This work may not be copied, distributed, displayed, published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the publisher’s Terms & Conditions.
Article Abstract
Objective: To evaluate the effect of 32-mg/d naltrexone sustained release and 360-mg/d bupropion sustained release (NB32) in overweight and obese patients with major depressive disorder (MDD).
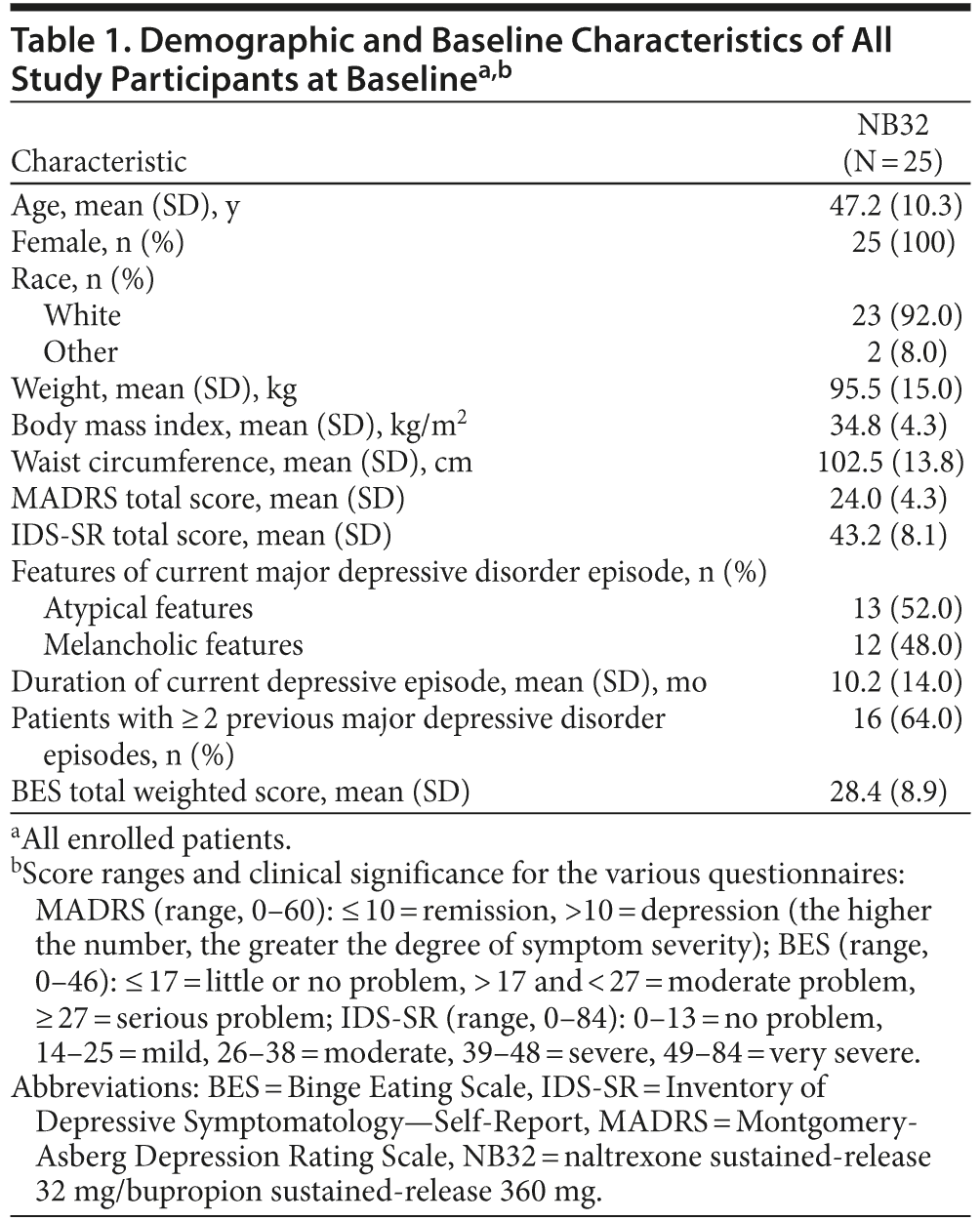
Method: Twenty-five female patients with a DSM-IV diagnosis of MDD, an Inventory of Depressive Symptomatology—Self-Report score > 26, and a body mass index ≥ 27 and ≤ 43 kg/m2 received up to 24 weeks of open-label treatment with NB32 with dietary and behavioral counseling (data collection: March 2008-July 2009). The primary endpoint was change from baseline in the Montgomery-Asberg Depression Rating Scale (MADRS) total score at 12 weeks; secondary endpoints included MADRS total score at week 24, change in weight, and Clinical Global Impressions-Improvement scale responder status (CGI-I score ≤ 2) at weeks 12 and 24 (modified intent-to-treat [mITT]: patients with ≥ 1 postbaseline MADRS total score on study drug; N = 23).
Results: MADRS scores showed significant reductions at weeks 12 and 24 (mITT-last observation carried forward [LOCF]: -13.1 ± 7.1 and -15.3 ± 8.1, respectively, P < .001 vs baseline for all). Mean ± SD weight loss was -4.0% ± 4.6% (mITT-LOCF) and -6.1% ± 4.7% (observed cases) at week 12 and -5.3% ± 6.5% (mITT-LOCF) and -9.2% ± 6.2% (observed cases) at week 24 (P < .001 vs baseline for all). By week 24, 95% of patients (mITT-LOCF) were responders (CGI-I score ≤ 2) and 70% were in remission (CGI-I score = 1). The safety/tolerability profile of NB32 was consistent with its individual components; the most common adverse events were nausea, constipation, headache, and insomnia, with no serious adverse events attributed to NB32.
Conclusions: Twenty-four weeks of open-label NB32 therapy with dietary and behavioral counseling was associated with improvement in depressive symptoms and reduced body weight in overweight/obese women with MDD.
Trial Registration: ClinicalTrials.gov Identifier: NCT00624858
Prim Care Companion CNS Disord 2013;15(3):doi:10.4088/PCC.12m01494
© Copyright 2013 Physicians Postgraduate Press, Inc.
Submitted: November 20, 2012; accepted February 18, 2013.
Published online: June 20, 2013.
Corresponding author: Susan L. McElroy, MD, Research Institute, Lindner Center of HOPE, 4075 Old Western Row Rd, Mason, OH 45040 ([email protected]).
Naltrexone/Bupropion Combination Therapy in Overweight or Obese Patients With Major Depressive Disorder: Results of a Pilot Study
ABSTRACT
Objective: To evaluate the effect of 32-mg/d naltrexone sustained release and 360-mg/d bupropion sustained release (NB32) in overweight and obese patients with major depressive disorder (MDD).
