Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders
Case Conference
The Banner Alzheimer’s Institute Case Conference is a weekly event in which physicians and staff discuss challenging and/or teaching cases of patients seen at the Institute’s Memory Disorders Clinic. These conferences are attended by a multidisciplinary group that includes Banner Alzheimer’s Institute dementia specialists, community physicians (internal medicine, family medicine, and radiology), physician assistants, social workers, nurses, medical students, residents, and fellows.
BANNER ALZHEIMER’ S INSTITUTE
The Banner Alzheimer’s Institute located in Phoenix, Arizona, has an unusually ambitious mission: to end Alzheimer’s disease without losing a generation, set a new standard of care for patients and families, and forge a model of collaboration in biomedical research. The Institute provides high-level care and treatment for patients affected by Alzheimer’s disease, dementia, and related disorders. In addition, the Institute offers extensive support services for families and many unique and rewarding research opportunities.
Prim Care Companion CNS Disord 2013;15(4):doi:10.4088/PCC.13alz01559
© Copyright 2013 Physicians Postgraduate Press, Inc.
Received: July 17, 2013; accepted July 17, 2013.
Published online: August 29, 2013.
AUTHORS
Roy Yaari, MD, MAS, a neurologist, is associate director of the Stead Family Memory Clinic of Banner Alzheimer’s Institute and a clinical professor of neurology at the College of Medicine, University of Arizona, Phoenix.
Anna D. Burke, MD, is a geriatric psychiatrist and dementia specialist at the Memory Disorders Clinic of Banner Alzheimer’s Institute.
Helle Brand, PA, is a physician assistant at the Stead Family Memory Clinic of Banner Alzheimer’s Institute.
James D. Seward, PhD, ABPP, is a clinical neuropsychologist at Banner Alzheimer’s Institute.
Pierre N. Tariot, MD, a geriatric psychiatrist, is director of Banner Alzheimer’s Institute and a research professor of psychiatry at the College of Medicine, University of Arizona, Phoenix.
Corresponding author: Roy Yaari, MD, MAS, Banner Alzheimer’s Institute, 901 E Willetta St, Phoenix, AZ 85006 ([email protected]).
CME Background
Original material is selected for credit designation based on an assessment of the educational needs of CME participants, with the purpose of providing readers with a curriculum of CME activities on a variety of topics from volume to volume. This special series of case reports about dementia was deemed valuable for educational purposes by the Publisher, Editor in Chief, and CME Institute Staff. Activities are planned using a process that links identified needs with desired results.
CME Objective
After studying this case, you should be able to:
- Diagnose and use appropriate interventions for an 84-year-old woman with cognitive decline, depression, and insomnia
Accreditation Statement
The CME Institute of Physicians Postgraduate Press, Inc., is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Credit Designation
The CME Institute of Physicians Postgraduate Press, Inc., designates this journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Creditâ„¢. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Note: The American Academy of Physician Assistants (AAPA) accepts certificates of participation for educational activities certified for AMA PRA Category 1 Creditâ„¢ from organizations accredited by ACCME or a recognized state medical society. Physician assistants may receive a maximum of 1.0 hour of Category I credit for completing this program.
Date of Original Release/Review
This educational activity is eligible for AMA PRA Category 1 Creditâ„¢ through August 31, 2016. The latest review of this material was August 2013.
Financial Disclosure
All individuals in a position to influence the content of this activity were asked to complete a statement regarding all relevant personal financial relationships between themselves or their spouse/partner and any commercial interest. The CME Institute has resolved any conflicts of interest that were identified. In the past year, Larry Culpepper, MD, MPH, Editor in Chief, has been a consultant for AstraZeneca, Forest, Janssen, Lundbeck, Merck, Pfizer, and Takeda and has been a member of the speakers/advisory board for Merck. No member of the CME Institute staff reported any relevant personal financial relationships. Faculty financial disclosure appears at the end of the article.
Ms A presented to the Stead Family Memory Clinic at the Banner Alzheimer’s Institute with her daughter, who supplemented the clinical history.
HISTORY OF PRESENT ILLNESS
Ms A is an 84-year-old woman who was first noted to have cognitive changes after an otherwise uneventful cardiac valve replacement surgery approximately 1 year prior to presentation. Postoperatively, she appeared “confused,” and, upon returning to her independent living facility, she was increasingly repetitive with stories and statements and no longer able to manage her finances. Her daughter took over the finances, but, in retrospect, noted that even prior to her surgery, Ms A would go to the bank “frequently” for help with balancing her checkbook.
Ms A often lost important objects such as her purse and required reminders for appointments. She wrote down appointment reminders for herself more frequently, but forgot where she wrote them. She began having trouble remembering the names of her grandchildren. On recent trips with her daughter, she was disoriented and unable to find the way to her hotel room.
Ms A was always focused on her appearance and beauty and continued to do very well with matching her clothes and accessories and applying makeup. She had no changes in personal hygiene or self-care. She managed her own medications without complications, but her daughter believed that she occasionally missed a dose. Ms A continued to drive a motor vehicle, but she self-restricted to short distances and daytime driving. Her daughter reported no safety concerns. Ms A was able to shop with a credit card; she did not use cash. She continued to play bridge routinely with a close group of friends, which she greatly enjoyed.
Mild depressive symptoms were reported, but no apathy was noted. Ms A was highly motivated to dress immaculately and meet her friends for bridge and outings. She had not displayed aggression, but she had been irritable and angry, mainly toward her daughter.
PAST MEDICAL HISTORY
Ms A had a history of hypertension, hypercholesterolemia, and hypothyroidism, which were well-managed. She was treated for osteoporosis and had a history of a remote hysterectomy, a squamous cell cancer removed from her face 2 years prior, and an aortic valve replacement 1 year prior.
ALLERGIES
Ms A had no known drug allergies.
MEDICATIONS
Ms A took rosuvastatin, valsartan, amlodipine, levothyroxine, ibandronate, and aspirin.
SOCIAL HISTORY
Ms A was a high school graduate and worked as a saleswoman. She very much enjoyed dance, bridge, music, and politics. She had been a widow for 25 years and lived in an independent living facility for the last 3 years. She had 4 adult children, 1 of whom lived locally. She drank limited amounts of red wine with dinner occasionally, with no history of alcohol abuse. There was no history of use of tobacco or illicit substances.
FAMILY HISTORY
There was no history of dementia in Ms A’s family, but her mother had “confusion” and “short-term memory problems” in the year preceding her death in her early 80s. Diabetes and hypertension were both prevalent in her family.
REVIEW OF SYSTEMS
A complete review of systems was unremarkable.
PHYSICAL EXAMINATION
Ms A had a blood pressure of 125/75 mm HG, pulse of 64 bpm, height of 60 inches, weight of 149 lb, and body mass index of 29.1 kg/m2. Her general physical examination was unremarkable except for a systolic murmur.
NEUROLOGIC EXAMINATION
Ms A’s neurologic examination was unremarkable except for broken smooth pursuits, positive snout reflex, positive bilateral palmomental reflex, and brisk but symmetric reflexes throughout.
Smooth pursuit can be tested by asking the patient to track a small moving target at a distance of about 1 m, while keeping his/her head stationary. Both horizontal and vertical smooth pursuit should be assessed. The target should be moved at a slow uniform speed, and the pursuit eye movements are observed to determine whether they are smooth or broken up by catch-up saccades or a fast movement of the eye. Because smooth pursuit requires the coordination of many brain regions, this is a nonspecific finding, but could be indicative of cerebral degeneration. Sudo et al (2010) reported that impaired smooth pursuit can be indicative of impaired intellectual and frontal lobe function and can be regarded as a primitive reflex (frontal release sign).
LABORATORIES/RADIOLOGY
Laboratory data were not available. No brain imaging had been performed.
A Mini-Mental State Examination score generally correlates with disease severity. Scores ≤ 9 points can indicate severe dementia, scores between 10-20 points can indicate moderate dementia, and scores > 20 points can indicate mild dementia (Mungas, 1991). Although MMSE scores must be interpreted in light of both the patient’s age and education, education is the primary demographic factor that affects scores. Therefore, whereas a cutoff of ≤ 23 is widely used in distinguishing between normal and abnormal performance, this cutoff may have less predictive ability in poorly educated individuals (Folstein et al, 1975).
On the basis of the information presented thus far, what would you expect the MMSE score to be?
Your Colleagues Who Attended the Banner Alzheimer’s Institute Case Conference Answered as Follows:
| A. 26-30 | 0% |
| B. 21-25 | 0% |
| C. 16-20 | 100% |
| D. 11-15 | 0% |
| E. < 11 | 0% |
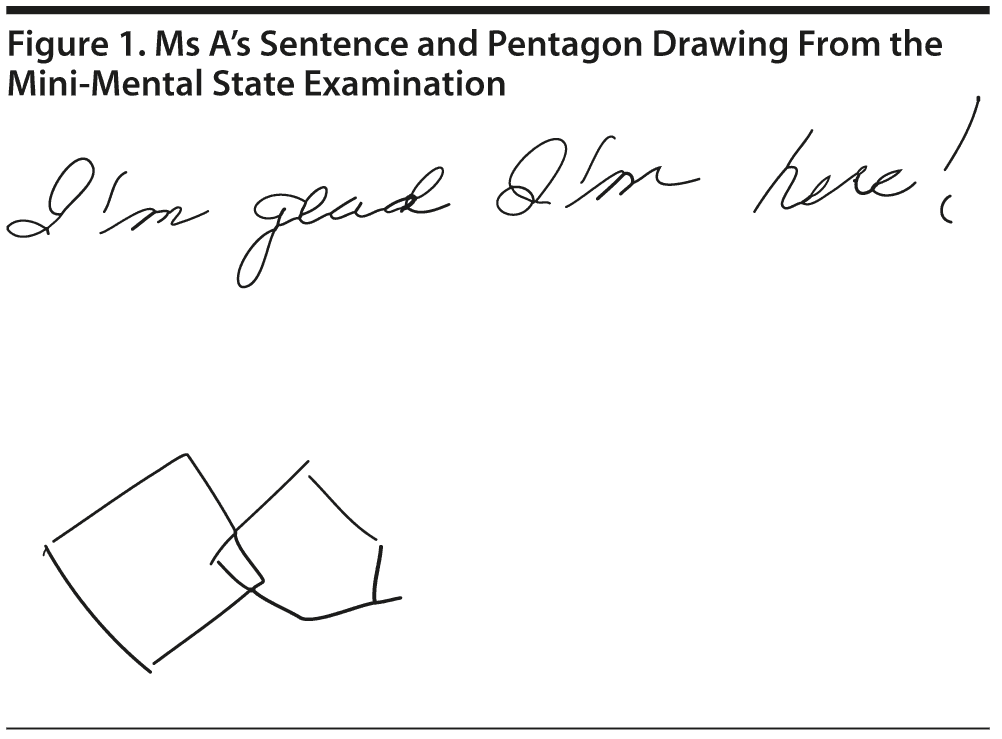
Ms A scored 22 points on the MMSE. Figure 1 shows Ms A’s sentence and pentagon drawing from the MMSE.
