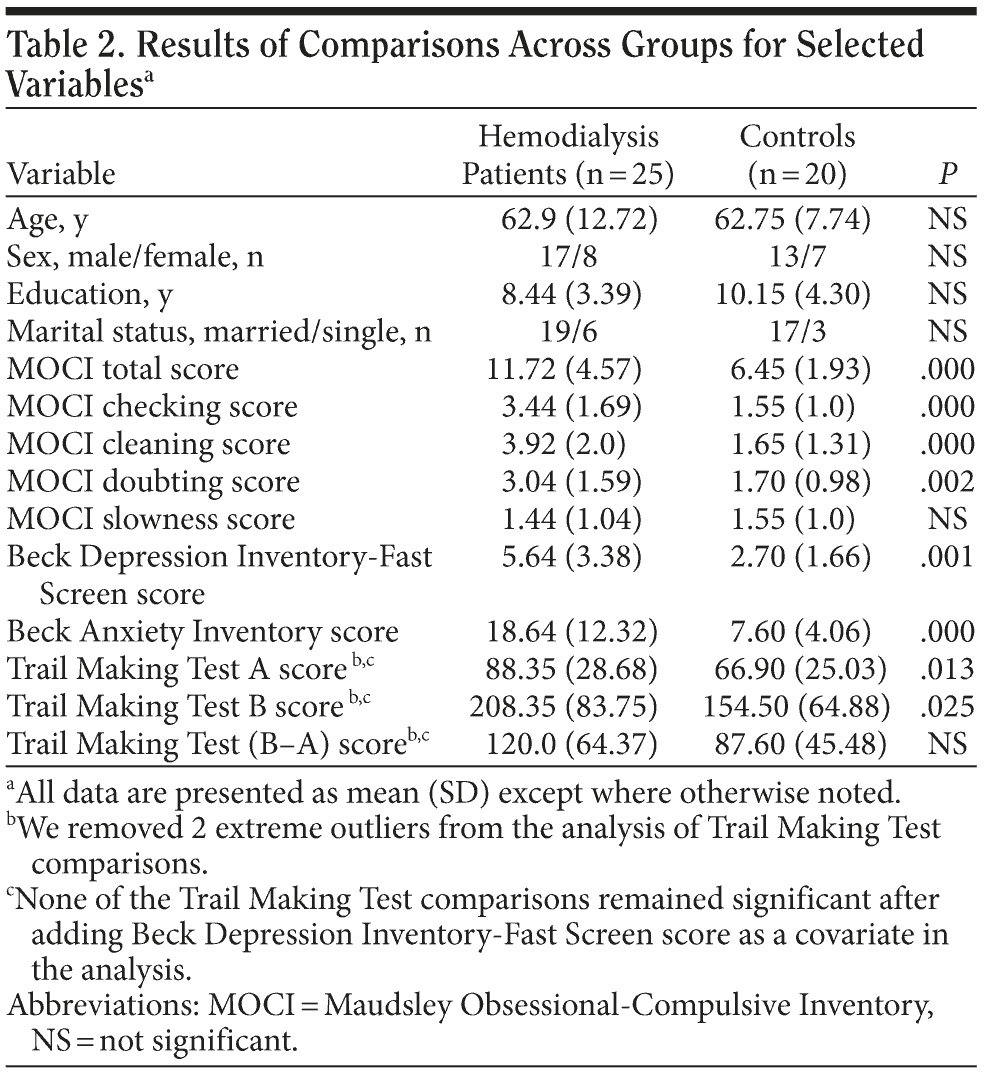
Results of comparisons across groups for selected variables are shown in Table 2. Patients showed significantly more obsessive traits than healthy controls on MOCI total score and in the checking, cleaning, and doubting subscales. Obsessional ruminations within the checking subscale were particularly prominent, affecting the majority of patients. Significant differences between groups occurred also on BDI and BAI scores. Unadjusted mean TMT scores were significantly worse in dialysis patients than in controls. However, none of the TMT comparisons remained significant after adding BDI-FS score as a covariate in the analysis.