Method: Twenty-five female patients with a DSM-IV diagnosis of MDD, an Inventory of Depressive Symptomatology—Self-Report score > 26, and a body mass index ≥ 27 and ≤ 43 kg/m2 received up to 24 weeks of open-label treatment with NB32 with dietary and behavioral counseling (data collection: March 2008-July 2009). The primary endpoint was change from baseline in the Montgomery-Asberg Depression Rating Scale (MADRS) total score at 12 weeks; secondary endpoints included MADRS total score at week 24, change in weight, and Clinical Global Impressions-Improvement scale responder status (CGI-I score ≤ 2) at weeks 12 and 24 (modified intent-to-treat [mITT]: patients with ≥ 1 postbaseline MADRS total score on study drug; N = 23).
Results: MADRS scores showed significant reductions at weeks 12 and 24 (mITT-last observation carried forward [LOCF]: -13.1 ± 7.1 and -15.3 ± 8.1, respectively, P < .001 vs baseline for all). Mean ± SD weight loss was -4.0% ± 4.6% (mITT-LOCF) and -6.1% ± 4.7% (observed cases) at week 12 and -5.3% ± 6.5% (mITT-LOCF) and -9.2% ± 6.2% (observed cases) at week 24 (P < .001 vs baseline for all). By week 24, 95% of patients (mITT-LOCF) were responders (CGI-I score ≤ 2) and 70% were in remission (CGI-I score = 1). The safety/tolerability profile of NB32 was consistent with its individual components; the most common adverse events were nausea, constipation, headache, and insomnia, with no serious adverse events attributed to NB32.
Conclusions: Twenty-four weeks of open-label NB32 therapy with dietary and behavioral counseling was associated with improvement in depressive symptoms and reduced body weight in overweight/obese women with MDD.
Trial Registration: ClinicalTrials.gov Identifier: NCT00624858
Prim Care Companion CNS Disord 2013;15(3):doi:10.4088/PCC.12m01494
© Copyright 2013 Physicians Postgraduate Press, Inc.
Submitted: November 20, 2012; accepted February 18, 2013.
Published online: June 20, 2013.
Corresponding author: Susan L. McElroy, MD, Research Institute, Lindner Center of HOPE, 4075 Old Western Row Rd, Mason, OH 45040 ([email protected]).
Over the past decade, the prevalence of obesity in the United States has continued to grow in both adults1 and children.2 In parallel, the 1-year prevalence of major depressive disorder (MDD) has been increasing,3 with an estimated 1-year prevalence of 6.7%4 and an estimated lifetime prevalence of 16.6%.5
Meta-analyses of both cross-sectional and longitudinal studies indicate that there is a significant and bidirectional association between obesity and depression, especially MDD.6,7 The longitudinal risk (unadjusted odds ratio [OR]) of developing depression in individuals with obesity is 1.55 (P < .001), while the presence of MDD also significantly increases the longitudinal risk of becoming obese (OR = 1.58, P < .001).7 The long-term risk of developing obesity appears to be especially high in adolescent girls with depression (OR = 2.57).8 The reasons for the significant bidirectional association between obesity and depression are uncertain but are most likely to be multifactorial, including both psychological and neurobiological pathways.
In addition to increasing the risk of developing MDD, clinical treatment outcomes are worse when MDD is accompanied by the presence of obesity. Obesity has been reported to predict poor antidepressant response in depression,9-13 more chronic episodes,14 and a higher risk of recurrence in patients with bipolar I disorder.15
A naltrexone sustained-release (SR) and bupropion SR combination therapy is currently under development for the treatment of obesity. Naltrexone is a competitive antagonist at the μ- and κ-opioid receptors that is approved in the United States for the treatment of opioid and alcohol dependence.16 Bupropion is a dopamine and norepinephrine reuptake inhibitor17 and is approved in the United States for the treatment of MDD, seasonal affective disorder, and nicotine dependence.18-20 Bupropion has also demonstrated modest efficacy as a monotherapy in the treatment of obesity,21 including obesity in patients with depressive symptoms.22
The naltrexone/bupropion combination is hypothesized to have actions in several brain regions key to regulation of food intake. Treatment is believed to modulate the mesolimbic dopaminergic reward pathways, which are involved in food intake behavior and mood states. Furthermore, this combination is believed to elicit complementary effects at the hypothalamus, which regulates energy homeostasis.23-25 Specifically, bupropion via combined dopaminergic and noradrenergic effects26-28 stimulates proopiomelanocortin (POMC) neurons in the arcuate nucleus of the hypothalamus that reduce appetite,24 and the addition of naltrexone is hypothesized to block β-endorphin-mediated POMC autoinhibition, thereby augmenting bupropion-stimulated POMC activation of these anorexigenic pathways.24 Thus, this novel combination treatment is believed to modulate the central nervous system to elicit sustained appetite reduction and enhanced control of eating behavior.
In 2 phase 2 weight loss studies in obese adults without MDD, combination treatment with naltrexone and bupropion reduced body weight compared with placebo and bupropion or naltrexone monotherapy.24,29 In nondepressed obese patients participating in phase 3 clinical trials, naltrexone/bupropion combination therapy with standard behavioral modification resulted in mean weight changes of −6.1% and −6.4% vs −1.3% and −1.2% with placebo,30,31 while mean body weight changes on a background of more intensive, group-based lifestyle intervention were -9.3% (naltrexone/bupropion) and −5.1% (placebo).32 The results of these studies, together with previous clinical trials of bupropion in depression, suggest that naltrexone/bupropion combination therapy may be uniquely suited to address the overlap of MDD and obesity. The primary objective of the current study was to obtain proof-of-concept data on the efficacy and safety of naltrexone/bupropion plus diet and exercise counseling in treating overweight or obese patients with MDD.
- There is a significant and bidirectional association between obesity and depression, especially major depressive disorder (MDD), and clinical outcomes are worse when MDD is accompanied by the presence of obesity.
- Combination therapy with naltrexone sustained-release and bupropion sustained-release has demonstrated substantial and sustained weight loss in overweight/obese nondepressed individuals; bupropion monotherapy is approved for the treatment of MDD.
- The results of this open-label study suggest that combination therapy with naltrexone/bupropion, along with dietary and behavioral counseling, may be a potentially effective treatment for MDD complicated by concurrent overweight or obesity.
METHOD
Study Population
Patients who were enrolled met all of the following inclusion criteria: female or male patients aged 18 to 65 years (inclusive), body mass index (BMI) ≥ 27 and ≤ 43 kg/m2, MDD without psychotic features according to DSM-IV criteria, and an Inventory of Depressive Symptomatology—Self-Report (IDS-SR) total score > 26 at screening and baseline. Patients were excluded from the study if they met any of the following criteria: a history of treatment with bupropion or naltrexone within the previous 12 months, weight loss or gain > 4 kg (9 lb) within the previous 3 months, surgical intervention for obesity, obesity of known endocrine origin, a history of drug or alcohol abuse or dependence (except nicotine dependence) within the previous 12 months, a history of nonresponse to 2 or more adequate courses of antidepressant therapy, any serious medical condition, and any clinically significant abnormalities in laboratory measures, vital signs, or electrocardiogram (ECG) (baseline ECG with corrected QT interval > 450 ms [males] and > 470 ms [females]). Female patients of childbearing potential were required to have a negative serum pregnancy test, to be using a medically acceptable form of contraception, and to be nonlactating.
