Metabolic, Digestive, and Reproductive Adverse Events Associated With Antimanic Treatment in Children and Adolescents: A Retrospective Cohort Study
Objective: To identify factors associated with incident metabolic and reproductive adverse events in children and adolescents.
Method: A retrospective cohort design evaluating Medicaid medical and pharmacy claims made in South Carolina between January 1996 and December 2005 was employed for 3,657 children and adolescents (aged 17 years old and younger) prescribed 1 of 3 antimanic medications (ie, lithium, carbamazepine, or valproic acid derivatives) and a random sample of 4,500 children and adolescents not treated with psychotropic medications.
Results: Compared to the control sample, the treated cohort was more likely to be diagnosed with obesity/weight gain (odds ratio [OR] = 1.89), type 2 diabetes mellitus (OR = 2.50), dyslipidemia (OR = 1.89), nausea (OR = 1.61), anorexia (OR = 3.85), and sexual/reproductive adverse events (OR = 2.04). Within the treated cohort, incident dyslipidemia was more likely for those prescribed carbamazepine (OR = 1.52) compared to valproate and coprescribed antipsychotics (OR = 1.47) or selective serotonin reuptake inhibitors (SSRIs) (OR = 1.49) compared to those not taking antipsychotics or taking serotonin-norepinephrine reuptake inhibitor/heterocyclic (SNRI/other) antidepressants. The odds of developing nausea/vomiting were higher for those prescribed carbamazepine (OR = 1.70) or lithium (OR = 1.49) compared to valproate, and those coprescribed psychostimulants (OR = 1.25) compared to those not taking psychostimulants. The odds of developing obesity/weight gain and type 2 diabetes mellitus were higher for those coprescribed SSRIs (ORs = 1.72, 2.58) or antipsychotics (ORs = 1.69, 1.77) compared to those taking SNRI/other antidepressants or not taking antipsychotics. Incident sexual/reproductive adverse events were more likely for those coprescribed SSRIs (OR = 2.02) compared to those taking SNRI/other antidepressants.
Conclusion: Commonly employed psychotropic agents are associated with clinically significant metabolic, digestive, and reproductive-related adverse events. Treatment decisions in young populations are usefully informed by the somatic consequences of the medication options.
Prim Care Companion J Clin Psychiatry 2010;12(4):e1-e8
© Copyright 2010 Physicians Postgraduate Press, Inc.
Submitted: September 18, 2009; accepted October 26, 2009
Published online: July 8, 2010 (doi:10.4088/PCC.09m00891ora).
Corresponding author: Jeanette M. Jerrell, PhD, Department of Neuropsychiatry and Behavioral Science, University of South Carolina School of Medicine, 3555 Harden St Ext, CEB 301, Columbia, SC 29203 ([email protected]).
Mood-stabilizing medications are frequently required for the treatment of complex mood disorder presentations in children and adolescents, eg, bipolar disorder. Recommended first-line treatments for pediatric bipolar disorder with manic or mixed features, with and without psychosis, are monotherapy with lithium, an anticonvulsant (either valproic acid derivatives or carbamazepine), or 1 of 3 second-generation antipsychotics (SGAs), olanzapine, quetiapine, or risperidone.1 One of the other mood stabilizers or SGAs could be used as an augmenting agent if the initial trial of monotherapy is only partially successful.1 Antidepressants may be coprescribed for depressive episodes.
The morbidity and mortality associated with pediatric bipolar disorder are mediated by the phenomenology of the illness, as well as disparate medical disorders and adverse health behaviors frequently encountered in these patients by adulthood, eg, cardiovascular disease, type 2 diabetes mellitus, obesity, and thyroid disease.2 Whereas efficacy evidence regarding mood stabilizers for children and adolescents is increasingly available from open-label trials and retrospective studies, there is only limited long-term safety and tolerability information available, especially from controlled clinical trials.3
Lithium, carbamazepine, and valproic acid derivatives have been associated with weight gain and related abnormalities in lipid (ie, elevated levels of cholesterol and triglycerides) and glucose metabolism in pediatric populations.4,5 Treatment-emergent adverse events related to lithium use, including nausea/vomiting, impotence/decreased libido, anorexia, and hypothyroidism,7 are often dose- and serum-concentration related.6 Carbamazepine also carries a milder increased risk of anorexia, diarrhea, and impotence/erectile dysfunction.7 Other tolerability problems reported for valproic acid derivatives are nausea/vomiting, especially in children and those with complicating factors (eg, metabolic diseases, mental retardation, or organic brain syndromes),5 menstrual dysfunction, and biochemical and clinical features suggestive of polycystic ovary syndrome.7
Clinical Points
- Commonly employed psychotropic agents are frequently combined in order to treat complex mood disorder presentations in children and adolescents.
- Mood-stabilizing medications are associated with clinically significant metabolic, digestive, and reproductive-related adverse events, especially when combined with certain antidepressants, antipsychotics, and psychostimulants.
- When evaluating the overall benefit-risk ratio of mood-stabilizing medications in children and adolescents, the primary care practitioner needs to give careful consideration to the possible metabolic disruptions, digestive symptoms, or sexual/reproductive side effects of these agents, especially when combined with other classes of agents.