The Montreal Cognitive Assessment (MoCA) is a 30-point test that assesses several cognitive domains. Because it is more challenging than the Mini-Mental State Examination, it has greater sensitivity for mild cognitive impairment and early stages of dementia. With a cutoff score < 26, the sensitivity for detecting mild cognitive impairment (N = 94) was 90% and the specificity was 87% (Nasreddine et al, 2005). Research has demonstrated that MoCA scores are highly correlated with education. It is recommended that education be taken into account when interpreting MoCA performance, but there are no formal specific cutoff scores for lower education at this time (Johns et al, 2008). This test is available online at http://mocatest.org/.
On the basis of the information presented thus far, what would you expect the MoCA score to be?
Your Colleagues Who Attended the Banner Alzheimer’s Institute Case Conference Answered as Follows:
| A. 26-30 | 0% |
| B. 21-25 | 17% |
| C. 16-20 | 83% |
| D. 11-15 | 0% |
| E. < 11 | 0% |
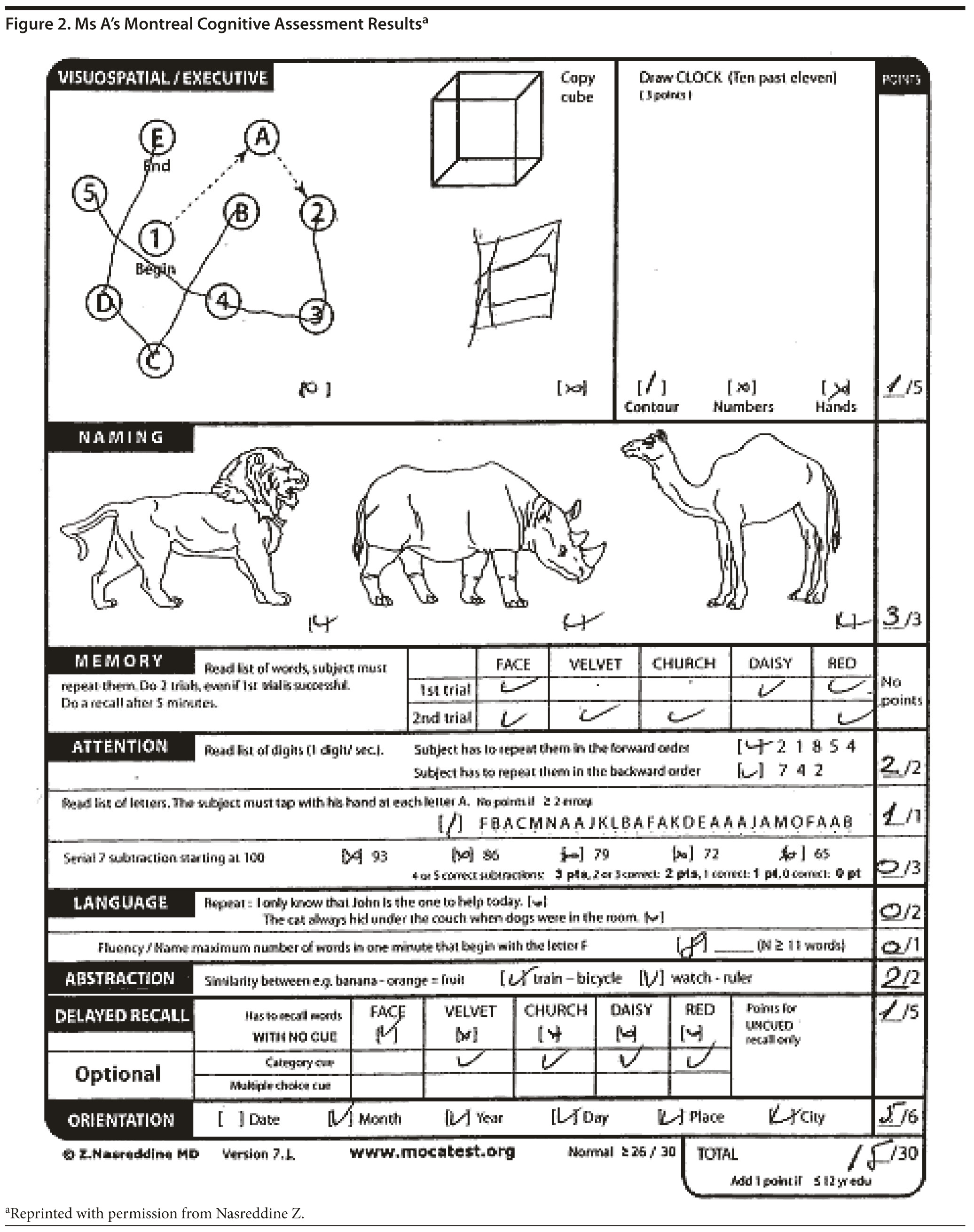
Ms A scored 15 points on the MoCA. Figure 2 provides Ms A’s MoCA results, and Figure 3 shows her clock drawing.
The DSM-IV defines dementia as multiple cognitive deficits that include memory impairment and at least 1 of the following cognitive disturbances: aphasia, apraxia, agnosia, or a disturbance in executive functioning. The cognitive deficits must be sufficiently severe to cause impairment in social or occupational functioning and must represent a decline from a previously higher level of functioning. A diagnosis of dementia should not be made if the cognitive deficits occur exclusively during the course of a delirium (American Psychiatric Association, 2000).
On the basis of the information presented thus far, do you believe that the patient meets criteria for dementia?
Your Colleagues Who Attended the Banner Alzheimer’s Institute Case Conference Answered as Follows:
| A. Yes | 100% |
| B. No | 0% |
Ms A has had a decline in cognitive ability in several cognitive domains that has affected her ability to conduct instrumental activities of daily life.
On the basis of the information presented thus far, what is the most likely diagnosis?
Your Colleagues Who Attended the Banner Alzheimer’s Institute Case Conference Answered as Follows:
| A. Frontotemporal dementia | 0% |
| B. Alzheimer’s disease | 18% |
| C. Mild cognitive impairment | 0% |
| D. Pseudodementia | 0% |
| E. Vascular dementia | 18% |
| F. Mixed dementia: Alzheimer’s disease and vascular dementia | 64% |
All conference attendees believed the etiology to be Alzheimer’s disease, vascular dementia, or a combination of the 2 as the most likely diagnosis. The clinical history of a cognitive decline after a cardiac surgery suggests the possibility of a vascular event. However, Ms A had a decline in cognition prior to her surgery.
Current clinical knowledge of the impact of surgery and anesthesia on subjects with Alzheimer’s disease is limited (Silbert et al, 2011). One study has implicated cardiac surgery as a risk factor for development of Alzheimer’s disease (Lee et al, 2005). However, other studies have shown no correlation with surgery and the risk of developing Alzheimer’s disease (Avidan et al, 2009 and Fischer et al, 2007). Postoperative cognitive dysfunction is a common complication in cognitively normal elderly patients, but a recent study suggests that postoperative cognitive dysfunction is not significantly associated with a higher risk of developing dementia (Steinmetz et al, 2013).
Despite the paucity of data, in the collective experience of Banner Alzheimer’s Institute providers, we have seen physical stressors (such as a surgery or hospitalization) result in a sharp decline in cognition in people with dementia or mild cognitive impairment. It is not uncommon for families to report that cognitive changes were first significantly noted after a surgery or hospitalization. Often, when asked, family will report subtle cognitive changes prior to the event. It is noted that Ms A did not have a postoperative delirium, but an episode of delirium can be the first sign of dementia in some patients (Rahkonen et al, 2000).
In April 2012, the US Food and Drug Administration approved use of florbetapir F-18 amyloid positron emission tomography imaging in patients with cognitive impairment who are being evaluated for Alzheimer’s disease and other causes of cognitive decline. Florbetapir F-18 is a radioactive diagnostic agent that binds to amyloid plaques, a hallmark characteristic of Alzheimer’s disease, and is detected using positron emission tomography images of the brain.
What further testing, if any, is indicated at this time?