Excluded concomitant medications included any psychotropic agents (with the exception of low-dose benzodiazepine or hypnotic sleep aids); any anorectic or weight loss agents; any over-the-counter dietary supplements or herbs with psychoactive, appetite, or weight effects; α-adrenergic blockers; dopamine agonists; clonidine; coumadin; theophylline; cimetidine; oral corticosteroids; cholestyramine or cholestipol; Depo-Provera; smoking cessation agents; and opioid or opioid-like medications.
Study Design
This single-center, open-label, 1-arm, prospective study of 24 weeks of treatment with 32-mg/d naltrexone SR and 360-mg/d bupropion SR (NB32) daily administered in divided doses was conducted between March 2008 and July 2009. The study protocol was approved by the University of Cincinnati Institutional Review Board (Cincinnati, Ohio), and each patient provided written informed consent prior to study entry. Study conduct was consistent with Good Clinical Practice standards and the Declaration of Helsinki. The study was registered in ClinicalTrials.gov (identifier: NCT00624858).
Study Treatment
The naltrexone/bupropion combination was provided as single tablets with 8 mg of naltrexone SR and 90 mg of bupropion SR, and participants were instructed to take 2 tablets with food twice a day (ie, morning and evening). Medication was initiated at one-quarter of the daily maintenance dose and increased weekly over the first 4 weeks (with the maintenance dose reached at the beginning of the fourth week). Patients who took < 70% of prescribed study drug for 2 consecutive visits were discontinued from the study. In addition to study medication, all patients received counseling at baseline and weeks 6, 12, and 16 in diet instruction, advice on behavior modification, and a prescription for exercise from a registered dietician. The exercise regimen consisted of progressively adding 5-10 minutes of walking 5-7 days/wk to the patient’s baseline exercise pattern.
Outcome Measures and Assessment Tools
The primary outcome measure was the 10-item clinician-rated Montgomery-Asberg Depression Rating Scale (MADRS).33 Secondary outcome measures included body weight, the Clinical Global Impressions-Improvement (CGI-I) scale,34 the 30-item subject-rated IDS-SR,35 the 16-item Binge Eating Scale (BES),36,37 and the Control of Eating Questionnaire (COEQ),38-40 which consists of 20 visual analog scales designed to assess changes in appetite, food craving, eating behavior, and mood. The MADRS was assessed at baseline and at weeks 1, 2, 3, 4, 6, 8, 12, 16, 20, and 24 (or early termination). The IDS-SR, which was used to secondarily measure depressive symptoms as well as to detect emergent suicidality, was assessed at screening, baseline, and weeks 4, 8, 12, 16, 20, and 24. The BES and COEQ were assessed at baseline and along with the CGI-I at weeks 4, 8, 12, and 24. Body weight (determined on the same scale with the patient wearing no shoes) was measured at each visit, and waist circumference was obtained at baseline, week 12, and week 24 (or early termination). Fasting blood samples for serum leptin and ghrelin levels and salivary cortisol were obtained at baseline, week 4 (cortisol only), week 12, and week 24 (or early termination). Safety assessments consisted of evaluation of treatment-emergent adverse events, vital signs, clinical laboratory measures, ECGs, and physical examinations.
Response was defined as CG-I score ≤ 2 (much or very much improved) and remission as CGI-I score = 1 (very much improved). Score ranges and clinical significance for the other questionnaires were MADRS score (range, 0-60) ≤ 10 = remission and > 10 = depression (the higher the number, the greater the degree of symptom severity); BES score (range, 0-46) ≤ 17 = little or no problem, > 17 and < 27 = moderate problem, and ≥ 27 = serious problem; and IDS-SR score (range, 0-84) 0-13 = no problem, 14-25 = mild, 26-38 = moderate, 39-48 = severe, and 49-84 = very severe.
Statistical Methods
Two populations were evaluated during this trial. The modified intent-to-treat (mITT) population included all patients with a baseline measurement and at least 1 postbaseline MADRS total score measurement while on study drug. The safety population included all patients who received at least 1 tablet of study drug and had at least 1 investigator contact/assessment at any time after beginning treatment.
The primary efficacy endpoint of this study was the change in MADRS total score from baseline to week 12. Secondary efficacy endpoints included the change in MADRS total score from baseline to week 24; the percent change in body weight and waist circumference from baseline to weeks 12 and 24; the proportions of patients experiencing response and remission on the CGI-I scale at weeks 12 and 24; the effects of NB32 on food cravings and binge eating as measured by COEQ and BES, respectively, at weeks 12 and 24; and changes in serum leptin, ghrelin, and salivary cortisol levels from baseline to weeks 12 and 24. The change in IDS-SR total score from baseline to week 24 was an additional endpoint of the study. Safety endpoints for the study included treatment-emergent adverse events and changes in clinical laboratory data, vital signs, and ECG parameters from baseline to subsequent visits.
For the categorical outcome variables, descriptive statistics included number and percentage of patients for each category. Percentages were calculated on the basis of patients with nonmissing data as the denominator (ie, those patients with missing data were excluded). For the continuous outcome variables, descriptive statistics included number of patients, mean, standard deviation, median, minimum, and maximum. The change from baseline was computed. Within-group t test statistics and 95% confidence intervals were calculated to evaluate whether the change from baseline was statistically significantly different from zero. The end point was defined as the last nonmissing postbaseline observation while on study drug (last observation carried forward [LOCF]).
RESULTS
Twenty-five women met study entry criteria and were enrolled, of whom 2 patients were not available for an on-drug MADRS assessment and therefore were not included in the mITT analysis population. Table 1 summarizes the demographic and clinical characteristics of all study participants at baseline. Fourteen patients (56%) completed 12 weeks and 13 patients (52%) completed 24 weeks of study treatment. Twelve patients (48%) discontinued treatment. Of these, 1 patient (4%) discontinued due to nonadherence with study drug, 1 (4%) was lost to follow-up before week 12, and the other 10 patients (40%) discontinued due to an adverse event, of whom 9 discontinued before week 12. Adverse events leading to withdrawal were dizziness, vomiting, back pain, feeling abnormal, somnolence, insomnia, pruritus, irritability, gastroenteritis, and mood swings.