A diverse array of metabolic/endocrine tolerability and safety concerns (ie, obesity/weight gain, type 2 diabetes mellitus, and dyslipidemia) are also attributable to frequently coprescribed SGAs in pediatric populations.8-14 The effect of antimanic and antipsychotic agents in combination on hyperprolactinemia and ensuing sexual/reproductive events may be more pronounced in postpubertal children and adolescents than in adults, prepubertal females, or males of any age.10
Furthermore, premarketing clinical trials indicate that some coprescribed antidepressant medications, ie, selective serotonin reuptake inhibitors (SSRIs), have a positive, often dose-related effect on a range of metabolic and digestive adverse events: nausea/vomiting, anorexia/weight loss, long-term obesity/weight gain, and metabolic disturbances, whereas serotonin-norepinephrine reuptake inhibitors (SNRIs) or heterocyclic antidepressants have a somewhat more pronounced effect on dyslipidemia, nausea/vomiting, and anorexia/weight loss.5,7,15-19 Hyperprolactinemia and the ensuing sexual/reproductive problems,18 such as galactorrhea, menstrual irregularities, and gynecomastia,5,20 are frequent side effects of the SSRIs5 compared to the newer SNRIs, heterocyclics, trazodone, or bupropion.5,7,19
There is a pressing need to better characterize the tolerability and safety profile of antimanic and other psychotropic agents commonly prescribed in young populations diagnosed with more severe forms of mood disorders. The expanding number of medications receiving US Food and Drug Administration approval for indication in both major depression and bipolar disorder and the frequent use of these agents off-label in children and adolescents underscore the importance of evaluating the therapeutic index of treatments in order that primary care practitioners can provide accurate information to patients and families, as well as appropriately select and sequence pharmacotherapies. Herein, the incidence/prevalence of disparate metabolic, digestive, and sexual/reproductive adverse events in an antimanic-treated cohort from South Carolina’s Medicaid system are compared with a random sample of children and adolescents served through Medicaid with no exposure to psychotropic medications, and the factors, eg, comorbid conditions, the coprescription of other psychotropic medications, and individual risk factors significantly related to the development of the metabolic, digestive, and sexual/reproductive adverse events are identified.
METHOD
Claims data for South Carolina’s Medicaid program were obtained through the state’s Office of Research and Statistics. Each Medicaid medical claim identifies a service encounter and gives the date of service and the International Classification of Diseases (ICD), Ninth Revision Clinical Modification diagnosis codes related to that visit (visit file). Pharmacy claims identified the medication dispensed and the date the prescription was filled (pharmacy file). A separate data file regarding eligibility was used to summarize the demographics for each patient (person file). The databases are frequently updated prior to being made available for analysis. This study was approved by the University of South Carolina Institutional Review Board as exempt from human subject research guidelines under 45 Code of Federal Regulations part 46.
Medical and pharmacy claims for the calendar years January 1, 1996, through December 31, 2005, were used to identify a cohort of child and adolescent patients (aged 17 years and under) enrolled in and eligible for Medicaid for a minimum of 9 months in each calendar year included in this analysis, who had a service encounter and were prescribed any of 3 medications used as mood stabilizers between January 1, 1998, and December 31, 2003. The date of first prescription of 1 of 3 antimanic/mood stabilizing medications (ie, valproic acid derivatives, lithium, or carbamazepine) in the Medicaid data set was defined as the selection encounter date. This process resulted in the identification of 3,657 patients for the treated cohort.
Out of the same population and from the same time period, medical and pharmacy claims were also used to identify a randomly selected group of child and adolescent patients (aged 17 years and younger) who were eligible for Medicaid 9 out of 12 months in all calendar years under study and had service encounters but no mental illness diagnosis and no prescriptions in the database for any class of psychotropic medication (antipsychotics, antidepressants, mood stabilizers, or psychostimulants). This process resulted in the identification of 40,660 patients who met the criteria. From this group, a random sample of 4,500 patients was selected to use as a representative control group. No matching procedure was employed, because we wanted to identify the adverse event and risk factor differences between the treated cohort and the untreated population-based, control sample and control for these differences in the statistical analyses.
Adverse Event Coding
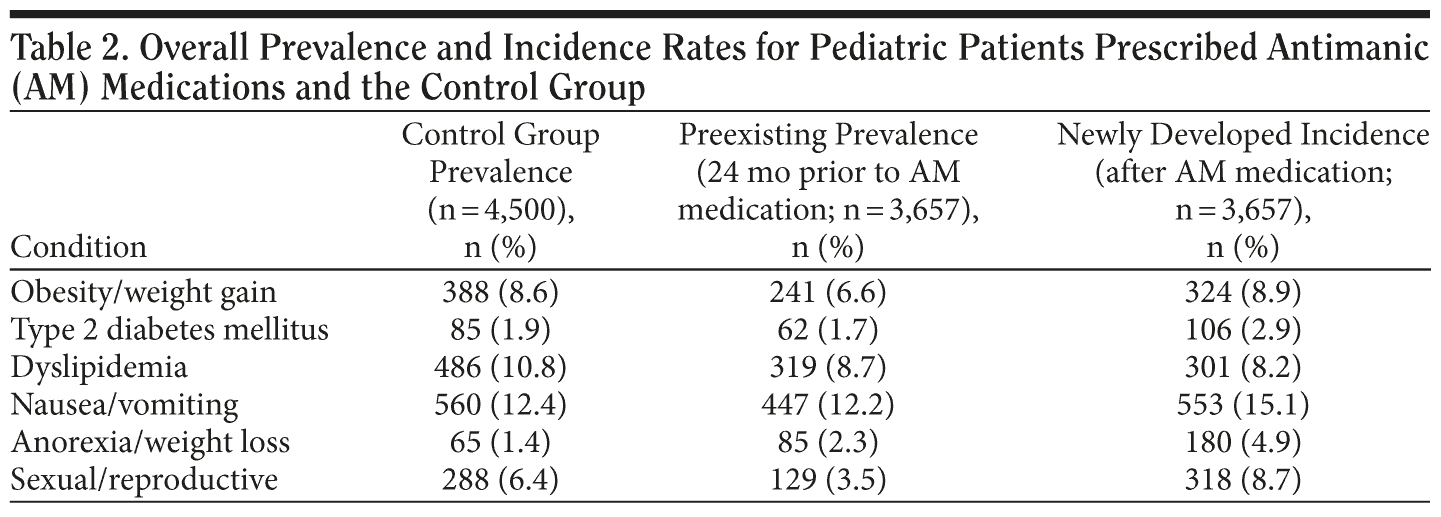
Metabolic, digestive, or sexual/reproductive medical conditions that were detected in the 24 months prior to each patient’s selection encounter date were coded as “preexisting” for this study. If the patient developed a medical condition subsequent to the prescription of the antimanic medication, new variables were created for these “incident” events. In the control group, detection of any of the metabolic, sexual/reproductive, or digestive medical conditions in a service billing record was coded. The following categories of conditions and events were evaluated: obesity or excessive weight gain (ICD-9 codes 278, 278.00, 278.01, 783.1, and 783.2); dyslipidemia (ICD-9 codes 272 and 272xx); type 2 diabetes mellitus (ICD-9 codes 250 and 250.00-251.92 with fifth digit = 0,2); anorexia or weight loss (ICD-9 codes 780.52, 783.0, and 783.21); nausea/vomiting (ICD-9 codes 787.01, 787.02, and 787.03); amenorrhea (ICD-9 code 626.0); oligomenorrhea (ICD-9 code 626.1); erectile dysfunction (ICD-9 codes 302.72 and 607.84); pituitary disorders, including hyperprolactinemia (ICD-9 code 253.xx); irregular menses (ICD-9 code 626.4); gynecomastia (ICD-9 codes 611.1 and 611.6); or galactorrhea (ICD-9 code 676). Data for both groups were monitored over the entire time they were in the Medicaid data set for 9 of 12 months per year for the development of the medical conditions of interest.