Your Colleagues Who Attended the Banner Alzheimer’s Institute Case Conference Answered as Follows:
| A. Neuropsychological testing | 0% |
| B. Structural brain scan (CT or MRI) | 0% |
| C. FDG-PET | 0% |
| D. CBC, CMP, vitamin B12, TSH, and structural brain scan (CT or MRI) | 54% |
| E. Florbetapir PET (amyloid imaging) | 0% |
| F. No further testing is indicated | 0% |
| G. CBC, CMP, vitamin B12, TSH, structural brain scan (CT or MRI), and neuropsychological testing | 46% |
Many conference attendees suggested ordering the basic tests for a routine dementia workup, which include CBC, CMP, vitamin B12, TSH, and structural brain imaging with either an MRI or CT (Knopman et al, 2001). Some conference attendees suggested neuropsychological testing in addition to the basic workup, arguing that results of such tests can clearly illustrate cognitive strengths and weakness that can be used as the basis of a care plan for the caregiver as well as the patient. Additionally, the neuropsychological testing can help assess whether the cognitive changes are more indicative of a subcortical vascular process versus a progressive neurodegenerative condition like Alzheimer’s disease.
Those opposed to neuropsychological testing stated that the clinical history and cognitive screening battery are sufficient for diagnosis, and neuropsychological testing will not provide additional significant information. Furthermore, hours of cognitive tests may be an additional burden on the patient.
Should the patient be initiated on a medication, or should current medications be adjusted?
Your Colleagues Who Attended the Banner Alzheimer’s Institute Case Conference Answered as Follows:
| A. Start an antidepressant | 0% |
| B. Initiate memantine | 0% |
| C. Initiate a cholinesterase inhibitor (donepezil, rivastigmine, or galantamine) | 50% |
| D. Discontinue rosuvastatin | 0% |
| E. No changes in medications at this time | 50% |
Although the workup had not been completed and the diagnosis of dementia etiology had not yet been established, many attendees endorsed initiation of a cholinesterase inhibitor with Alzheimer’s disease as the presumptive diagnosis. Evidence in the literature suggests that cholinesterase inhibitors may be useful for vascular dementia (Birks et al, 2013); however, they are not approved by the US Food and Drug Administration for this purpose. Others opted to wait for the diagnosis to be established prior to initiation of cognitive medications.
Ms A drove locally, only short distances during daylight hours. Her daughter, a reliable informant, was consistently a passenger in the vehicle with her at least once every 2 weeks and reported no driving safety concerns. What action, if any, should be recommended regarding driving at this time?
Your Colleagues Who Attended the Banner Alzheimer’s Institute Case Conference Answered as Follows:
| A. The patient must pass a formal on-road driving assessment in order to continue driving | 72% |
| B. Driving should stop immediately | 0% |
| C. Continue driving, as long as her daughter continues to closely monitor (at least every 2 weeks) | 28% |
| D. Refer the patient to a driving education class (AAA or AARP) | 0% |
Most attendees endorsed a formal on-road driving assessment at this time, while some felt that driving could continue as long as Ms A’s daughter monitored her mother’s driving closely. Should the daughter feel that driving is no longer safe, she could contact the physician to have the driving stopped, as opposed to confronting Ms A herself.
Physicians are often challenged by the attempt to identify those patients who are a safety risk, while avoiding driving cessation prematurely. No clinical tool exists that accurately predicts driving safety in dementia, but the American Academy of Neurology has published guidelines (Iverson et al, 2010). Driving should be reviewed at each visit, addressing the topic of planning for after driving cessation. Should the patient pass a formal driving assessment, it should be repeated every 6 to 12 months to assess future change. Likewise, it would be prudent for the daughter to monitor Ms A’s driving regularly.
IMPRESSION OF THE TREATING PHYSICIAN AT THE FIRST VISIT
Ms A is an 84-year-old woman who presented for a cognitive evaluation. The patient’s clinical history and cognitive findings are consistent with a dementia. The most likely etiology of her dementia is Alzheimer’s disease, but other etiology such as vascular is possible. Further testing is indicated. Ms A has mild depressive features. She is living alone in an independent living facility and continues to drive.
Note: At the Banner Alzheimer’s Institute, the Geriatric Depression Scale (Heisel et al, 2005) is regularly used to monitor for depression. In this case, the scale was not employed; but, in retrospect, it may have been useful to detect and track depressive symptoms. In this case, depressive features were assessed by interviewing the patient and caregiver at each appointment.
Plan
- Obtain an MRI of the brain.
- Obtain outside laboratory results from her other physicians (specifically, CBC, CMP, TSH, and vitamin B12). If any further testing is required, Ms A and her daughter will be notified.
- Initiate donepezil 5 mg once daily in the morning for 4 weeks and then 10 mg thereafter; discussed potential side effects of this medication and how this medication is administered (in the morning with food).
- Memantine is not indicated at this time.
- Monitor depressive features; an antidepressant is not indicated at this time.
- Discussed the importance of continuing to optimize physical, social, and mental activity.
- Discussed the Family and Community Services at Banner Alzheimer’s Institute and the caregiver classes and support groups that they provide; Ms A’s daughter was referred to a caregiver education class.
- Discussed driving. Ms A’s daughter agreed to monitor driving at least every 2 weeks. If safety issues are observed, then driving will stop immediately. Ms A understood and agreed. Ms A’s daughter can contact the physician should driving need to stop.
- Discussed the possibility of clinical trial participation.
- Follow up in approximately 6 weeks.
The most common side effects of cholinesterase inhibitors (donepezil, rivastigmine, and galantamine) include cholinergic side effects of diarrhea, nausea, vomiting, and bradycardia (can be symptomatic with syncope [Farlow et al]).
FOLLOW-UP APPOINTMENT 2 MONTHS AFTER THE INITIAL VISIT
Since the last visit, Ms A and her daughter stated that she was doing “terrific.” Ms A had initiated the donepezil, and although she initially had intermittent diarrhea, she was able to titrate to 10 mg without incident. Since starting donepezil, Ms A felt that her cognition had improved. Her daughter agreed. Ms A was delighted that her bridge game improved. Her daughter observed that Ms A was completing sentences better and also noted that her mother had been bragging about her improved bridge playing among her friends. Ms A reported that she was driving less, but her daughter did not endorse any safety concerns. Since the last visit, Ms A had an MRI of the brain and a CBC, CMP, vitamin B12, and TSH, all of which were unremarkable.
Impression
Ms A is an 84-year-old woman with early stages of Alzheimer’s disease dementia. She has had improvements with donepezil. She continues to live alone in independent living and continues to drive, although less so.
Plan
- Continue donepezil 10 mg daily.
- Discussed the importance of optimizing physical, social, and mental activity.
- Referred Ms A’s daughter to meet with a social worker for counseling regarding adjustment to a chronic illness, but she politely declined.
- Discussed clinical trial participation, but Ms A was not interested.
- Follow up in 6 months.
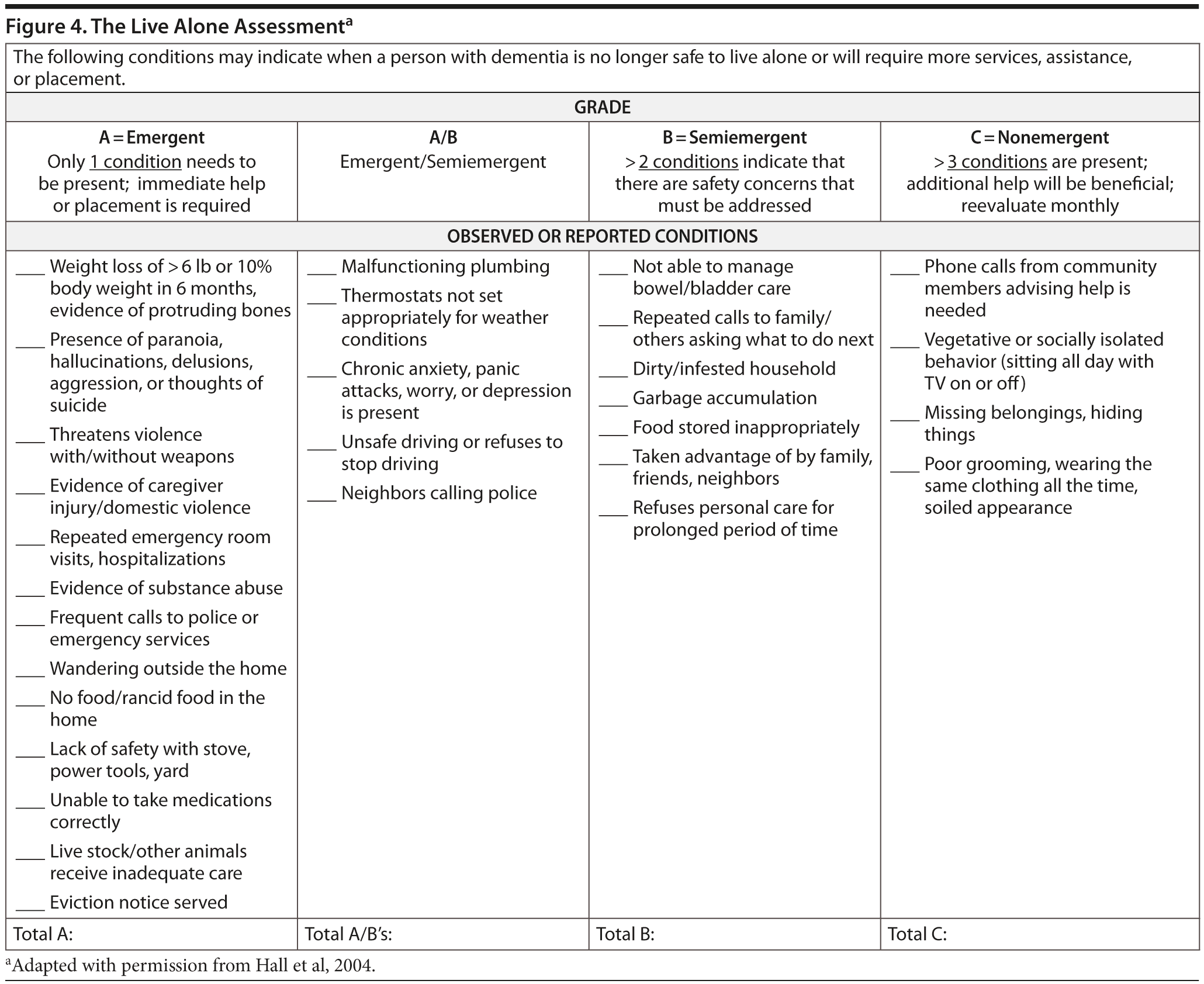
Note: A conference attendee suggested, and most conference attendees were in agreement, that, at this point, the Live Alone Assessment (Hall et al, 2004; Figure 4) should be given to Ms A’s daughter to help monitor the living situation, which was not done at the time of Ms A’s presentation. According to the Live Alone Assessment, at this point, the patient may continue to live independently. It should also be noted that the obvious improvement observed by Ms A and her daughter is not a typical response. Although patients derive benefit from cholinesterase inhibitors, most patients do not have an overtly obvious positive response.
