Prim Care Companion CNS Disord 2022;24(4):21cr03117
To cite: Midi I, Okay BB, Cetin SZ, et al. Intractable seizure with hypoglycemia: a mysterious retroperitoneal fıbrosarcoma. Prim Care Companion CNS Disord. 2022;24(4):21cr03117.
To share: https://doi.org/10.4088/PCC.21cr03117
© 2022 Physicians Postgraduate Press, Inc.
aDepartment of Neurology, School of Medicine, Marmara University, Istanbul, Turkey
bSchool of Medicine, Marmara University, Istanbul, Turkey
cDepartment of Radiology, School of Medicine, Marmara University, Istanbul, Turkey
*Corresponding author: Ipek Midi, Prof Dr, Department of Neurology, Marmara University, School of Medicine, Çarşı Mah, Tarihi Ayazma Cad, Ayazma Park Apt No: 27 Daire, 18 kat: 8 Kartal, Istanbul, Turkey 34876 ([email protected]).
We describe a male patient with an abdominal fibrosarcoma who experienced recurrent episodes of agitation and convulsions related to hypoglycemic attacks. Many conditions may mimic or cause epileptic seizures, one of which is hypoglycemia. Hypoglycemia can lead to acute disorders of consciousness, epilepsy, and psychosis. This situation can be caused by an insulinoma or fibrosarcoma, which should be considered in the differential diagnosis of intractable seizures.
Case Report
A 49-year-old man was admitted to the emergency department (ED) with severe agitation and repeated seizures with hyperkinetic-like features. On admission, he was unresponsive. Diazepam was administered to the patient to control the seizures and his agitated condition. In the following period, the same attacks occurred every 30 minutes and lasted 15 minutes before stopping. It was difficult to control the patient’s movements even with 4 or 5 people. Intravenous diphenylhydantoin (18 mg/kg) was administered, and levetiracetam and haloperidol infusions were also given, but the patient’s clinical situation did not change. We decided to monitor the patient in the intensive care unit (ICU) with the administration of midazolam infusion.
We learned that the patient’s first attacks occurred at night, especially between 3:00 and 5:00 am, characterized by uncontrolled movements of the extremities without a stereotype and unresponsiveness. Seizures had started to occur with increased frequency over the past 2 months. The duration of the attacks was generally 15 or 20 minutes, but the patient took 30 minutes to recover after each episode.
An electroencephalograph (EEG) recording taken in another hospital before admission to our ED was within normal limits. A cardiac evaluation with 24-hour Holter monitoring was normal, and echocardiogram findings showed pulmonary hypertension. The previous history of the patient included trauma when he was 8 years old. There was no epilepsy in his family history.
Cranial magnetic resonance imaging showed encephalomalacia in the temporal lobe, which was related to the childhood trauma, and mega cisterna magna. Diffusion-weighted imaging revealed bilateral frontal lobe diffusion restriction, which may have been related to the seizure activity. To address the patient’s agitation, haloperidol and quetiapine were added to the treatment. When he was being monitored in the ICU, the patient extubated himself more than once because of uncontrolled agitation. The midazolam infusion (45 mg/kg) was tapered with the guidance of EEG recordings.
The pressure of the lumbar puncture (LP) was 260 mm H2O, the cerebrospinal fluid (CSF) protein level was 68 mg/dL, and the glucose level was 8.7 mg/dL; the serum glucose level for the same period was 22 mg/dL. LP was repeated, and the values for CSF glucose and protein were 86 mg/dL and 48 mg/dL, respectively.
During the physical examination, gynecomastia was noted, and the patient was consulted by endocrine doctors. C-peptide and insulin levels were investigated, but the results were within normal levels.
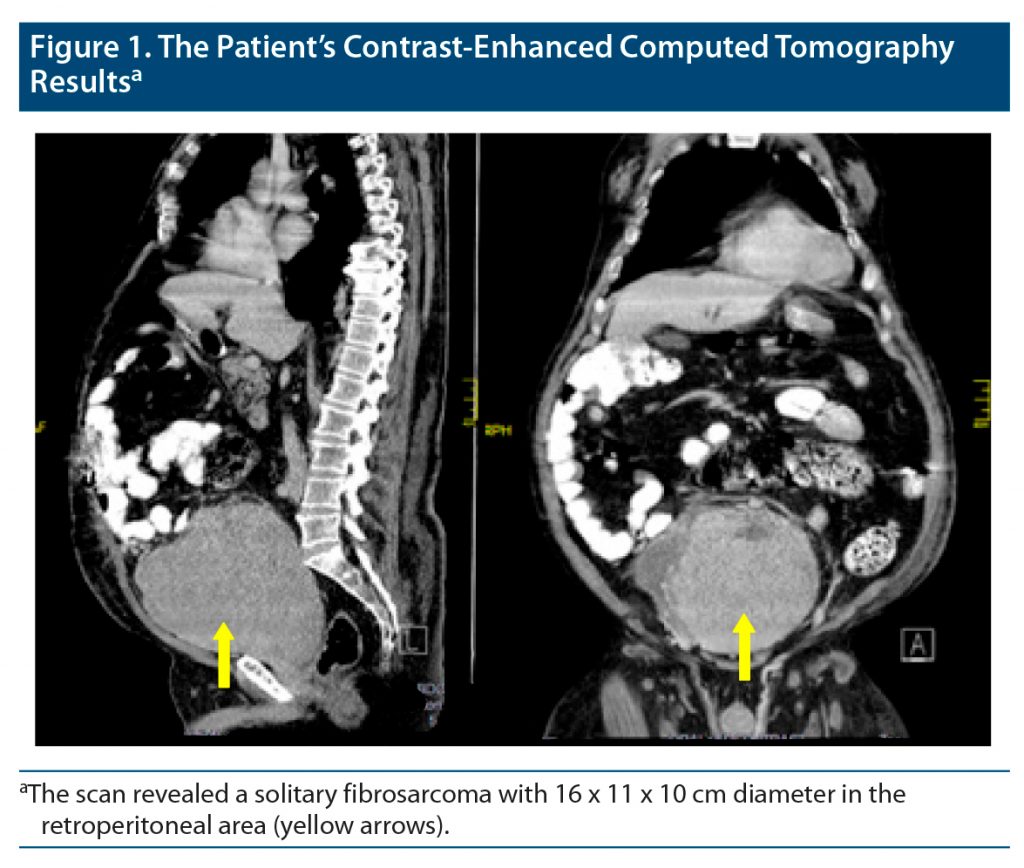