Analyses
To address research questions regarding differences in prevalence of the metabolic, digestive, and sexual/reproductive conditions/events in the treated cohort and control sample, 6 multiple logistic regression equations were constructed to assess the relative odds associated with developing each adverse event using the control group as the primary comparator and 3 individual risk factors (ie, sex, ethnicity, and age), dichotomously coded as male/female, African American/other, and age ≤ 12/age ≥ 13, to identify related covariates.
To identify factors associated with the incidence of metabolic, digestive, and sexual/reproductive events in the treated cohort of pediatric patients prescribed antimanic agents, including the role of comorbid medical conditions and concomitant psychotropic medications on the development of these conditions, 6 separate multiple logistic regression equations were constructed to assess the relative odds associated with developing each adverse event under scrutiny using carbamazepine and lithium coded separately as the main antimanic agent covariates (compared to valproic acid derivatives) and coprescriptions of SSRI antidepressants (compared to SNRIs), psychostimulants, or antipsychotics (compared to no prescriptions of these agents) as additional covariates of interest, controlling for the 3 dichotomously coded individual risk factors (ie, sex, ethnicity, and age).
Coprescribed medications were grouped together (categorized) because we were not specifically interested in the effect of individual medications, and existing safety and tolerability information demonstrates similar patterns of reactions/events for SSRIs, SNRIs, SGAs, and psychostimulants. Antidepressants were categorized as SSRIs for citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, and sertraline or SNRIs/heterocyclics for bupropion, duloxetine, maprotiline, mirtazapine, nefazodone, trazodone, or venlafaxine.5 Second-generation antipsychotics coded in the analyses were aripiprazole, ziprasidone, quetiapine, risperidone, or olanzapine. In this cohort, no child was prescribed haloperidol or fluphenazine. Psychostimulants coded in the analyses were methylphenidate, dextroamphetamine, and amphetamine salts.
Since patients with epilepsy/seizure disorder manifest mood symptoms that are highly similar to those in bipolar disorder, and there are similarities/overlap in the pharmacologic treatment of both disorders,21-23 we included/coded epilepsy/seizure disorder comorbid with any other psychiatric disorder treated with an antimanic medication as a separate covariate in the analyses to determine if there was any differential development of the metabolic or endocrine conditions being examined. Patients with epilepsy/seizure disorder as the only neuropsychiatric disorder being treated were omitted from the antimanic-treated cohort.
RESULTS