FOLLOW-UP APPOINTMENT 8 MONTHS AFTER THE INITIAL VISIT
Ms A stated that she was “very happy”; however, her daughter noted that she had become somewhat “mean.” Ms A and her daughter agreed that the donepezil continued to be associated with improved cognition. Ms A continued to be active socially and physically, with continued careful detail to her appearance. She played bridge 3 times a week, played video games (bowling), walked, and danced. Ms A reported some difficulty falling asleep at night; however, a glass of wine helps. She stopped driving on her own volition without specific reason.
Cognitive Testing
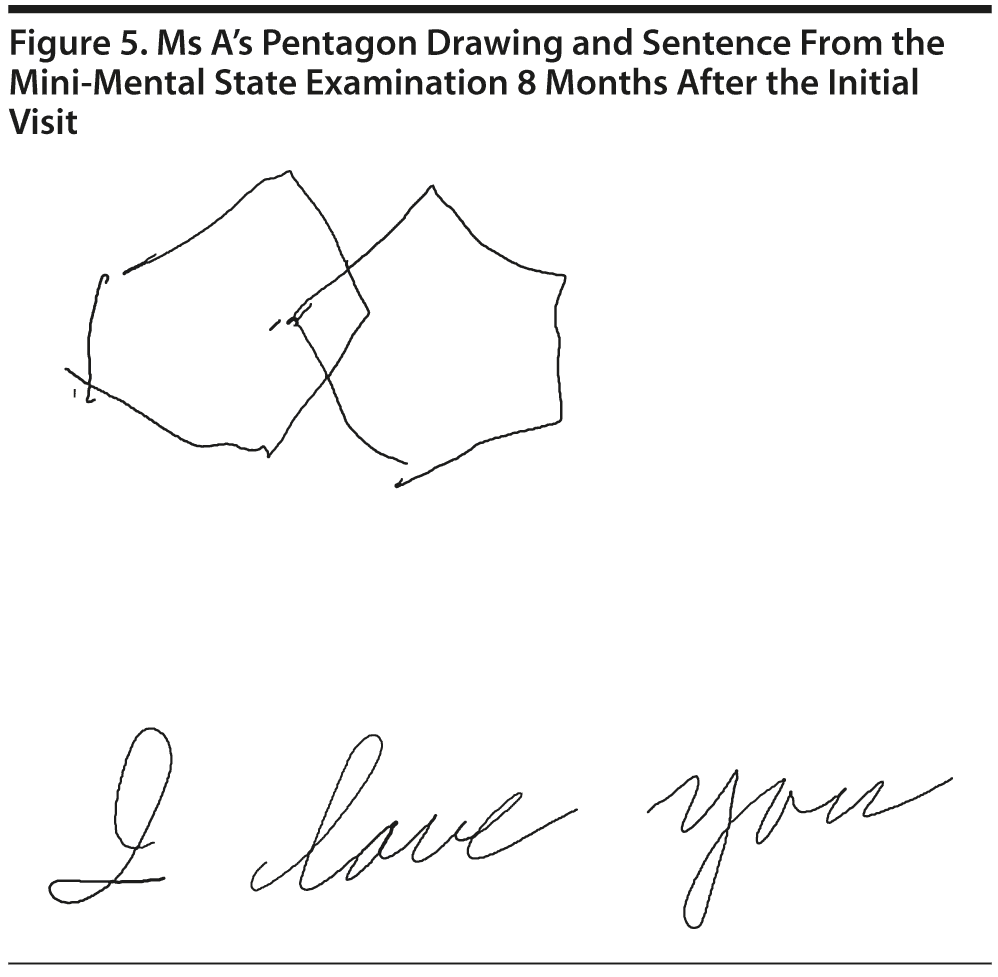
Ms A scored 24 points on the MMSE. Figure 5 shows Ms A’s pentagon drawing and sentence from the MMSE.
Impression
Ms A is an 85-year-old woman with early stages of Alzheimer’s disease dementia, who is stable on donepezil. She lives alone in independent living. She is no longer driving. Ms A’s daughter noted that her mother has become more “mean” and irritable. Ms A stated that she was “very happy” and does not wish to start an antidepressant.
Plan
- Continue donepezil.
- Monitor the patient’s mood and consider initiation of a selective serotonin reuptake inhibitor, such as citalopram, if indicated.
- Follow up in 6 months.
Note: Irritability may reflect more subtle functional and cognitive changes, less resilience, and gradually decreasing ability to live alone.
FOLLOW-UP APPOINTMENT 15 MONTHS AFTER THE INITIAL VISIT