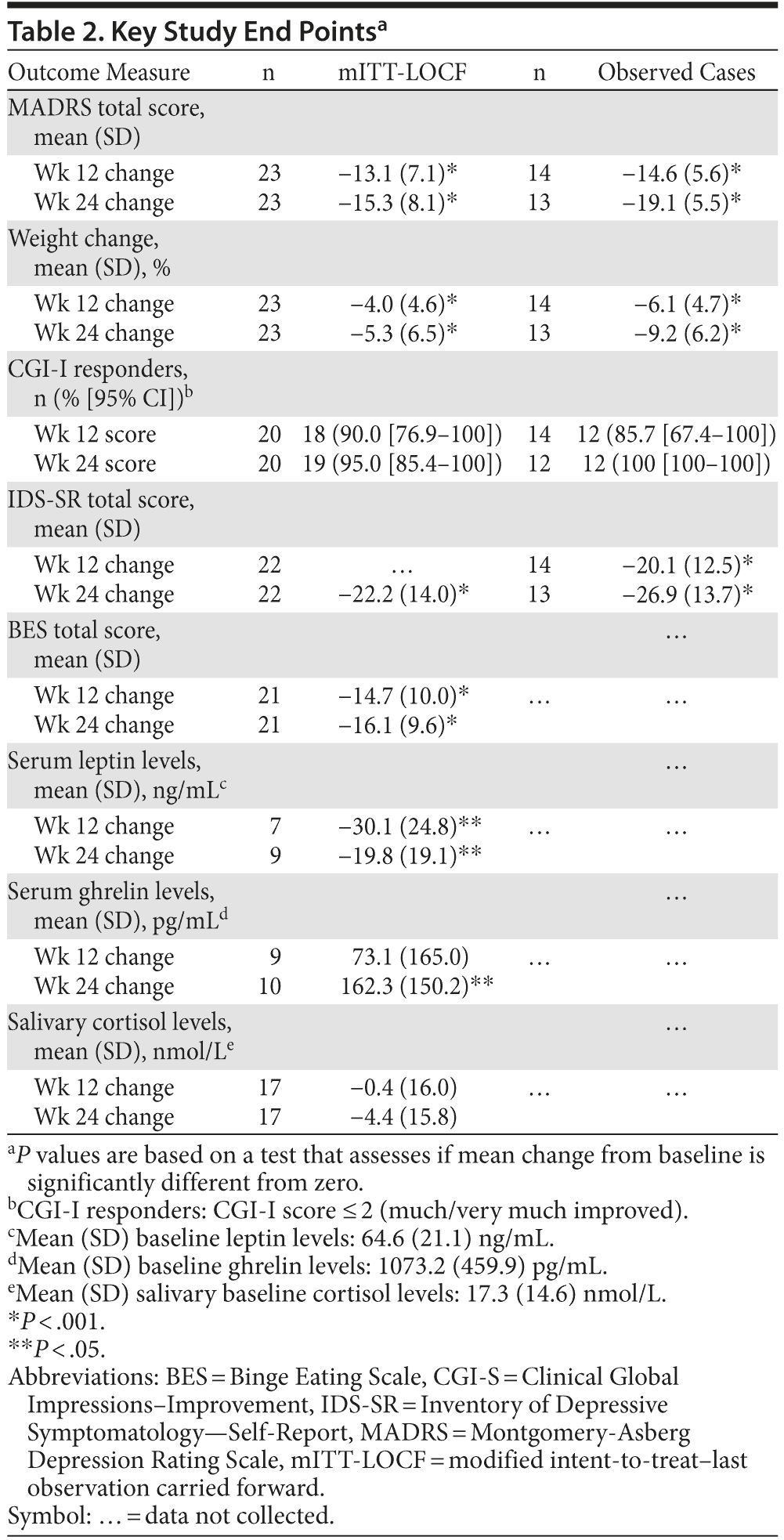
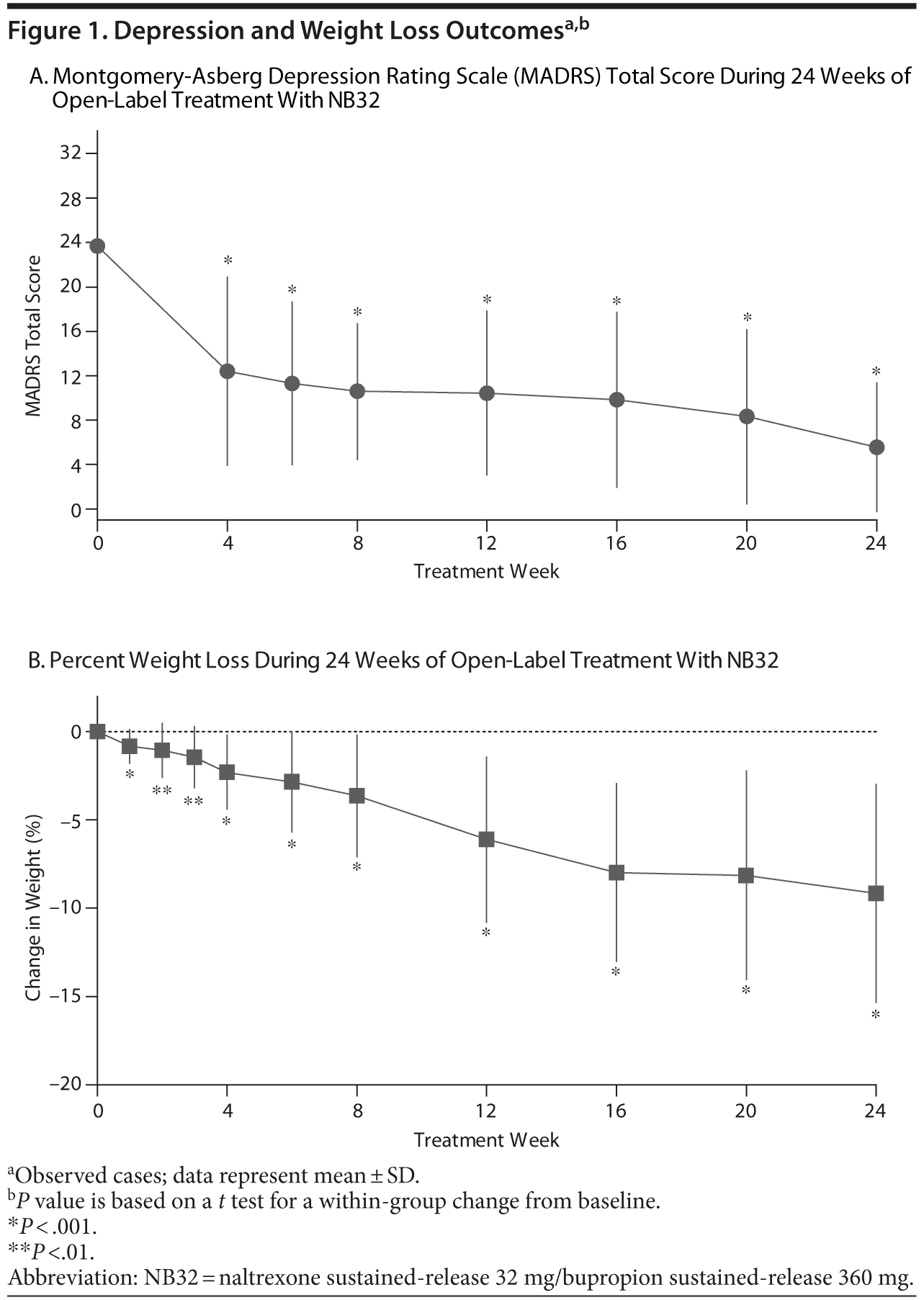
Open-label treatment with NB32 in combination with dietary and behavioral counseling resulted in improvement on the primary efficacy measure, the mean ± SD MADRS total score, at week 12 in the mITT-LOCF analysis set (−13.1 ± 7.1, P < .001) and on an observed case basis (−14.6 ± 5.6, P < .001, Table 2). Treatment with NB32 was also associated with improvement in the MADRS total score at week 24 in the mITT-LOCF analysis set (−15.3 ± 8.1, P < .001) and on an observed case basis (−19.1 ± 5.5, P < .001, Table 2). The reduction in MADRS total score over time is summarized in Figure 1A for observed cases at each assessment time point. Response (CGI-I score ≤ 2, much/very much improved) rates were 90.0% at week 12 and 95.0% at week 24 in the mITT-LOCF analysis set and 85.7% at week 12 and 100% at week 24 on an observed case basis (Table 2). Remission (CGI-I score = 1) with mITT-LOCF and observed case analyses, respectively, was achieved by 55% and 57% of patients by week 12 and 70% and 92% of patients by week 24. Results for the IDS-SR also showed improvement at week 24 with both the mITT-LOCF and observed case analyses (Table 2).