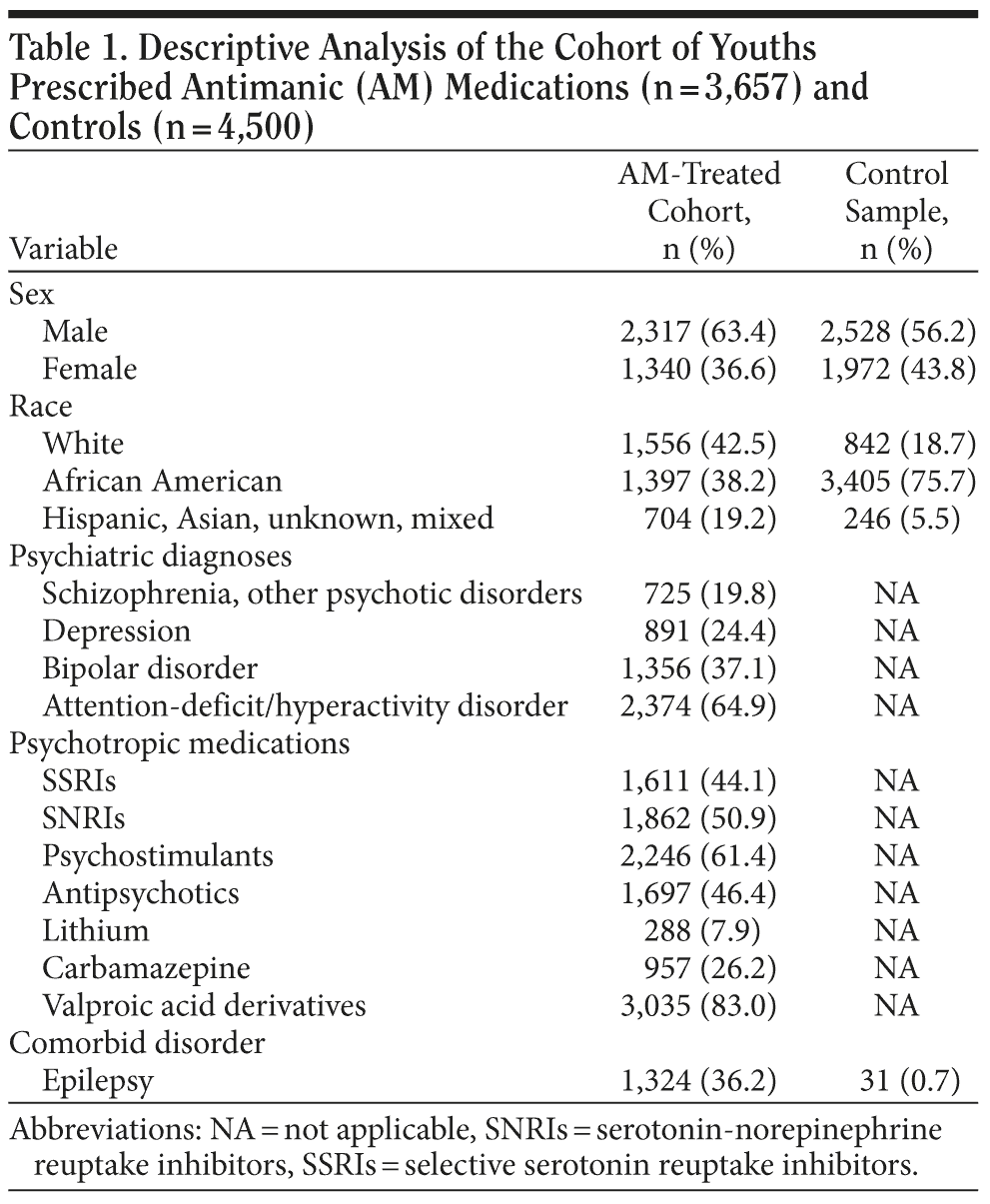
The treated cohort was primarily male and white compared to the control sample, which was primarily male and African American. The antimanic-treated cohort was being treated primarily for bipolar disorder and attention-deficit/hyperactivity disorder ([ADHD] Table 1), with a mean age of 10.3 (SD = 3.4) years of age at the time of antimanic initiation (selection date). Valproic acid derivatives (primarily valproate) were prescribed to 83% of these children and adolescents, along with contemporary antidepressant agents (SNRIs/others) (51%), psychostimulants (61%), and antipsychotics (46%). Antidepressants prescribed to more than 10% of the treated cohort were fluoxetine (n = 525; 14.4%), paroxetine (n = 643; 17.6%), and sertraline (n = 833; 22.8%) (categorized as SSRIs) and mirtazapine (an SNRI; n = 612; 16.7%), bupropion (a norepinephrine-dopamine reuptake inhibitor; n = 605; 16.5%), imipramine (a tricyclic antidepressant; n = 373; 10.2%), and trazodone (predominantly a 5-HT2A receptor antagonist; n = 518; 14.2%) (categorized as SNRIs). Psychostimulants prescribed to more than 10% of the treated cohort were methylphenidate (n = 1,835; 50.2%) and dextroamphetamine (n = 1,691; 46.2%). Atomoxetine, a selective norepinephrine reuptake inhibitor, was also prescribed for treatment of comorbid ADHD (n = 693; 19.0%). Antipsychotics prescribed to more than 10% of the treated cohort were olanzapine (n = 593; 16.2%), quetiapine (n = 606; 16.6%), or risperidone (n = 1,270; 34.7%).
Although the patients in the antimanic-treated cohort were 2 to 3 years older at selection into the cohort (start date of the antimanic medication) than those in the control sample, data were compiled on the treated cohort for 2 years prior to their selection date for analysis of the preexisting conditions, making their mean age at start date in the data set more comparable to the control group (7.6 years).
Comparison of Treated Cohort and Untreated Control Sample
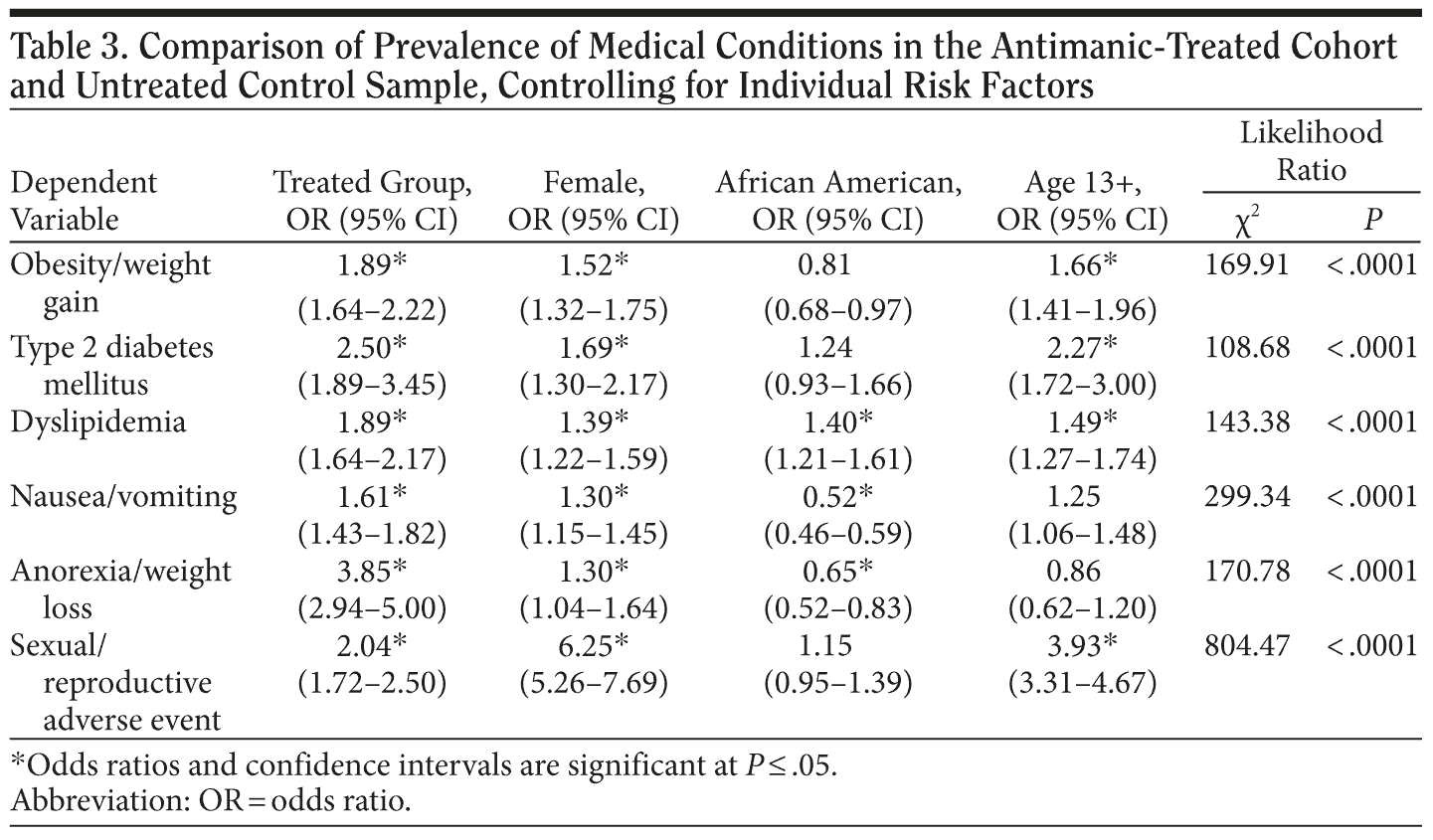
The incidence/prevalence rates for the conditions examined are presented in Table 2, along with the prevalence rates of these conditions in the untreated control sample. Table 3 presents statistical comparisons of the treated cohort and untreated control sample, controlling for individual risk factor (ie, age, sex, and ethnicity) differences. The cohort treated with antimanic medications and other psychotropic agents was more likely to have been diagnosed with obesity/weight gain (odds ratio [OR] = 1.89), type 2 diabetes mellitus (OR = 2.50), dyslipidemia (OR = 1.89), nausea (OR = 1.61), anorexia (OR = 3.85), and sexual/reproductive adverse events (OR = 2.04). Individual risk factor differences are presented to further inform provider practice, eg, to the uniform susceptibility of females to these medical conditions and adverse events.