The first LP glucose level and the night measurements of glucose in the ICU showed that the patient had severe hypoglycemia during some periods. We decided to search for possible intraabdominal tumors as the underlying pathology, and abdominal computed tomography was performed. Radiologists reported that there was a 16 x 11 x 10 cm mass in the retroperitoneal area (Figure 1). The pathology of the mass was consistent with vimentin-positive high-grade fibrosarcoma with classic type. We searched for metastatic lesions in any other part of the body, but none were found.
Discussion
Misdiagnosis and delay in treatment are common in patients with hypoglycemia. This condition can lead to permanent neurologic deficits or fatal coma. Hypoglycemia can be caused by mass lesions such as insulinomas or fibrosarcomas. Fibrosarcomas originate from mesenchymal cells.1 These tumors can be primary or secondary and can show characteristics of malignancy. Extrapancreatic tumors are generally related to hypoglycemia.
Secondary hypoglycemia related with mesenchymal tumors has been reported in gastrointestinal cancers. To understand the mechanism of repeated hypoglycemia, we must know the biochemistry of insulin-like growth factor (IGF). The IGF system comprises ligands and receptors.2,3 Although insulin, IGF-I, and IGF-II have their specific receptors, the ligands bind with varying affinity to other receptors that share 50% amino acid homology. Further, 70% to 80% of circulating IGF is normally bound to IGF-binding protein-3 (IGFBP-3), which prevents hypoglycemia. Some tumors such as non-islet cell tumors may overproduce large amounts of IGF-II. IGFBP-3 receptors are insufficient to bind this excess amount of IGF-II.4–6 This mechanism of repeated hypoglycemia can be confirmed through elevated IGF-II levels, serum insulin levels, C-peptide, and growth hormone concentrations, and the clinical situation of the patient will be consistent with hypoglycemia.
After surgically removing the mass, the clinical condition returns to normal, and patients become seizure free. Although our patient’s C-peptide and insulin levels were within normal limits, this may be explained by the samples not being taken in a hypoglycemic period. After the operation, the patient’s clinical condition subsequently deteriorated. The tumor recurred 5 months later, and the patient died 8 months after the operation. Abdominal mass lesions are a rare cause of hypoglycemia, but these patients are generally admitted to emergency units, and these lesions should be taken into consideration as an underlying pathology.
Published online: July 28, 2022.
Relevant financial relationships: None.
Funding/support: None.
Patient consent: Informed consent was obtained to publish the case report, and information has been de-identified to protect anonymity.
References (6)

- Antonescu CR, Erlandson RA, Huvos AG. Primary fibrosarcoma and malignant fibrous histiocytoma of bone–a comparative ultrastructural study: evidence of a spectrum of fibroblastic differentiation. Ultrastruct Pathol. 2000;24(2):83–91. PubMed CrossRef
- LeRoith D, Roberts CT Jr. The insulin-like growth factor system and cancer. Cancer Lett. 2003;195(2):127–137. PubMed CrossRef
- Zapf J, Futo E, Peter M, et al. Can “big” insulin-like growth factor II in serum of tumor patients account for the development of extrapancreatic tumor hypoglycemia? J Clin Invest. 1992;90(6):2574–2584. PubMed CrossRef
- Fukuda I, Hizuka N, Ishikawa Y, et al. Clinical features of insulin-like growth factor-II producing non-islet-cell tumor hypoglycemia. Growth Horm IGF Res. 2006;16(4):211–216. PubMed CrossRef
- Cotterill AM, Holly JM, Davies SC, et al. The insulin-like growth factors and their binding proteins in a case of non-islet-cell tumor-associated hypoglycemia. J Endocrinol. 1991;131(2):303–311. PubMed CrossRef
- Alkemade GM, Bakker M, Rikhof B, et al. Hypoglycemia in a patient with a big “big”-IGF-II-producing tumor. J Clin Endocrinol Metab. 2013;98(8):3113–3114. PubMed CrossRef
Enjoy this premium PDF as part of your membership benefits!