Open-label treatment with NB32 in combination with dietary and behavioral counseling was also associated with mean weight loss in the mITT-LOCF analysis set at week 12 (−4.0% ± 4.6%, P < .001) and week 24 (−5.3% ± 6.5%, P < .001, Table 2). On an observed case analysis, mean weight loss at weeks 12 and 24 was −6.1% ± 4.7% (P < .001) and −9.2% ± 6.2% (P < .001), respectively. Mean weight continued to decline over the 24-week course of treatment (Figure 1B). Mean waist circumference was decreased at week 24 (−1.6 ± 4.2 cm, P = .16) but not at week 12 (+0.3 ± 1.7 cm, P = .47) in the mITT-LOCF analysis. A correlation was observed at week 24 between the percent change in body weight and the change in the MADRS total score (mITT-LOCF, r = 0.43, P = .041). Of note, an exploratory analysis demonstrated no difference in percentage of weight loss between patients with atypical versus melancholic features of depression, despite similar improvement in MADRS scores (data not shown).
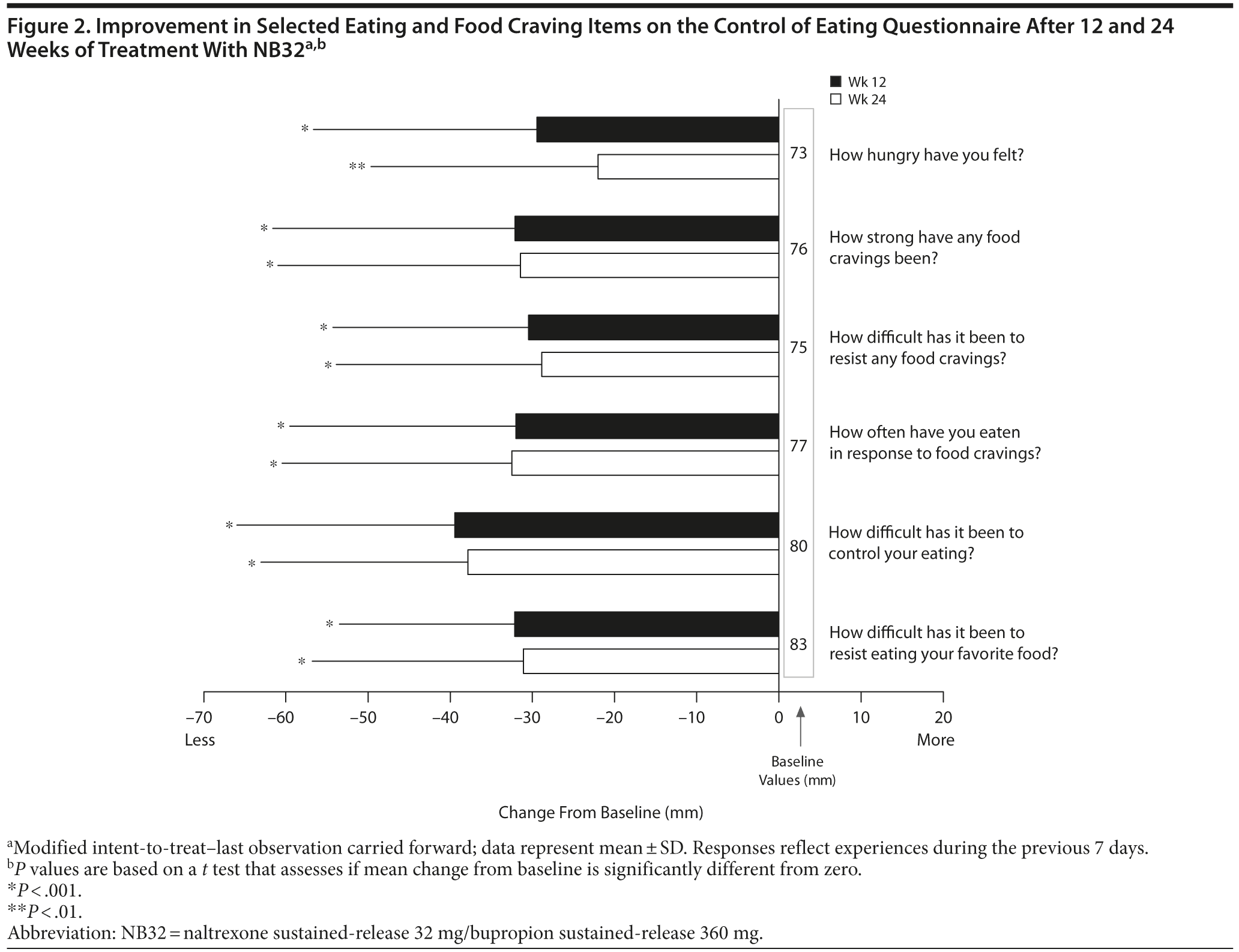
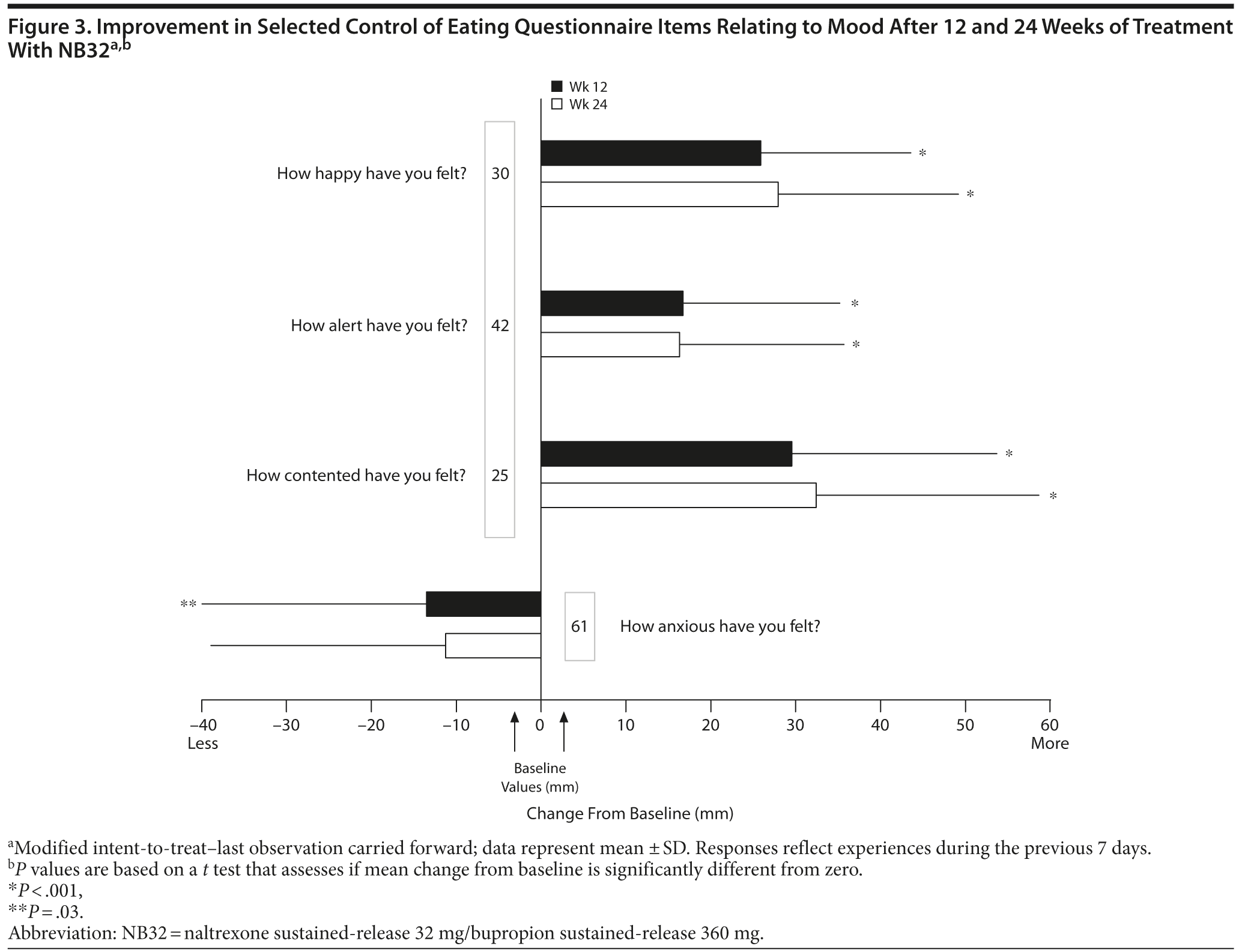
Open-label treatment with NB32 was also associated with improvement in the BES total score on the mITT-LOCF analysis at week 12 (−14.7 ± 10.0, P < .001) and week 24 (−16.1 ± 9.6, P < .001). Similarly, NB32 was associated with improvement in all food cravings and general control of eating items at both week 12 and week 24 (mITT-LOCF, P < .01, Figure 2). In the mITT-LOCF analysis, treatment with NB32 was associated with improvement at week 12 in specific COEQ food craving items, such as desire to eat sweet foods (−24.5 ± 25.9, P < .001), desire to eat tasty foods that are not sweet (−28.3 ± 29.5, P < .001), craving for chocolate or chocolate-flavored food (−25.7 ± 27.4, P < .001), and desire to eat starchy foods (−25.2 ± 28.3, P < .001). Desire to eat fruit or dairy foods (cheese, yogurt, milk) did not change. Treatment with NB32 was also associated with improvement at week 12 in mood-related items on the COEQ, including happiness (25.9 ± 17.7, P < .001), alertness (16.7 ± 18.5, P < .001), contentment (29.5 ± 24.2, P < .001), and anxiety (−13.5 ± 26.4, P = .03, Figure 3). Week 24 results were generally similar, with the exception of anxiety, which trended toward improvement (P = .077).