Treated Cohort Analyses
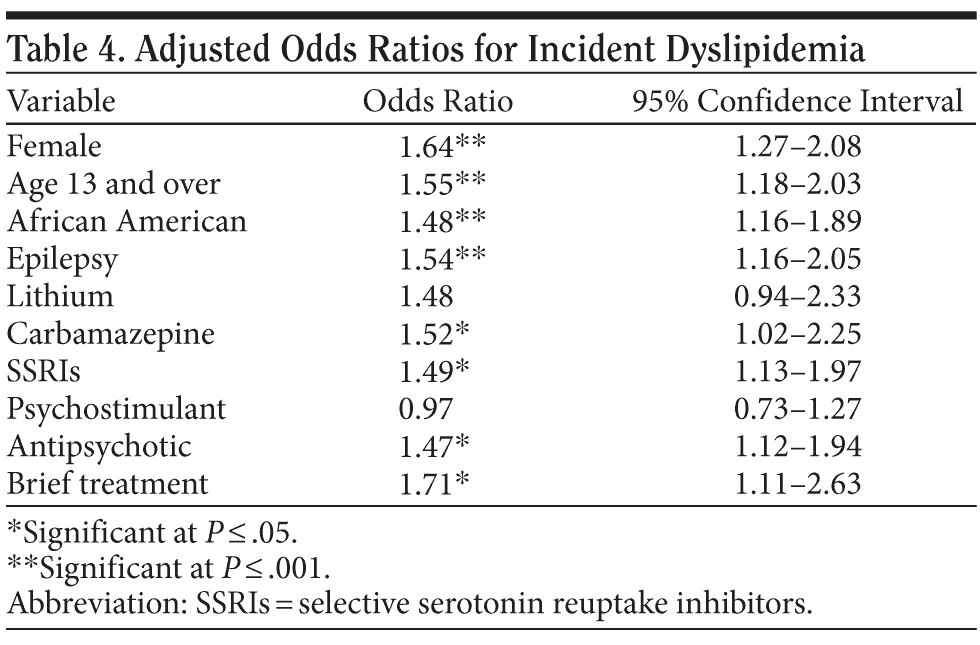
Within the antimanic-treated cohort, the odds of developing incident dyslipidemia were higher for those prescribed carbamazepine (OR = 1.52) compared to those taking valproate, those coprescribed SSRIs (OR = 1.60) or antipsychotics (OR = 1.47) compared to those taking SNRIs or not taking antipsychotics, those with comorbid epilepsy (OR = 1.54), or those in brief treatment (OR = 1.71) compared to long-term treatment with these agents, controlling for individual risk factor differences (Table 4).
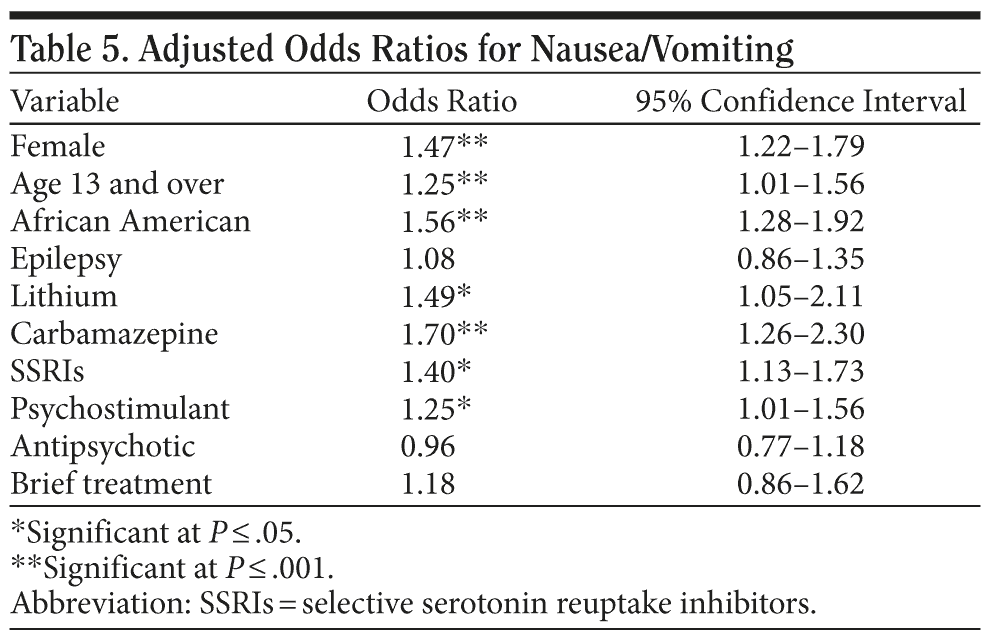
The incidence rates of developing nausea or vomiting that required medical intervention were higher for those taking carbamazepine (OR = 1.70) or lithium (OR = 1.49) compared to those taking valproate, those coprescribed SSRI antidepressants (OR = 1.49) or antipsychotics (OR = 1.47) compared to those taking SNRIs or not taking antipsychotics, controlling for individual risk factor differences (Table 5).