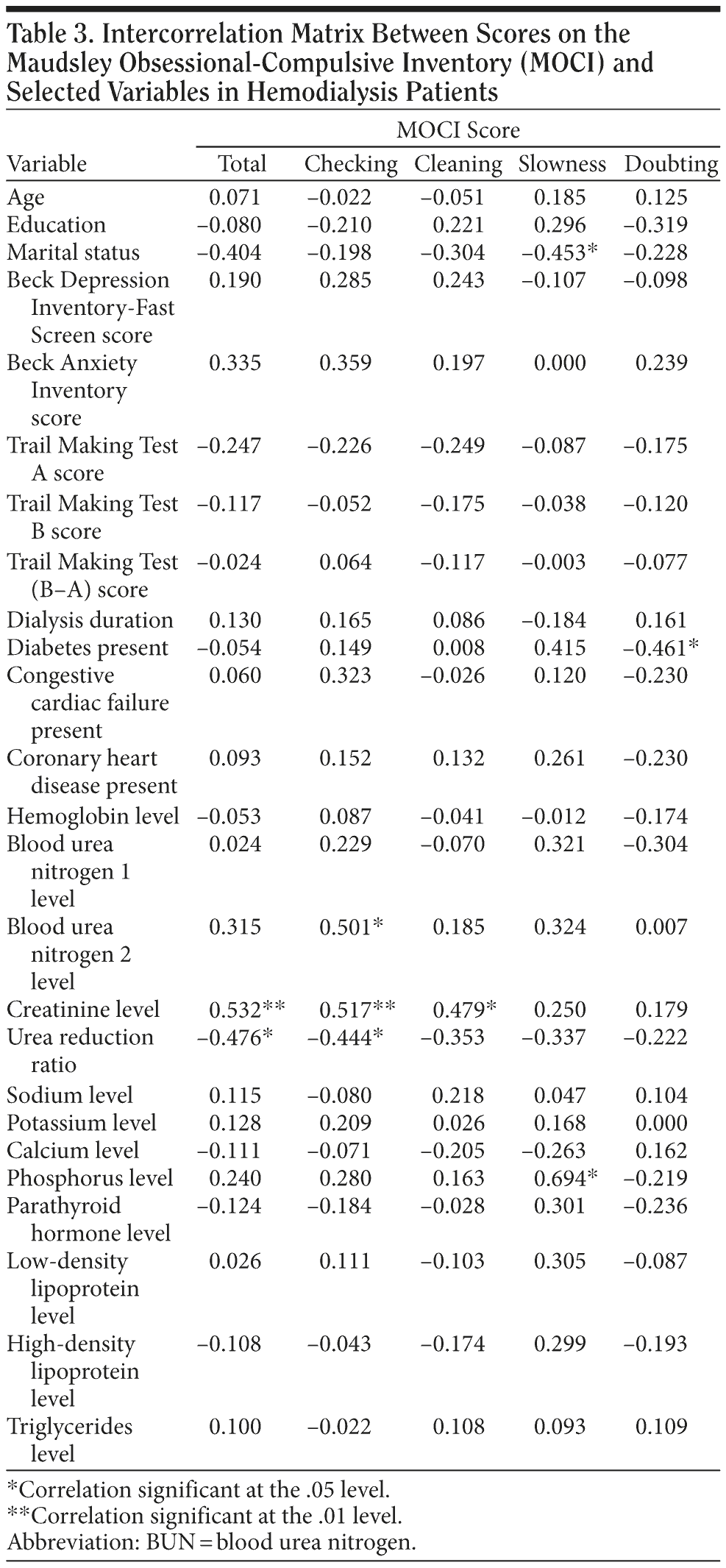
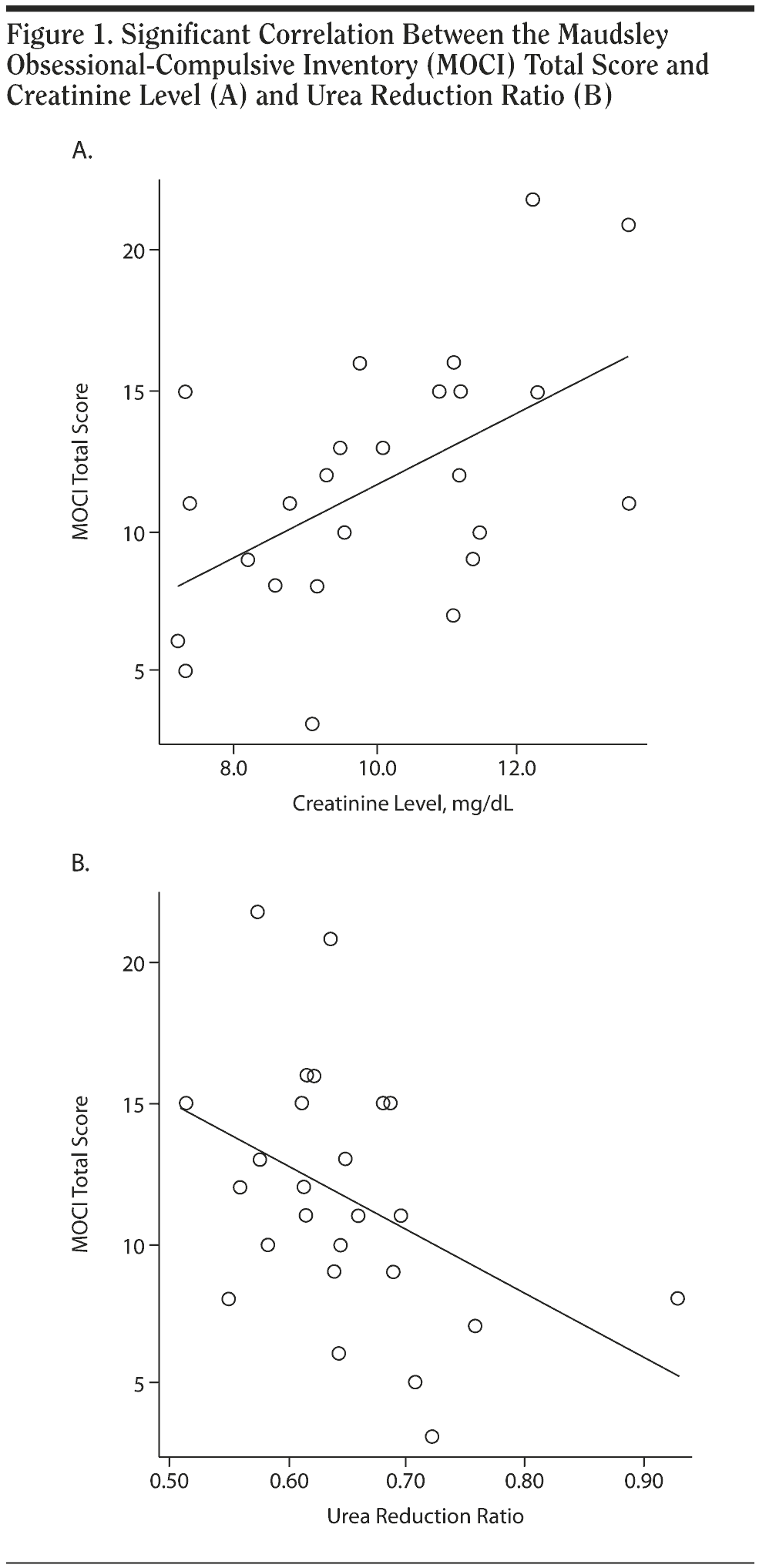
Correlation coefficients between MOCI scores and other parameters in patients are presented in Table 3. The MOCI total score did not correlate with marital status, education level, duration of hemodialysis, or other psychological instrument scores in patients. By contrast, the MOCI total score was associated with the level of creatinine (r = 0.532, P = .006) and showed an inverse correlation with the urea reduction ratio (URR) (r = -0.476, P = .016) in patients. Correlations between MOCI total score and creatinine level and between MOCI total score and URR remained significant even after controlling for duration of hemodialysis. Furthermore, significant correlations of MOCI with these parameters loaded primarily on the checking subscale and secondarily on the cleaning subscale. Checking subscore correlated with levels of creatinine (r = 0.517, P = .008), URR (r = -0.444, P = .026), and postdialysis urea (r = 0.501, P = .011). Significant correlations are shown in Figure 1 in the form of scatterplots. Despite the significant association between MOCI score and serum creatinine levels in patients, no significant correlations were detected with estimates of residual renal function in either patients or controls. No significant associations were found between MOCI total score and the other psychiatric ratings or cognitive scores and other clinical factors, including important comorbid conditions and laboratory values. Marginally significant correlations of MOCI slowness and doubting subscale scores occurred, however, with marital status and diabetes, respectively (.04 < P < .05). Hyperphosphatemia in patients also correlated with increased slowness subscale scores (P = .019).
The total obsessionality score and the 4 factor scores did not correlate with BDI-FS and BAI scores. On the other hand, BDI-FS scores demonstrated a significant correlation with BAI scores (r = 0.616, P = .001). As with the BDI, some of the items of the BAI measure physical symptoms of anxiety that overlap with symptoms of uremia. Therefore, these items were clustered, and cognitive anxiety was separated from somatic anxiety. No different results were obtained when analyses were repeated with the cognitive anxiety instead of the full anxiety scale.