Treatment with NB32 was not associated with any clinically meaningful effects on laboratory or ECG measures. There was no clinically meaningful change in mean systolic blood pressure (mean change from baseline at week 24: 0.4 ± 12.3 mm Hg), diastolic blood pressure (mean change from baseline at week 24: 0.9 ± 9.3 mm Hg), or pulse rate (mean change from baseline at week 24: 0.3 ± 9.0 bpm) in the safety analysis set (using LOCF method) at week 24. The most common adverse events reported during the study (number of patients experiencing each event) were nausea (n = 12, 48%), constipation (n = 8, 32%), headache (n = 8, 32%), insomnia (n = 8, 32%), dizziness (n = 7, 28%), and hot flush (n = 7, 28%), the majority of which were considered moderate in severity. There were no serious adverse events related to study drug. Ghrelin, leptin, and cortisol levels did not show consistent meaningful changes from baseline (Table 2).
DISCUSSION
This was an exploratory, open-label study that evaluated the efficacy and safety of 24 weeks of NB32 plus dietary and behavioral counseling for the treatment of MDD in overweight or obese patients. No specific psychosocial treatment of depression was provided other than general support throughout the study. Treatment with NB32 was associated with a > 50% mean reduction in depression symptom severity by week 6 as measured by the MADRS. This level of improvement was sustained through week 24. Consistent with this level of reduction in depression symptom severity, CGI-I responder rates were 85.7% by week 12 and 100.0% by week 24 in patients completing 24 weeks of therapy.
Parallel to the improvement in depressive symptoms, treatment with NB32 was associated with a clinically meaningful weight loss (−9.2 ± 6.2%, −9.4 ± 6.4 kg [20.7 ± 14.1 lb]) in patients completing 24 weeks of therapy. In addition to weight loss, patients also reported reduction in binge-eating symptomatology and an improvement in control of eating accompanied by a reduction in food cravings and appetite.