The odds of incident obesity/excessive weight gain were higher for those coprescribed SSRIs (OR = 1.72; CI = 1.32-2.26) or antipsychotics (OR = 1.69; CI = 1.29-2.21) compared to those taking SNRIs or not taking antipsychotics, controlling for individual risk factor differences. The incidence rates of developing type 2 diabetes mellitus were also higher for those coprescribed SSRIs (OR = 2.58; CI = 1.58-4.20) or antipsychotics (OR = 1.77; CI = 1.12-2.81) compared to those taking SNRIs or not taking antipsychotics, controlling for individual risk factor differences. However, the odds of incident type 2 diabetes mellitus were lower for those prescribed psychostimulants (OR = 0.60; CI = 0.38-0.93). The odds of developing anorexia or weight loss serious enough to require medical treatment were higher for those with comorbid epilepsy (OR = 1.63; CI = 1.14-2.33), controlling for individual risk factor differences.
The odds of developing hyperprolactinemia and/or the ensuing sexual/reproductive adverse events were higher for those coprescribed SSRIs (OR = 2.02; CI = 1.49-2.74) compared to those taking SNRIs, those with preexisting comorbid metabolic disorders of type 2 diabetes mellitus and dyslipidemia (ORs = 1.90; CI = 1.37-2.64), and those with other comorbid endocrine disorders (OR = 5.01; CI = 3.46-7.24), controlling for individual risk factor differences.
DISCUSSION
Based on the existing literature, we expected the metabolic, digestive, and sexual/reproductive conditions examined to be more prevalent and differentially distributed across the 3 antimanic agents prescribed to young patients in this database. We further expected that adverse event hazards would differentially exist when agents were prescribed as monotherapy or in combination with either antipsychotics or antidepressants. Individuals receiving antimanic treatment with the 3 agents investigated herein were more likely to have diagnosed incident obesity/weight gain, type 2 diabetes mellitus, dyslipidemia, nausea, anorexia, and sexual/reproductive events related to hyperprolactinemia than Medicaid patients without mental illness or treatment with psychotropic medications.
Moreover, females were differentially affected for each type of adverse event. The adolescent population was more likely to experience an adverse event when compared to the child cohort. Except for dyslipidemia and anorexia/weight loss, however, patients with comorbid seizure disorder/epilepsy demonstrated no significant differences from those without, which is important for practitioners to know.
Within the treated cohort, incident dyslipidemia was significantly associated with being prescribed carbamazepine compared to valproate and with being coprescribed SSRIs rather than SNRIs or antipsychotic agents compared to taking no antipsychotics. The capacity for carbamazepine to adversely affect lipid homeostasis has been reported in other studies.7-14
Our finding regarding the metabolic hazards of antidepressants in this younger population is also consistent with observations in adult populations. For example, coprescribed paroxetine (an SSRI prescribed to 17% of these patients) and mirtazapine (an SNRI/heterocyclic prescribed to 17% of these patients) exert an unfavorable effect on serum lipid parameters (ie, triglycerides and low-density lipoprotein cholesterol), whereas fluoxetine and sertraline (SSRIs prescribed to 17% and 22% of these patients, respectively) appear to have no direct effect on the lipid milieu.24,25
The association between nausea/vomiting and prescription of carbamazepine or lithium is also consonant with the relatively high rates at which these side effects have been reported in pivotal and effectiveness trials.7,20 While premarketing clinical trials indicate that some SSRIs have a dose-related effect on nausea/vomiting,5,7,15-19 there is no consistent evidence that antipsychotics induce this side effect.
Obesity/excessive weight gain and type 2 diabetes mellitus were also found to be more closely associated with coprescribed SSRIs and SGAs, with no differential effect for the antimanic agents investigated. These findings comport with those from previous studies in adult and pediatric populations for the SSRI agents and antipsychotics.5,7-9,11,12,14,15,18,24-26 The SSRI paroxetine24-26 and the SGAs risperidone (prescribed to 35% of these patients) and olanzapine (prescribed to 16%) are highly associated with incident type 2 diabetes and excessive weight gain.11 However, since valproic acid derivatives, which have been associated with metabolic hazards when prescribed as monotherapy and in combination with antipsychotics,10 were prescribed to a large percentage of individuals in this treated cohort (83%), no differential effect on metabolic indices was evident for any antimanic agent.
As reported elsewhere, sexual/reproductive adverse events related to hyperprolactinemia were more often noted in individuals coprescribed SSRIs. This association remained after controlling for coprescription of antipsychotic medication as well as comorbid endocrinopathies.7,14,17,18,24,26 No differential effect for valproic acid derivatives was identified in relation to the SSRI effect,27 which may be due to the low percentage of women taking valproic acid derivatives who experience menstrual or thyroid dysfunction (≤ 20%). Furthermore, the lack of association of sexual/reproductive events for those coprescribed SGAs was, perhaps, because olanzapine and quetiapine, agents not associated with sustained hyperprolactinemia, were used to the same extent as risperidone.4,8-11,13,14,28,29
It is important to distinguish adverse reactions to the prescribed pharmacotherapy from the psychopathological features of the illness being treated. Using a secondary database, we have tried to identify preexisting conditions, individual risk factors, or the medications prescribed for other mental illnesses being treated and either eliminate them from the analysis of incident adverse events or statistically control for them as important sources of outcome variation. However, we are presenting findings for adverse events/side effects that are commonly monitored as such, and we defer to the treating clinicians to determine if a presenting symptom/condition is a side effect or a clinical feature of the illness being treated.