Ms A presented alone (her living facility provided transportation). Her daughter was contacted prior to and after the appointment. Ms A and her daughter reported that she continued to do very well. Ms A presented a list of current medications that included the addition of citalopram. She did not know the dose, when it was initiated, or who prescribed it. (Her daughter was not aware that, apparently, this medication was prescribed recently by Ms A’s primary care physician.) Ms A was confused about her medications but insisted that she was able to self-administer them without errors. Ms A’s daughter believed that her mother was able to manage medications appropriately but did not regularly double-check.
Ms A’s daughter related that her mother was very “negative” and was displaying growing apathy. There was a slight decline in personal hygiene (not brushing teeth and bathing as frequently as prior).
Cognitive Testing
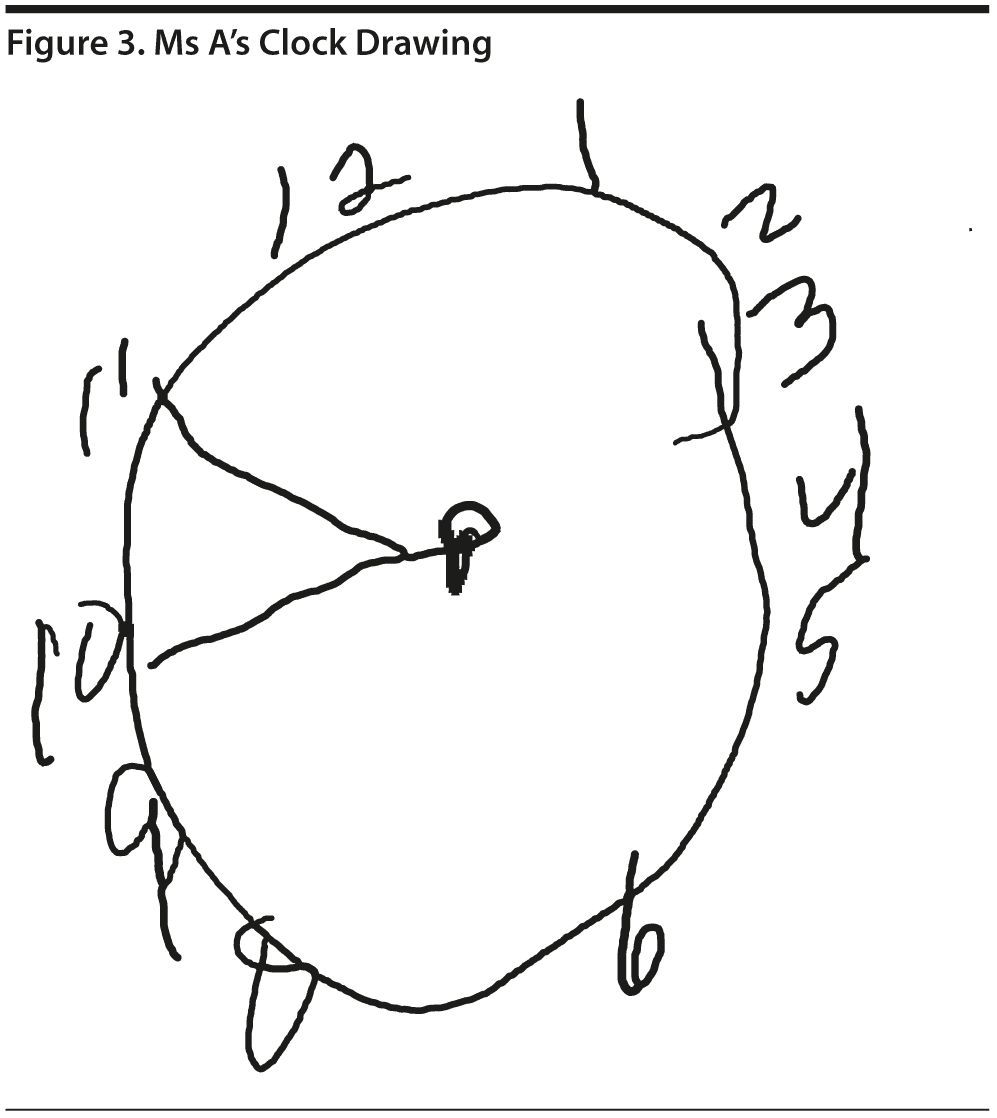
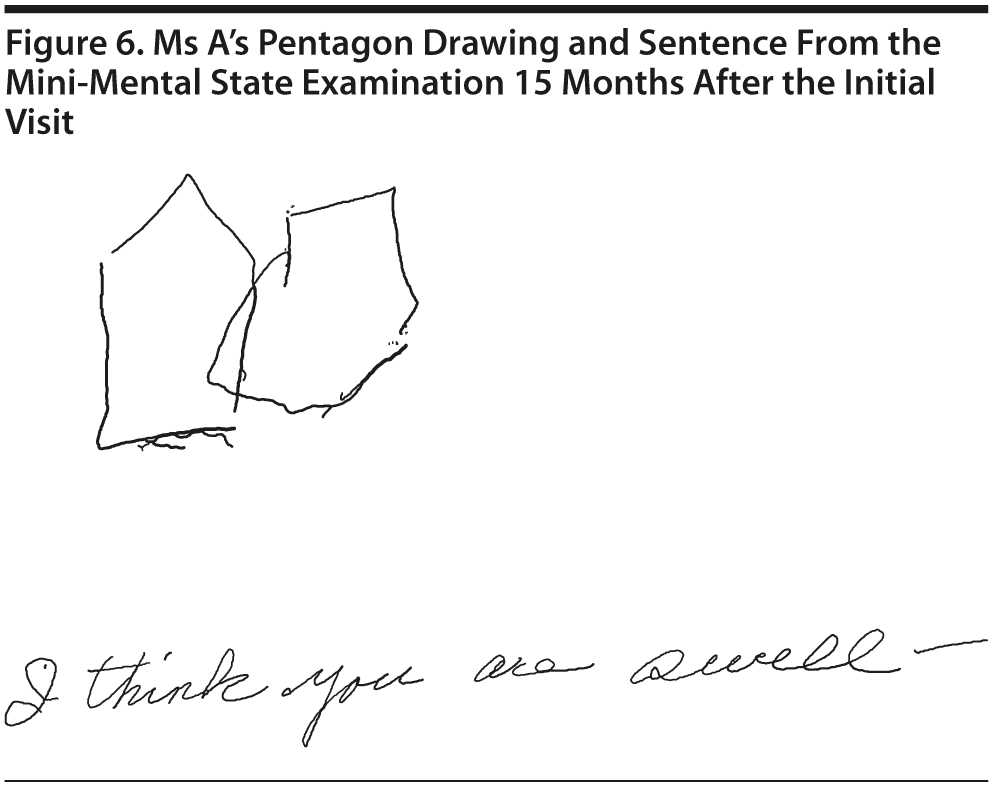
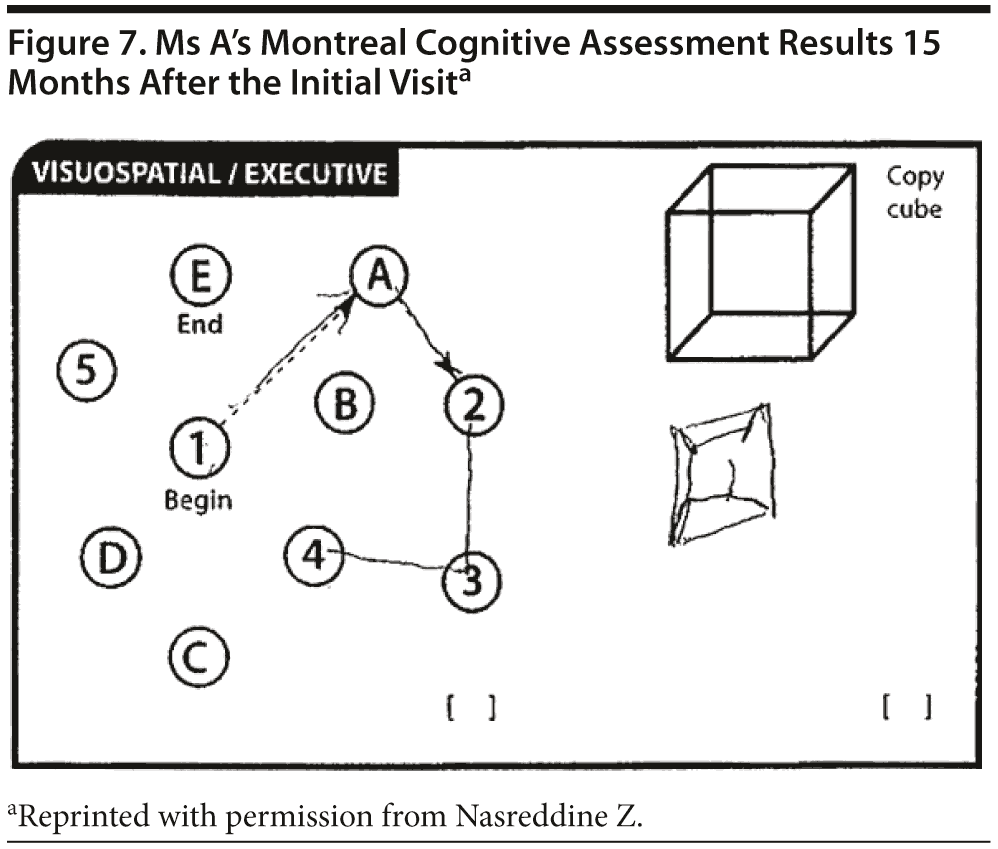
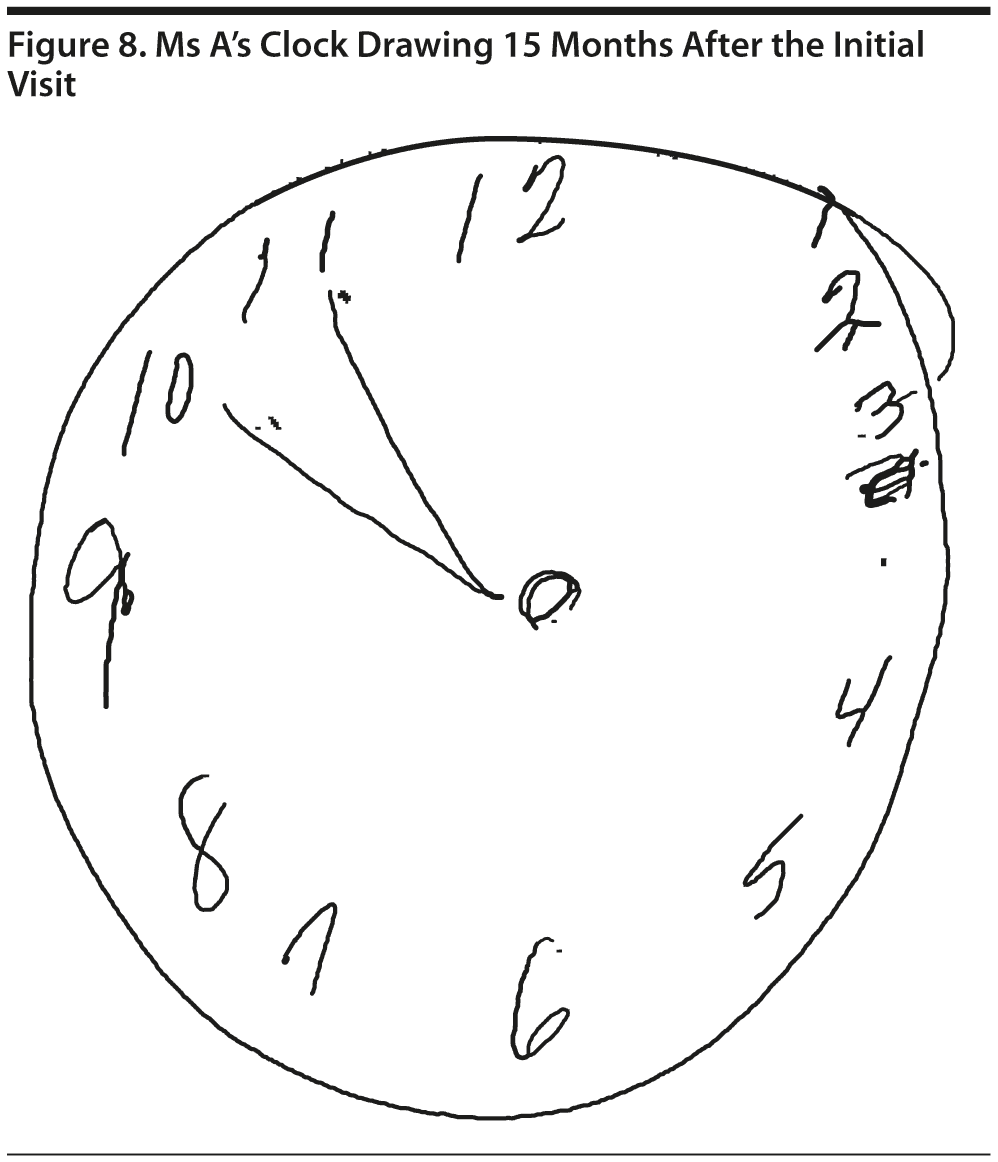
Ms A scored 22 points on the MMSE. Figure 6 shows Ms A’s sentence and pentagon drawing from the MMSE. She had a partial MoCA (Figure 7), in which she was unable to correctly do the trails test, cube copy, or clock drawing (Figure 8).
Impression
Ms A is an 86-year-old woman in the early stages of Alzheimer’s disease dementia, who is stable on donepezil. She lives alone in an independent living facility and no longer drives. She has become more “negative” and has been prescribed citalopram. Although the primary care physician started the citalopram and was monitoring the depressive symptoms, in retrospect, this could have been more closely monitored with a Geriatric Depression Scale and more frequent office visits to the Banner Alzheimer’s Institute.
Plan
- Continue donepezil 10 mg daily.