DISCUSSION
Our results suggest that OCS constitute an important aspect of the psychiatric profile of patients undergoing hemodialysis treatment. These psychiatric symptoms may be an unrecognized problem in the hemodialysis population, perhaps neglected and untreated in the face of other medical priorities.
End-stage renal disease represents a paradigm of generalized metabolic deficit that also involves the brain. Thus, in one respect, the relatively high obsessionality ratings in hemodialysis patients could have an organic substrate. Increased obsessive-compulsive symptomatology has been associated with organic brain damage.2 Neural correlates of OCS may be selectively sensitive to high serum levels of toxic substances retained in uremia’s passing the blood-brain barrier and causing damage to crucial brain structures or exerting functional influences on neurotransmission. The fact that the severity of OCS in patients was related to the level of creatinine and to the URR during hemodialysis indicates that these behavioral symptoms may rely on the degree of these metabolic abnormalities associated with kidney disease or adequacy of treatment. Nevertheless, no direct causal relationship can be established, and other factors could simultaneously account for both poorer response to dialysis and higher obsessive-compulsive symptomatology. Higher psychiatric morbidity in patients admitted to chronic hemodialysis programs may reflect the high burden of comorbid conditions, although no correlations were found, at least with diabetes and cardiovascular disease. In this respect, one could well hypothesize the converse relationship, ie, that the initial presence of OCS may have affected clinical parameters, in which case increased pathological preoccupations would lead to neglect of self-care and reduced compliance with dietary guidance and prescribed treatment.
Assuming the existence of a biologic contribution to increased OCS in uremic patients, it is important that future research assesses psychiatric symptoms in patients with both earlier stages of chronic kidney disease and kidney failure before and after introduction of hemodialysis to elicit the impact of treatment, if any. Structural brain abnormalities and high loads of cerebrovascular lesions have been associated with hemodialysis treatment itself.14,15 The significant correlation, however, of lower URRs with higher MOCI scores indicates that reported obsessive-compulsive behavior is rather linked to ESRD or underdialysis than to dialysis itself. It is still possible that the findings of the current study may reflect a more general change seen in chronic disease and not restricted to ESRD or dialysis.
Psychiatric symptoms in patients undergoing dialysis can alternatively be regarded as dynamic behavioral adaptations representing patients’ attempts to cope with disease and threats of disability and death. Behavioral rigidity should, on this perspective, be appreciated as an indication of a strong need for control and autonomy.
Our results of high depressive symptomatology in hemodialysis patients are in line with previous research.3,4 Cognitive dysfunction is a well-described feature in the dialysis population.11,12 In our study, however, worse performance in visuospatial attention and psychomotor speed was rather attributed to the higher level of affective disturbance in hemodialysis subjects compared to controls. Despite the frequent co-occurrence of OCD and anxiety or affective disorders,16 the MOCI score was related to neither anxiety nor depressive symptoms in our hemodialysis patients. Our study had 84% power to detect a significant correlation that had a coefficient of 0.5 at a single-sided α = .05 level, assuming a same-directional correlation, or 75% if double-sided. Our study was not adequately powered to detect more modest, yet significant, correlations between MOCI score and BDI-FS or BAI scores. The absence of such significant correlations could, on the other hand, be explained by assuming that excess anxiety or depression diathesis could be counterbalanced when alternatively channeled into the form of obsessive-compulsive behavior in an effort to handle the expression of the former situations.
In justifying the use of the particular questionnaire used in our study, self-reported measures such as the MOCI exhibit certain advantages in OCD assessment over physician-rated instruments such as the YBOCS.13 Self-reported measures can be completed quickly without challenging participants’ patience, and people may feel more comfortable completing them independently rather than engaging in a discussion with the clinician. Since OCD patients are said to be secretive about their disorder,17 the use of self-reported measures may be preferable to guard against the underreporting of symptoms that is sometimes observed during a clinician-administered interview. Moreover, self-reported inventories are free of the bias introduced by the personal experience of the rater and could, in this view, be regarded as more objective. Furthermore, one major advantage of MOCI is that it controls for an acquiescent response set.6 It examines various types of OCS by means of an internal control of items with repeated content. We cannot exclude the possibility, however, that some of the participants had difficulties in understanding the format of the questionnaire or regarded the evaluation of their responses as embarrassing to them. Moreover, we did not use structured diagnostic interviews to supplement the assessment of the participants in our study and did not apply Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, criteria.1 The MOCI does not by any means serve to diagnose OCD, so it cannot be concluded from our study that fully developed OCD has a higher prevalence among hemodialysis patients.