The perspective provided by this longitudinal database has several strengths: (1) the cohort represents a large, heterogeneous group of children and adolescents with varying periods of exposure to antidepressant and other psychotropic medications; (2) there is sufficient power in the treated cohort and untreated sample sizes to detect somewhat low-incidence conditions and combine these conditions into related groupings; (3) previous studies have found that, although Medicaid databases provide much less detailed information on individuals than a structured research interview, the physician diagnoses and utilization data are more reliable than client or family self-reports30,31; and (4) the outcomes of disparate digestive, metabolic, and sexual/reproductive events related to antidepressant and concomitant medication use are clinically relevant and of substantial public health importance.
These results also need to be interpreted with several limitations in mind: (1) the data were not obtained through controlled trial methods but through a secondary administrative data set using observational techniques in a retrospective cohort design; (2) structured research and clinical interviews were not employed to confirm any of the assigned medical disorders or patient compliance with the prescribed medication regimen; (3) dosage information in the pharmacy file was not a reliable indicator of prescribed or actual daily dosage, so this clinical information was omitted from the presentation but is clearly important to interpreting the adverse events noted; (4) the reporting of adverse events was based on spontaneous reporting to a physician and may be an underestimate; (5) the detection of any adverse event was also dependent on the ICD-9 codes being properly submitted to Medicaid, so adverse events are also likely to be an underestimate; (6) these results report associations and, as a result, directions of causality cannot be inferred; (7) key risk factors such as family history of metabolic disorders were not available in the database and are not modeled in these analyses; and (8) there is no way to estimate how representative this Medicaid cohort is in relation to those in other states and service systems.
During the last decade, the rate of diagnosing bipolar disorder among pediatric populations utilizing primary care and specialty outpatient services has significantly increased.32 This analysis draws further attention to the long-term safety profiles of antimanic and coprescribed psychotropic agents in pediatric populations to enhance and guide clinical decision support. These results indicate that antimanic medications are associated with adverse events in community-based care settings, and they are germane to the overall appraisal of the benefits and risks of this class of agents, especially when prescribed in tandem with other classes of agents. When evaluating the overall benefit-risk ratio of antimanic medications in children and adolescents, the primary care practitioner needs to give careful consideration to possible metabolic disruptions, digestive symptoms, or sexual/reproductive side effects of these agents, especially in individuals with comorbid metabolic/endocrine conditions and those receiving concomitant psychotropic medications.
Drug names: amphetamine salts (Adderall and others), aripiprazole (Abilify), atomoxetine (Strattera), bupropion (Aplenzin, Wellbutrin, and others), carbamazepine (Carbatrol, Equetro, and others), citalopram (Celexa and others), dextroamphetamine (Dexedrine and others), duloxetine (Cymbalta), escitalopram (Lexapro), fluoxetine (Prozac and others), fluvoxamine (Luvox and others), haloperidol (Haldol and others), imipramine (Tofranil, Surmontil, and others), lithium (Eskalith, Lithobid, and others), methylphenidate (Ritalin and others), mirtazapine (Remeron and others), olanzapine (Zyprexa), paroxetine (Paxil, Pexeva, and others), quetiapine (Seroquel), risperidone (Risperdal and others), sertraline (Zoloft and others), trazodone (Oleptro and others), valproate (Depacon and others), valproic acid (Stavzor, Depakene, and others), venlafaxine (Pristiq, Effexor, and others), ziprasidone (Geodon).
Author affiliation: Department of Neuropsychiatry and Behavioral Science, University of South Carolina School of Medicine, Columbia (Dr Jerrell); and Department of Psychiatry and Pharmacology, University of Toronto, Ontario, Canada (Dr McIntyre).
Potential conflicts of interest: Dr McIntyre has received research funding from AstraZeneca, GlaxoSmithKline, Merck, Servier, and Wyeth and is a consultant and speaker for AstraZeneca, Eli Lilly, GlaxoSmithKline, Lundbeck, Organon, Ortho-McNeil-Janssen, Oryx, Pfizer, Prestwick, and Wyeth. Dr Jerrell has no conflicts of interest to disclose regarding the publication of these results.
Funding/support: Data analysis was supported by a State Mental Health Data Infrastructure Grant (SAMHSA SM54192).
Disclaimer: The views expressed do not necessarily represent those of the funding agency or official findings of the South Carolina Department of Health and Human Services (Medicaid).
Acknowledgment: The authors thank Ling Li, MSPH, a graduate student in the Biostatistics Department of the Arnold School of Public Health, University of South Carolina, Columbia, for performing the analyses. Ms Li reports no financial or other relationship relevant to the subject of this article.
REFERENCES
1. Kowatch RA, Fristad M, Birmaher B, et al. Child Psychiatric Workgroup on Bipolar Disorder. Treatment guidelines for children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2005;44(3):213-235. PubMed doi:10.1097/00004583-200503000-00006
2. Krishnan KRR. Psychiatric and medical comorbidities of bipolar disorder. Psychosom Med. 2005;67(1):1-8. PubMed doi:10.1097/01.psy.0000151489.36347.18
3. Azorin JM, Findling RL. Valproate use in children and adolescents with bipolar disorder. CNS Drugs. 2007;21(12):1019-1033. PubMed doi:10.2165/00023210-200721120-00005
4. Correll CU. Weight gain and metabolic effects of mood stabilizers and antipsychotics in pediatric bipolar disorder: a systematic review and pooled analysis of short-term trials. Am Acad Child Adolesc Psychiatry. 2007;46(6):687-700. PubMed doi:10.1097/chi.0b013e318040b25f
5. Gold Standard, Inc. Carbamazepine, lithium, valproic acid derivatives, citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, and sertraline: adverse reactions. http://www.mdconsult.com. Accessed January 30, 2008.
6. Fedorowicz VJ, Fombonne E. Metabolic side effects of atypical antipsychotics in children: a literature review. J Psychopharmacol. 2005;19(5):533-550. PubMed doi:10.1177/0269881105056543
7. Hirschfeld RMA. The role of atypical antipsychotics in the treatment of bipolar disorder. J Clin Psychiatry. 2005;66(suppl 3):3-4.