- Advised that Ms A should have medication oversight, but at this time, her daughter did not think this was required. Her daughter was asked to check whether medications are appropriately managed. The danger of medication errors was discussed.
- Continue citalopram (the daughter will call with the prescribed dose, which the patient did not know).
- The daughter continued to decline meeting with a social worker to discuss the living situation and other caregiver issues.
- Follow up in 6 months.
Note: Ms A’s daughter e-mailed the clinic 2 weeks later stating that she is monitoring her mother’s medications and that “she does well” in taking them. The Banner Alzheimer’s Institute generally recommends that a family member set up a weekly pill box and then check on a weekly basis. If a patient refuses to use a pill box, then family should count the pills in the containers. If family is not involved on a regular basis, then checking the refills with the pharmacy could be beneficial. The best alternative would be for the patient to have medications administered to him or her. In this case, both the patient and daughter refused. The patient refused in order to preserve independence, and the daughter refused due to cost.
FOLLOW-UP APPOINTMENT 19 MONTHS AFTER THE INITIAL VISIT
Ms A presented alone (her living facility provided transportation). Her daughter was contacted before and after the visit. Her mood was better. Family initiated daily phone calls to Ms A to remind her to take her medications. She continued to play bridge and video bowling and to participate in social activities.
Impression
Ms A is an 86-year-old woman in the early stages of dementia due to Alzheimer’s disease. Her donepezil dose is stable, and she lives alone in an independent living facility. She has had some depressive features in the past that, at the current time, appear to be well-controlled with citalopram. Family calls daily to remind Ms A to take her medications.
Plan
- Continue donepezil 10 mg daily.
- Continue citalopram 20 mg daily.
- Follow up in approximately 6 months.
FOLLOW-UP APPOINTMENT 25 MONTHS AFTER THE INITIAL VISIT
Ms A presented alone (her living facility provided transportation). Her daughter was contacted before and after the visit. Since the last visit, Ms A reported a decline in energy and worsening insomnia. She stated that she was “out of my routine” of exercising and social activities. She had the desire to resume her activities but reported that fatigue prevented her from doing so. She related that one night she thought she saw a raccoon tail under her bed, but when help came, no raccoon was found. Ms A’s general physical and neurologic examinations were unchanged from prior evaluations, although she appeared to be less energetic than in the past.
Cognitive Testing
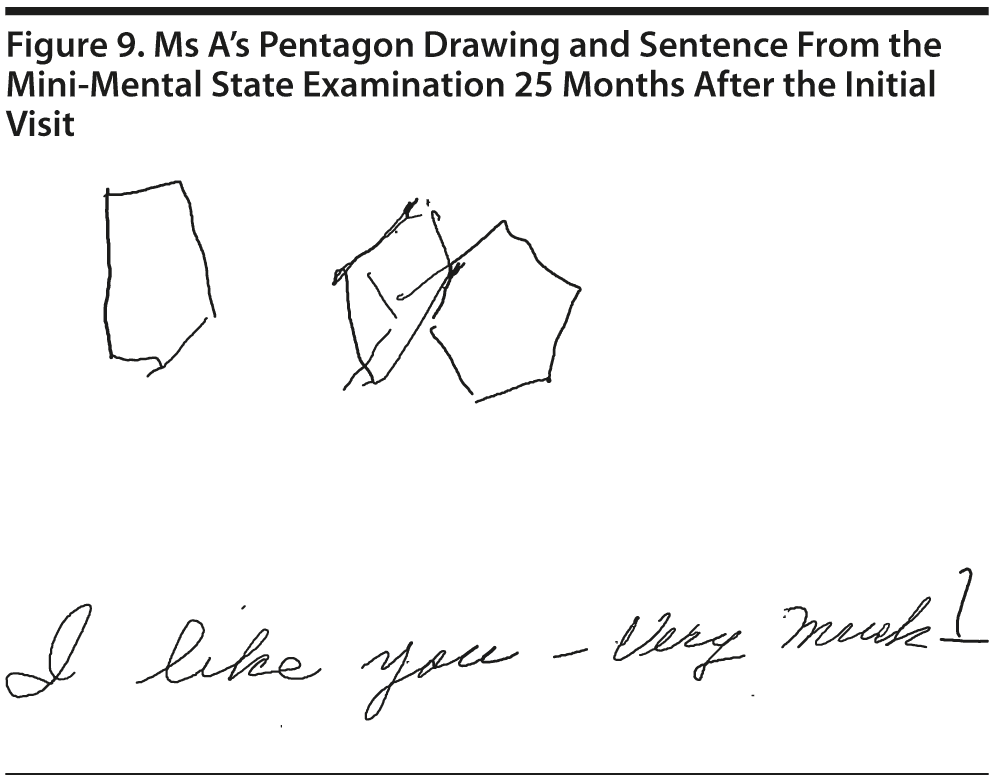
Ms A scored 23 points on the MMSE. Figure 9 shows Ms A’s pentagon drawing and sentence from the MMSE.
Impression
Ms A is an 86-year-old woman with dementia due to Alzheimer’s disease. She is stable on donepezil and citalopram. She has increased fatigue with insomnia. She may have had a recent visual hallucination versus a vivid dream.
Plan
- Continue donepezil 10 mg daily.
- Continue citalopram 20 mg daily.
- Discussed in length ways to increase Ms A’s energy by improving her sleep; discussed the importance of limiting evening liquids and caffeine, exercising and being active during the day, limiting daytime naps, and sunlight; and recommended that she stop falling asleep with the television on. Ms A refused a medication for insomnia such as trazodone, but this should be considered in the future if insomnia persists. Her daughter will continue to call to remind her mother to take medications and to engage in her social activities and exercise.
- Encouraged Ms A’s daughter to meet with a social worker to discuss the living situation. It is not clear whether Ms A is taking her medications correctly. Increasing her level of care or hiring someone to administer medications was recommended.
- Monitor a possible visual hallucination versus a vivid dream.
- Follow up in 3 months.
SOCIAL WORKER APPOINTMENT WITH the DAUGHTER (the PATIENT WAS NOT PRESENT)
Although the daughter disagreed with the diagnosis of Alzheimer’s disease, she reported that she and other family members have observed continued decline in her mother’s memory and functioning. However, she also reported that her mother had very good social skills and reveled in her ability to engage people socially.
The staff in her independent living facility “loves her,” and she took an active informal role as hostess within the dining room. She very much appreciated that role and was proud of herself in succeeding at it.
The daughter noted an increase of sleep during the day. Her mother was having more difficulty keeping track of time and often called her daughter numerous times to ask about the date or the time, sometimes as early in the morning as 3 am. Ms A’s daughter had also noted that her mother has forgotten bridge dates as well.
Many aspects of caregiver education were discussed, including the current living situation and how additional care now and in the future may support the patient in being at her best. Ms A’s daughter felt strongly that if she were to move her mother to assisted living or memory care that “it would kill her,” indicating this was not an option. It was suggested to hire a “personal assistant” to help her engage in a weekly routine and medication administration.
FOLLOW-UP APPOINTMENT 29 MONTHS AFTER THE INITIAL VISIT
Ms A presented alone (her living facility provided transportation). Her daughter was contacted before and after the visit. The patient reported no changes since the last visit. She continued to have trouble falling asleep and was increasingly tired. Her primary care physician monitors her TSH level, which was normal.
Impression
Ms A is an 87-year-old woman with Alzheimer’s disease dementia. She stated that she was having insomnia and stayed up very late watching television and then had difficulty waking up in the morning. Ms A has had 1 or 2 hallucinations versus vivid dreams. She complained of fatigue during the day. It is not clear if she is taking her medications appropriately, as no one is monitoring.
Plan
- Continue donepezil 10 mg daily.
- Continue citalopram 20 mg daily.
- Advocated that she stop watching television at night, as it was preventing her from sleeping and could be the cause of the hallucinations versus vivid dreams. It was recommended that she read a book instead if she has trouble falling asleep (especially a boring book).
- Follow up in approximately 3 to 4 months.
FOLLOW-UP APPOINTMENT 33 MONTHS AFTER THE INITIAL VISIT
Ms A presented alone (her living facility provided transportation). Her daughter was contacted on the phone prior to the appointment. Ms A reported that she was doing “wonderfully.” She stated that she was involved in activities and, overall, quite happy. She visited with family frequently as well. Since the last visit, family called her nightly to remind her to turn off the television. She was sleeping much better and had not had any further hallucinations versus vivid dreams.
Cognitive Testing
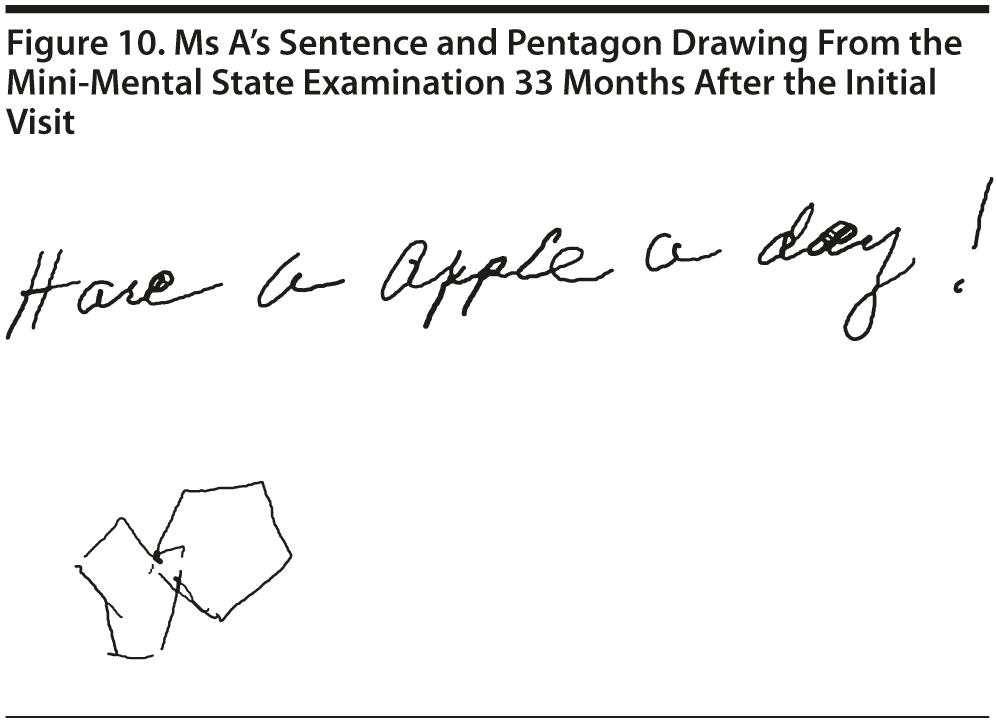
Ms A scored 25 points on the MMSE. Figure 10 shows Ms A’s sentence and pentagon drawing from the MMSE.