Within this framework, our study serves mainly as a preliminary investigation of the issue, and the findings warrant further replication in larger, multicenter samples. Our results have important clinical implications encouraging further research for potential use of screening instruments in hemodialysis settings to detect clinically significant obsessive-compulsive behavior requiring psychiatric consultation and treatment. It would also be of importance to evaluate the impact of increased OCS on the quality of life of patients, already diminished by significant other morbidities.
Author affiliations: Hemodialysis Unit, General Hospital of Amfissa, Amfissa, Greece (Drs Lyros, Dendias, Siavelis, and Triantafyllou); and Department of Neurology, Neuropsychology Section, University of Patras, Patras, Greece (Drs Lyros, Messinis, and Papathanasopoulos).
Potential conflicts of interest: None reported.
Funding/support: None reported.
Previous presentation: Findings from this study were presented in poster and in abstract form at the 2nd European Federation of Neuropsychiatry Congress; November 22-24, 2007; Florence, Italy.
REFERENCES
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. Washington, DC: American Psychiatric Association; 1994.
2. Coetzer BR. Obsessive-compulsive disorder following brain injury: a review. Int J Psychiatry Med. 2004;34(4):363-377. doi:10.2190/XENN-NNWT-7N2K-R26A PubMed
3. Cukor D, Coplan J, Brown C, et al. Depression and anxiety in urban hemodialysis patients. Clin J Am Soc Nephrol. 2007;2(3):484-490. doi:10.2215/CJN.00040107 PubMed
4. Kimmel PL. Depression in patients with chronic renal disease: what we know and what we need to know. J Psychosom Res. 2002;53(4):951-956. doi:10.1016/S0022-3999(02)00310-0 PubMed
5. Murray CJL, Lopez AD. The Global Burden of Disease. Boston, MA: Harvard University Press; 1996.
6. Hodgson RJ, Rachman S. Obsessional-compulsive complaints. Behav Res Ther. 1977;15(5):389-395. doi:10.1016/0005-7967(77)90042-0 PubMed
7. Beck AT, Steer RA, Brown GK. BDI-Fast Screen for Medical Patients: Manual. San Antonio, TX: Harcourt Assessment/The Psychological Corporation; 2000.
8. Beck AT, Epstein N, Brown G, et al. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56(6):893-897. doi:10.1037/0022-006X.56.6.893 PubMed
9. Shua-Haim J, Koppuzha G, Gross J. A simple scoring system for clock drawing in patients with Alzheimer’s disease. J Am Geriatr Soc. 1996;44(3):335. PubMed
10. Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. 4th ed. New York, NY: Oxford University Press; 2004.
11. Kurella M, Chertow GM, Luan J, et al. Cognitive impairment in chronic kidney disease. J Am Geriatr Soc. 2004;52(11):1863-1869. doi:10.1111/j.1532-5415.2004.52508.x PubMed
12. Murray AM, Tupper DE, Knopman DS, et al. Cognitive impairment in hemodialysis patients is common. Neurology. 2006;67(2):216-223. doi:10.1212/01.wnl.0000225182.15532.40 PubMed
13. Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive-Compulsive Scale, I: development, use and reliability. Arch Gen Psychiatry. 1989;46(11):1006-1011. PubMed
14. Pereira AA, Weiner DE, Scott T, et al. Cognitive function in dialysis patients. Am J Kidney Dis. 2005;45(3):448-462. doi:10.1053/j.ajkd.2004.10.024 PubMed
15. Fazekas G, Fazekas F, Schmidt R, et al. Brain MRI findings and cognitive impairment in patients undergoing chronic hemodialysis treatment. J Neurol Sci. 1995;134(1-2):83-88. doi:10.1016/0022-510X(95)00226-7 PubMed
16. T×¼kel R, Polat A, Ozdemir O, et al. Comorbid conditions in obsessive-compulsive disorder. Compr Psychiatry. 2002;43(3):204-209. doi:10.1053/comp.2002.32355 PubMed
17. Jenike MA. Obsessive-compulsive and related disorders: a hidden epidemic. N Engl J Med. 1989;321(8):539-541. PubMed