Reliable estimates of the proportion of patients with MDD who are overweight or obese are not currently available, but this comorbid subgroup may represent more than one-third of all patients with MDD.1 The degree of antidepressant response observed with NB32 in this population is notable because the presence of obesity in individuals with MDD is associated with greater treatment resistance to the usual antidepressant therapies,9-13 an overall tendency to higher recurrence rates,15 and higher levels of depression chronicity.14 Interestingly, we observed a correlation between improvement in depression symptom severity and weight change at week 24. This result is consistent with a previous report in which patients treated with bupropion monotherapy who experienced greater reductions in weight had a greater degree of depressive symptom improvement.22 The current sample was not large enough to examine the extent to which the benefit of NB32 was due to direct or indirect effects on depression and weight. The current results, however, suggest that in this combination therapy, the addition of naltrexone does not minimize the antidepressant effects of bupropion in overweight and obese patients. Additionally, adults with MDD and comorbid obesity may benefit from a therapeutic approach that includes treatment of both conditions.
Within the constraints of an open-label study in a small sample, the safety profile of NB32 in overweight or obese depressed patients appears comparable to that evidenced in larger controlled studies in obese nondepressed patients.30-32 As reported previously,30-32 the predominant adverse events are either gastrointestinal (nausea, constipation) or linked to the central nervous system (dizziness, headache, insomnia). Whether the adverse event profile in depressed obese patients differs from that observed in nondepressed obese patients will require larger, controlled trials. It should be noted that while the prescribing information for sustained-release formulations of bupropion approved for depression and smoking cessation caution against use in eating disorder populations due to concern for seizure risk (based on findings with the immediate-release formulation),41,42 there were no instances of seizure with NB32 use in this study despite the study sample’s elevated baseline BES score (indicating a severe problem with binge eating).
Major limitations of the current study include the lack of a randomized, double-blind, placebo-controlled design; a small sample size limited to female patients; the high rate (48%) of treatment discontinuation; and conduct of the study at only 1 clinical site. Given the often marked placebo effects in depression studies, the efficacy results reported here should be interpreted cautiously. As with the safety and tolerability results, corroboration of these findings will require further evaluation in appropriately powered, blinded, controlled studies.
In conclusion, the results of this open-label study suggest that combination therapy with naltrexone/bupropion, along with dietary and behavioral counseling, may be a potentially effective treatment for MDD complicated by concurrent overweight or obesity. Because of the high prevalence of both MDD and obesity in the primary care setting, further evaluation of the efficacy and safety of the naltrexone/bupropion combination in large, multicenter, randomized, placebo-controlled trials appears warranted.
Drug names: bupropion (Wellbutrin, Aplenzin, and others), cimetidine (Tagamet and others), clonidine (Catapres, Duraclon, and others), naltrexone (Vivitrol, ReVia, and others).
Author affiliations: Research Institute, Lindner Center of HOPE, Mason, Ohio (Dr McElroy); Department of Psychiatry and Behavioral Neuroscience, University of Cincinnati College of Medicine, Cincinnati, Ohio (Drs McElroy and Guerdjikova); and Orexigen Therapeutics, Inc, La Jolla, California (Drs Burns, Kim, Harris-Collazo, Landbloom, and Dunayevich). Dr Kim is currently employed by Zafgen. Dr Harris-Collazo is currently employed by Santarus. Dr Landbloom is currently employed by Merck. Dr Dunayevich is currently employed by Amgen.
Potential conflicts of interest: In the past year, Dr McElroy has served as a consultant to or on the scientific advisory boards of Alkermes, Allergan, Brackett, Corcept, and Shire; has served as a principal or coinvestigator on research studies sponsored by Agency for Healthcare Research and Quality, Alkermes, Cephalon, Forest, Jazz, Marriott Foundation, National Institute of Mental Health, Orexigen Therapeutics, Shire, Takeda, and Transcept; and is inventor on US patent no. 6,323,236 B2, Use of Sulfamate Derivatives for Treating Impulse Control Disorders, and, along with the patent’s assignee, University of Cincinnati (Cincinnati, Ohio), has received payments from Johnson & Johnson Pharmaceutical Research and Development, LLC, which as exclusive rights under the patent. Dr Kim has served as a consultant to Alkermes, Cebix, Dimedica, GI Dynamics, and MetaCon; has served on the speakers or advisory boards of Alkermes and Viridian Healthcare; and is a stock shareholder in Orexigen and Vivus. Dr Burns is an employee of Orexigen. Dr Landbloom is a stock shareholder in Amgen. Drs Guerdjikova, Harris-Collazo, and Dunayevich report no conflicts of interest related to the subject of this article.
Funding/support: This study was funded by Orexigen Therapeutics, Inc.
Role of sponsor: Orexigen Therapeutics, Inc, contributed to the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript.
Previous presentations: Data presented at the 70th Annual Scientific Meeting of the American Diabetes Association; June 25-29, 2010; Orlando, Florida ▪ 11th International Congress on Obesity; July 11-15, 2010; Stockholm, Sweden ▪ the 28th Annual Scientific Meeting of The Obesity Society; October 8-12, 2010; San Diego, California.
Acknowledgment: Edward Schweizer, MD (Paladin Consulting Group, Princeton, New Jersey), provided editorial assistance, which was funded by Orexigen Therapeutics, Inc, in the preparation of an early draft of the manuscript.