Impression
Ms A is an 87-year-old woman with Alzheimer’s disease dementia. She is stable on donepezil and citalopram and lives alone in an independent living facility. She has had depressive features in the past, which appear to be well-controlled. She has had insomnia in the past, which has improved by turning off the television at night. Ms A has no complaints today.
Plan
- Continue donepezil 10 mg daily.
- Continue citalopram 20 mg daily.
- Continue to monitor the patient’s mood and insomnia.
- Follow up in approximately 6 months.
FOLLOW-UP APPOINTMENT 39 MONTHS AFTER THE INITIAL VISIT
Ms A presented alone (her living facility provided transportation). Her daughter was contacted before and after the visit. Ms A’s family called her nightly to remind her to turn off the TV. She started sleeping better at night and was no longer fatigued during the day. She resumed much of her daily activities and exercise and continued to play bridge. She related that her memory was getting worse, and it was frustrating for her. Ms A had an 8-lb weight loss since her last appointment.
Cognitive Testing
Ms A scored 19 points on the MMSE and 13 points on the MoCA.
Impression
Ms A is an 87-year-old woman with Alzheimer’s disease dementia. She is stable on donepezil and lives alone in an independent living facility. She has had depressive features in the past, which wax and wane, but appear to be generally well-controlled. Insomnia has improved by turning off the television at night. She is losing weight.
Plan
- Continue donepezil 10 mg daily.
- Continue citalopram 20 mg daily.
- Monitor the patient’s mood and insomnia.
- Instructed Ms A’s daughter to increase her mother’s caloric intake. Given the cognitive changes with weight loss and worsening memory, it is very likely that Ms A is not going to all meals at her assisted living facility. She will need reminders for meals.
- Encouraged Ms A’s daughter to come to future appointments with the patient.
- Referred her daughter to meet with a social worker to discuss planning for the future, especially placement options, as the patient may no longer be appropriate for independent living. Her daughter reported that if she were to move her mother to assisted living or memory care, “it would kill her.” Her daughter politely refused to meet with the social worker.
- Follow up in 6 months.
Note: Weight loss can often be associated with side effects of medication, depression, or other medical issues. In dementia patients who are living alone, weight loss can result from not remembering to fix or get meals for themselves or sleeping longer hours and missing calories during the day. There was potential for significant decompensating in Ms A if more adequate support was not provided.
FOLLOW-UP APPOINTMENT 43 MONTHS AFTER THE INITIAL VISIT
Ms A presented with her daughter. She had lost another 6 lb. The family hired help to administer Ms A’s morning medications, as reminder phone calls were no longer working. She was very depressed and stopped playing bridge because she felt that her friends “don’ t want me there.” She had stayed in bed and was less socially interactive.
Given the weight loss, depression, and need for reminders for medications and activities, what was the next best step regarding her current living situation?
Your Colleagues Who Attended the Banner Alzheimer’s Institute Case Conference Answered as Follows:
| A. Consider calling Adult Protective Services if her daughter refuses | 10% |
| B. Consider addition of mirtazapine, which could help improve appetite (weight) and mood and potentially further preserve independence | 40% |
| C. Consider addition of memantine, which could help improve cognition and potentially further preserve independence | 50% |
| D. No intervention; continue independent living and wait for a crisis; at the time of crisis, the patient can be placed in a higher level of care | 0% |
| E. Slowly transition to assisted living | 0% |
Follow-Up Appointment 43 MONTHS AFTER THE INITIAL VISIT (Continued)
Impression
The patient is an 88-year-old woman with Alzheimer’s disease dementia. She is stable on donepezil and lives alone in an independent living facility with hired help to administer medications. Depressive features have significantly worsened with weight loss.
Plan
- Continue donepezil 10 mg daily.
- Memantine may be indicated and considered at a future visit.
- Initiate mirtazapine 15 mg at bedtime to help treat the depressive features, as well as stimulate appetite.
- Continue citalopram 20 mg daily, as it appears to be providing some benefit.
- Discussed increasing caloric intake. It was explained that, if the patient continues to lose weight, then she will need to move to an assisted living facility, wherein she will be brought to her meals.
- Monitor the patient’s weight.
- Follow up in 8 weeks.
FOLLOW-UP APPOINTMENT 46 MONTHS AFTER THE INITIAL VISIT
Ms A presented with her daughter. Since the last visit, Ms A had done exceptionally well. She initiated mirtazapine, and her mood had significantly improved. She was eating well and gained 8 lb. She had resumed playing bridge, and her spirits were much better. Overall, she was much happier, and her demeanor was better. Her memory continued to decline.
Cognitive Testing
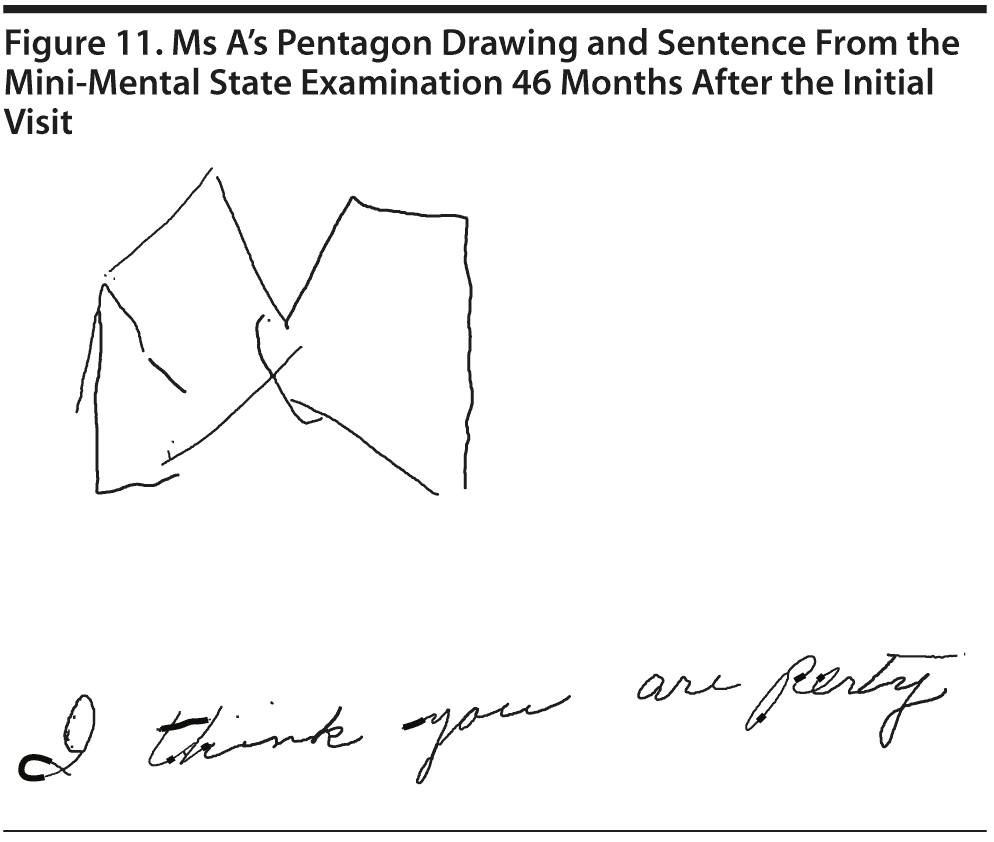
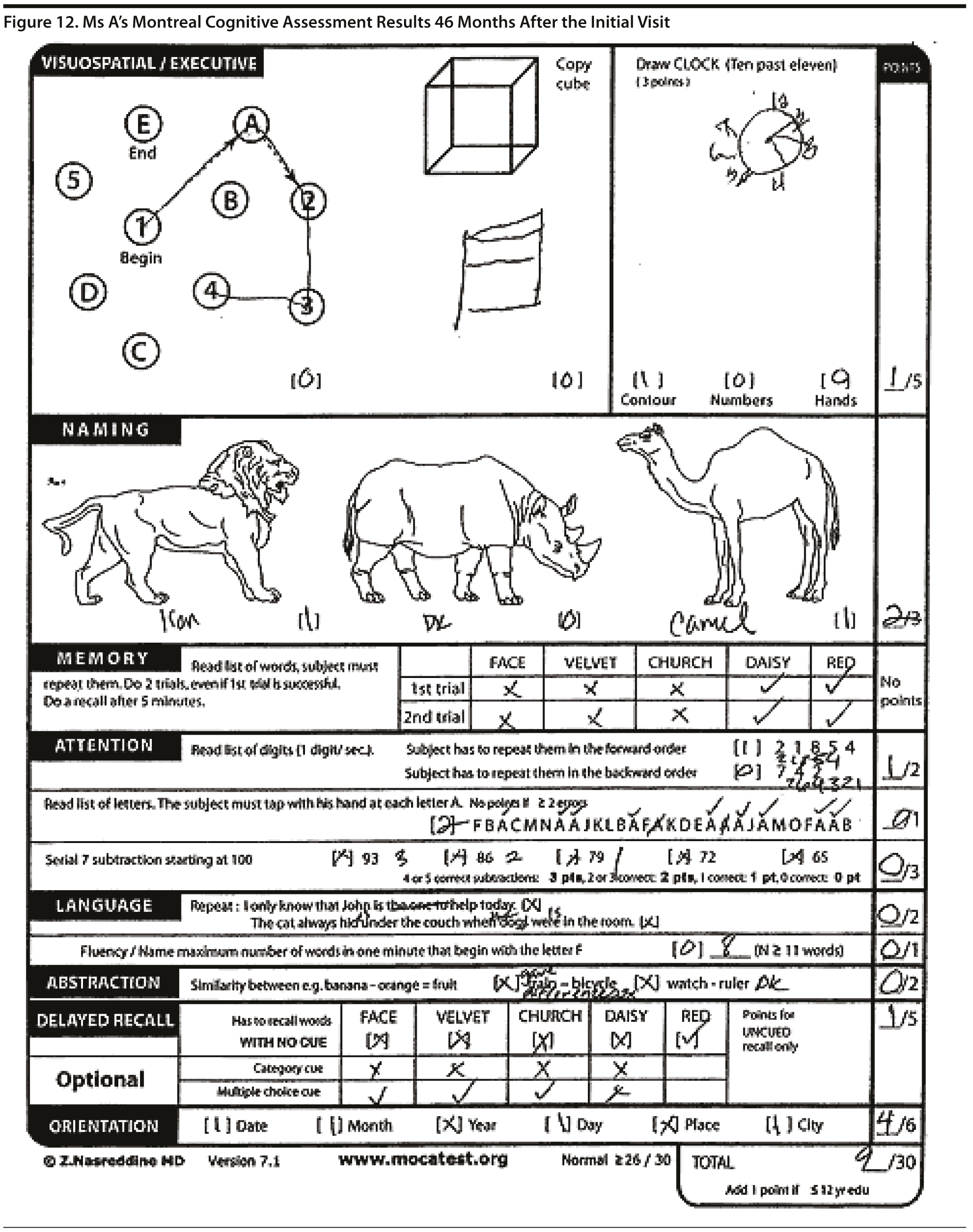
Ms A scored 20 points on the MMSE and 9 points on the MoCA. Figure 11 shows Ms A’s pentagon drawing and sentence from the MMSE. Figure 12 shows Ms A’s MoCA results.