8. Byerly M, Suppes T, Tran QV, et al. Clinical implications of antipsychotic-induced hyperprolactinemia in patients with schizophrenia spectrum or bipolar spectrum disorders: recent developments and current perspectives. J Clin Psychopharmacol. 2007;27(6):639-661. PubMed doi:10.1097/jcp.0b013e31815ac4e5
9. Cheng-Shannon J, McGough JJ, Pataki C, et al. Second-generation antipsychotic medications in children and adolescents. J Child Adolesc Psychopharmacol. 2004;14(3):372-394. PubMed doi:10.1089/cap.2004.14.372
10. Correll CU, Carlson HE. Endocrine and metabolic adverse effects of psychotropic medications in children and adolescents. J Am Acad Child Adolesc Psychiatry. 2006;45(7):771-791. PubMed
11. Correll CU, Penzner JB, Parikh UH, et al. Recognizing and monitoring adverse events of second-generation antipsychotics in children and adolescents. Child Adolesc Psychiatr Clin N Am. 2006;15(1):177-206. PubMed doi:10.1016/j.chc.2005.08.007
12. Goodnick PJ, Rodriguez L, Santana O. Antipsychotics: impact on prolactin levels. Expert Opin Pharmacother. 2002;3(10):1381-1391. PubMed doi:10.1517/14656566.3.10.1381
13. McIntyre RS, Konarski JZ. Tolerability profiles of atypical antipsychotics in the treatment of bipolar disorder. J Clin Psychiatry. 2005;66(suppl 3):28-36. PubMed
14. Toren P, Ratner S, Laor N, et al. Benefit-risk assessment of atypical antipsychotics in the treatment of schizophrenia and comorbid disorders in children and adolescents. Drug Saf. 2004;27(14):1135-1156. PubMed doi:10.2165/00002018-200427140-00005
15. Burke MJ, Lincoln J. Mood disorders. In: Bakel RE, Bope ET, eds. Conn’s Current Therapy 2006. Philadelphia, PA: WB Saunders Co; 2006:1361-1370.
16. Chen JL, Spinowitz N, Karwa M. Hypertriglyceridemia, acute pancreatitis, and diabetic ketoacidosis possibly associated with mirtazapine therapy: a case report. Pharmacotherapy. 2003;23(7):940-944. PubMed doi:10.1592/phco.23.7.940.32725
17. Cohen D, Gerardin P, Mazet P, et al. Pharmacological treatment of adolescent major depression. J Child Adolesc Psychopharmacol. 2004;14(1):19-31. PubMed doi:10.1089/104454604773840454
18. Hu XH, Bull SA, Hunkeler EM, et al. Incidence and duration of side effects and those rated as bothersome with SSRI treatment for depression: patient report versus physician estimate. J Clin Psychiatry. 2004;65(7):959-965. PubMed doi:10.4088/JCP.v65n0712
19. Masand PS, Gupta S. Long-term side effects of newer-generation antidepressants: SSRIs, venlafaxine, nefazodone, bupropion, and mirtazapine. Ann Clin Psychiatry. 2002;14(3):175-182. PubMed doi:10.3109/10401230209147454
20. Ellsworth AJ, Witt DM, Dugdale DC, et al. Mosby’s Medical Drug Reference. St. Louis, MO: Mosby; 2006.
21. Harden CL, Goldstein MA. Mood disorders in patients with epilepsy: epidemiology and management. CNS Drugs. 2002;16(5):291-302. PubMed doi:10.2165/00023210-200216050-00002
22. Post RM, Weiss SR. Convergences in course of illness and treatments of the epilepsies and recurrent affective disorders. Clin EEG Neurosci. 2004;35(1):14-24. PubMed
23. Mula M, Schmitz B, Jauch R, et al. On the prevalence of bipolar disorder in epilepsy. Epilepsy Behav. 2008;13(4):658-661. PubMed doi:10.1016/j.yebeh.2008.08.002
24. McIntyre RS, Soczynska JK, Konarski JZ, et al. The effect of antidepressants on glucose homeostasis and insulin sensitivity: synthesis and mechanisms. Expert Opin Drug Saf. 2006;5(1):157-168. PubMed doi:10.1517/14740338.5.1.157
25. Andersohn F, Schade R, Suissa S, et al. Long-term use of antidepressants for depressive disorders and the risk of diabetes mellitus. Am J Psychiatry. 2009;166(5):591-598. PubMed doi:10.1176/appi.ajp.2008.08071065
26. McIntyre RS, Soczynska JK, Konarski JZ, et al. The effect of antidepressants on lipid homeostasis: a cardiac safety concern? Expert Opin Drug Saf. 2006;5(4):523-537. PubMed doi:10.1517/14740338.5.4.523
27. Isojärvi JIT, Laatikainen TJ, Pakarinen AJ, et al. Polycystic ovaries and hyperandrogenism in women taking valproate for epilepsy. N Engl J Med. 1993;329(19):1383-1388. PubMed doi:10.1056/NEJM199311043291904
28. Kim KS, Pae CU, Chae JH, et al. Effects of olanzapine on prolactin levels of female patients with schizophrenia treated with risperidone. J Clin Psychiatry. 2002;63(5):408-413. PubMed
29. Kryzhanovskaya LA, Robertson-Plouch CK, Xu W, et al. The safety of olanzapine in adolescents with schizophrenia or bipolar I disorder: a pooled analysis of 4 clinical trials. J Clin Psychiatry. 2009;70(2):247-258. PubMed doi:10.4088/JCP.08m03538
30. Holmberg SK, Kane C. Health and self-care practices of persons with schizophrenia. Psychiatr Serv. 1999;50(6):827-829. PubMed
31. Mark TL, Buck JA, Dilonardo JD, et al. Medicaid expenditures on behavioral health care. Psychiatr Serv. 2003;54(2):188-194. PubMed doi:10.1176/appi.ps.54.2.188
32. Moreno C, Laje G, Blanco C, et al. National trends in the outpatient diagnosis and treatment of bipolar disorder in youth. Arch Gen Psychiatry. 2007;64(9):1032-1039. PubMed doi:10.1001/archpsyc.64.9.1032