REFERENCES
1. Flegal KM, Carroll MD, Ogden CL, et al. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303(3):235-241. PubMed doi:10.1001/jama.2009.2014
2. Singh GK, Kogan MD, van Dyck PC. Changes in state-specific childhood obesity and overweight prevalence in the United States from 2003 to 2007. Arch Pediatr Adolesc Med. 2010;164(7):598-607. PubMed doi:10.1001/archpediatrics.2010.84
3. Compton WM, Conway KP, Stinson FS, et al. Changes in the prevalence of major depression and comorbid substance use disorders in the United States between 1991-1992 and 2001-2002. Am J Psychiatry. 2006;163(12):2141-2147. PubMed doi:10.1176/appi.ajp.163.12.2141
4. Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617-627. PubMed doi:10.1001/archpsyc.62.6.617
5. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602. PubMed doi:10.1001/archpsyc.62.6.593
6. de Wit L, Luppino F, van Straten A, et al. Depression and obesity: a meta-analysis of community-based studies. Psychiatry Res. 2010;178(2):230-235. PubMed doi:10.1016/j.psychres.2009.04.015
7. Luppino FS, de Wit LM, Bouvy PF, et al. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry. 2010;67(3):220-229. PubMed doi:10.1001/archgenpsychiatry.2010.2
8. Blaine B. Does depression cause obesity? a meta-analysis of longitudinal studies of depression and weight control. J Health Psychol. 2008;13(8):1190-1197. PubMed doi:10.1177/1359105308095977
9. Khan A, Schwartz KA, Kolts RL, et al. BMI, sex, and antidepressant response. J Affect Disord. 2007;99(1-3):101-106. PubMed doi:10.1016/j.jad.2006.08.027
10. Kloiber S, Ising M, Reppermund S, et al. Overweight and obesity affect treatment response in major depression. Biol Psychiatry. 2007;62(4):321-326. PubMed doi:10.1016/j.biopsych.2006.10.001
11. Papakostas GI, Petersen T, Iosifescu DV, et al. Obesity among outpatients with major depressive disorder. Int J Neuropsychopharmacol. 2005;8(1):59-63. PubMed doi:10.1017/S1461145704004602
12. Uher R, Mors O, Hauser J, et al. Body weight as a predictor of antidepressant efficacy in the GENDEP project. J Affect Disord. 2009;118(1-3):147-154. PubMed doi:10.1016/j.jad.2009.02.013
13. Oskooilar N, Wilcox CS, Tong ML, et al. Body mass index and response to antidepressants in depressed research subjects. J Clin Psychiatry. 2009;70(11):1609-1610. PubMed doi:10.4088/JCP.09l05226blu
14. Murphy JM, Horton NJ, Burke JD Jr, et al. Obesity and weight gain in relation to depression: findings from the Stirling County Study. Int J Obes (Lond). 2009;33(3):335-341. PubMed doi:10.1038/ijo.2008.273
15. Fagiolini A, Kupfer DJ, Houck PR, et al. Obesity as a correlate of outcome in patients with bipolar I disorder. Am J Psychiatry. 2003;160(1):112-117. PubMed doi:10.1176/appi.ajp.160.1.112
16. Srisurapanont M, Jarusuraisin N. Opioid antagonists for alcohol dependence. Cochrane Database Syst Rev. 2005;(1):CD001867. PubMed
17. Foley KF, DeSanty KP, Kast RE. Bupropion: pharmacology and therapeutic applications. Expert Rev Neurother. 2006;6(9):1249-1265. PubMed doi:10.1586/14737175.6.9.1249
18. Jefferson JW. Bupropion extended-release for depressive disorders. Expert Rev Neurother. 2008;8(5):715-722. PubMed doi:10.1586/14737175.8.5.715
19. Richmond R, Zwar N. Review of bupropion for smoking cessation. Drug Alcohol Rev. 2003;22(2):203-220. PubMed doi:10.1080/09595230100100642
20. Hays JT, Ebbert JO. Bupropion for the treatment of tobacco dependence: guidelines for balancing risks and benefits. CNS Drugs. 2003;17(2):71-83. PubMed doi:10.2165/00023210-200317020-00001
21. Anderson JW, Greenway FL, Fujioka K, et al. Bupropion SR enhances weight loss: a 48-week double-blind, placebo- controlled trial. Obes Res. 2002;10(7):633-641. PubMed doi:10.1038/oby.2002.86
22. Jain AK, Kaplan RA, Gadde KM, et al. Bupropion SR vs placebo for weight loss in obese patients with depressive symptoms. Obes Res. 2002;10(10):1049-1056. PubMed doi:10.1038/oby.2002.142
23. Sinnayah P, Wallingford NM, Evans AE, et al. Bupropion and naltrexone interact synergistically to decrease food intake in mice. Obesity (Silver Spring). 2007;15(9):A179.
24. Greenway FL, Whitehouse MJ, Guttadauria M, et al. Rational design of a combination medication for the treatment of obesity. Obesity (Silver Spring). 2009;17(1):30-39. PubMed doi:10.1038/oby.2008.461
25. Stahl SM. Stahl’s Essential Psychopharmacology. 3rd ed. New York, NY: Cambridge University Press; 2008.
26. Hasegawa H, Meeusen R, Sarre S, et al. Acute dopamine/norepinephrine reuptake inhibition increases brain and core temperature in rats. J Appl Physiol. 2005;99(4):1397-1401. PubMed doi:10.1152/japplphysiol.00435.2005
27. Billes SK, Cowley MA. Inhibition of dopamine and norepinephrine reuptake produces additive effects on energy balance in lean and obese mice. Neuropsychopharmacology. 2007;32(4):822-834. PubMed doi:10.1038/sj.npp.1301155
28. Billes SK, Cowley MA. Catecholamine reuptake inhibition causes weight loss by increasing locomotor activity and thermogenesis. Neuropsychopharmacology. 2008;33(6):1287-1297. PubMed doi:10.1038/sj.npp.1301526
29. Greenway FL, Dunayevich E, Tollefson G, et al; NB-201 Study Group. Comparison of combined bupropion and naltrexone therapy for obesity with monotherapy and placebo. J Clin Endocrinol Metab. 2009;94(12):4898-4906. PubMed doi:10.1210/jc.2009-1350
30. Apovian CM, Aronne LJ, Rubino DM, et al. A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II). Obesity (Silver Spring). 2013 Feb 14 [Epub ahead of print]. doi:10.1002/oby.20309 PubMed
31. Greenway FL, Fujioka K, Plodkowski RA, et al; COR-I Study Group. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376(9741):595-605. PubMed doi:10.1016/S0140-6736(10)60888-4
32. Wadden TA, Foreyt JP, Foster GD, et al. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: the COR-BMOD trial. Obesity (Silver Spring). 2011;19(1):110-120. PubMed doi:10.1038/oby.2010.147
33. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134(4):382-389. PubMed doi:10.1192/bjp.134.4.382
34. Guy W. ECDEU Assessment Manual for Psychopharmacology, Revised Edition. Rockville, MD: National Institute of Mental Health, Psychopharmacology Research Branch; 1976.
35. Rush AJ, Gullion CM, Basco MR, et al. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med. 1996;26(3):477-486. PubMed doi:10.1017/S0033291700035558
36. Gormally J, Black S, Daston S, et al. The assessment of binge eating severity among obese persons. Addict Behav. 1982;7(1):47-55. PubMed doi:10.1016/0306-4603(82)90024-7
37. Gladis MM, Wadden TA, Foster GD, et al. A comparison of two approaches to the assessment of binge eating in obesity. Int J Eat Disord. 1998;23(1):17-26. PubMed doi:10.1002/(SICI)1098-108X(199801)23:1<17::AID-EAT3>3.0.CO;2-4
38. Wilcox CS, Oskooilar N, Erickson JS, et al. An open-label study of naltrexone and bupropion combination therapy for smoking cessation in overweight and obese subjects. Addict Behav. 2010;35(3):229-234. PubMed doi:10.1016/j.addbeh.2009.10.017
39. Flint A, Raben A, Blundell JE, et al. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord. 2000;24(1):38-48. PubMed doi:10.1038/sj.ijo.0801083
40. Krishna R, Gumbiner B, Stevens C, et al. Potent and selective agonism of the melanocortin receptor 4 with MK-0493 does not induce weight loss in obese human subjects: energy intake predicts lack of weight loss efficacy. Clin Pharmacol Ther. 2009;86(6):659-666. PubMed doi:10.1038/clpt.2009.167
41. Wellbutrin XL tablets (bupropion hydrochloride) [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2010.
42. Zyban sustained-release tablets (bupropion hydrochloride). [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2012:1-34.
This PDF is free for all visitors!