Impression
Ms A is an 88-year-old woman with Alzheimer’s disease dementia. She is stable on donepezil and lives alone in an independent living facility with help administering medications. Depression and weight loss have improved with mirtazapine. Insomnia is well-controlled. Ms A appears to be doing well with independent living at this time.
Plan
- Continue donepezil 10 mg daily.
- Continue mirtazapine 15 mg daily.
- Continue citalopram 20 mg daily.
- Consider memantine in the future; her family wished to hold of on prescribing memantine for now.
- Monitor Ms A’s weight, depression, and insomnia.
- Recommended continued increased caloric intake.
- Monitor living situation. At the last visit, it appeared that assisted living was indicated, but with recent adjustments, Ms A is able to continue in independent living at this time.
- Follow up in 6 months.
Update
Four months since the last visit, Ms A continued to do very well. She had no further weight loss, depression was controlled, and there was no further insomnia. She required reminders for most appointments. She continued to live independently.
Disclosure of off-label usage
The authors have determined that, to the best of their knowledge, no investigational information about pharmaceutical agents that is outside US Food and Drug Administration-approved labeling has been presented in this article.
FINANCIAL DISCLOSURE
Dr Yaari is a consultant for Amedisys Home Health. Dr Tariot has served as a consultant for Abbott, AC Immune, Adamas, Avanir, Boehringer-Ingelheim, Chase, Chiesi, Eisai, Elan, MedAvante, Merz, Neuroptix, Otsuka, and Sanofi-Aventis; has received consulting fees and research support from AstraZeneca, Avid, Bristol-Myers Squibb, Eli Lilly, Genentech, GlaxoSmithKline, Janssen, Medivation, Merck, Pfizer, Roche, and Toyama; has received research support only from Baxter, Functional Neuromodulation, GE, and Targacept; has received other research support from Alzheimer’s Association, Arizona Department of Health Services, National Institute of Mental Health, and National Institute on Aging; is a stock shareholder in Adamas; and is listed as a contributor to a patent owned by the University of Rochester (Rochester, New York) for “Biomarkers of Alzheimer’s Disease.” Drs Burke and Seward and Ms Brand have no personal affiliations or financial relationships with any commercial interest to disclose relative to the activity.
FUNDING/SUPPORT
None reported.
DISCLAIMER
The opinions expressed are those of the authors, not of Banner Health or Physicians Postgraduate Press.
clinical points
- Depression and insomnia must be recognized and promptly treated in patients with early-stage dementia.
- Weight loss in patients with Alzheimer’s disease may be associated with medication side effects, depression, or other medical issues.
- Driving and the ability to live independently should be closely monitored in patients with Alzheimer’s dementia.
This CME activity is expired. For more CME activities, visit cme.psychiatrist.com.
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders
REFERENCEs
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Arlington, VA: American Psychiatric Association; 2000.
Avidan MS, Searleman AC, Storandt M, et al. Long-term cognitive decline in older subjects was not attributable to noncardiac surgery or major illness. Anesthesiology. 2009;111(5):964-970. PubMed doi:10.1097/ALN.0b013e3181bc9719
Birks J, McGuinness B, Craig D. Rivastigmine for vascular cognitive impairment. Cochrane Database Syst Rev. 2013;5:CD004744. PubMed
Different dementias may be associated with various physical examination findings. However, most often, the physical examination is normal in the early stages. Some subtle general findings can include frontal release signs such as a positive snout, glabellar reflex, or palmomental reflex (Links et al, 2010).
Farlow MR, Miller ML, Pejovic V. Treatment options in Alzheimer’s disease: maximizing benefit, managing expectations. Dement Geriatr Cogn Disord. 2008;25(5):408-422. PubMed doi:10.1159/000122962
Fischer P, Wallner H, Jungwirth S, et al. Cumulative exposure to general anesthesias and cognitive dysfunction at age 75 in the Vienna Transdanube Aging “VITA” study. J Neuropsychiatry Clin Neurosci. 2007;19(1):21-26. PubMed doi:10.1176/appi.neuropsych.19.1.21
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. doi:10.1016/0022-3956(75)90026-6 PubMed
Hall G, Bossen A, Specht J. Live Alone Assessment. Iowa City, Iowa: The University of Iowa College of Nursing, 2004.
Heisel MJ, Flett GL, Duberstein PR, et al. Does the Geriatric Depression Scale (GDS) distinguish between older adults with high versus low levels of suicidal ideation? Am J Geriatr Psychiatry. 2005;13(10):876-883. PubMed
Iverson DJ, Gronseth GS, Reger MA, et al. Quality Standards Subcomittee of the American Academy of Neurology. Practice parameter update: evaluation and management of driving risk in dementia: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2010;74(16):1316-1324. PubMed doi:10.1212/WNL.0b013e3181da3b0f
Lee TA, Wolozin B, Weiss KB, et al. Assessment of the emergence of Alzheimer’s disease following coronary artery bypass graft surgery or percutaneous transluminal coronary angioplasty. J Alzheimers Dis. 2005;7(4):319-324. PubMed
Links KA, Merims D, Binns MA, et al. Prevalence of primitive reflexes and Parkinsonian signs in dementia. Can J Neurol Sci. 2010;37(5):601-607. PubMed
Knopman DS, DeKosky ST, Cummings JL, et al. Report of the Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: diagnosis of dementia (an evidence-based review). Neurology. 2001;56(9):1143-1153. PubMed doi:10.1212/WNL.56.9.1143
McShane R, Areosa Sastre A, Minakaran N. Memantine for dementia. Cochrane Database Syst Rev. 2006;(2):CD003154. PubMed
Memantine is US Food and Drug Administration approved for moderate-to-severe Alzheimer’s disease. Memantine is beneficial when used alone or when added to a cholinesterase inhibitor (McShane et al, 2006; Tariot et al, 2004).
Mungas D. In-office mental status testing: a practical guide. Geriatrics. 1991;46(7):54-58, 63, 66. PubMed
Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695-699. PubMed doi:10.1111/j.1532-5415.2005.53221.x
Rahkonen T, Luukkainen-Markkula R, Paanila S, et al. Delirium episode as a sign of undetected dementia among community dwelling elderly subjects: a 2-year follow-up study. J Neurol Neurosurg Psychiatry. 2000;69(4):519-521. PubMed doi:10.1136/jnnp.69.4.519
Silbert B, Evered L, Scott DA, et al. Anesthesiology must play a greater role in patients with Alzheimer’s disease. Anesth Analg. 2011;112(5):1242-1245. PubMed doi:10.1213/ANE.0b013e3182147f5b
Steinmetz J, Siersma V, Kessing LV, et al; ISPOCD Group. Is postoperative cognitive dysfunction a risk factor for dementia? a cohort follow-up study. Br J Anaesth. 2013;110(suppl 1):i92-i97. PubMed doi:10.1093/bja/aes466
Sudo K, Mito Y, Tajima Y, et al. Smooth-pursuit eye movement: a convenient bedside indicator for evaluating frontal lobe and intellectual function. In Vivo. 2010;24(5):795-797. PubMed
Tariot PN, Farlow MR, Grossberg GT, et al; Memantine Study Group. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial. JAMA. 2004;291(3):317-324. PubMed doi:10.1001/jama.291.3.317
Enjoy this premium PDF as part of your membership benefits!