Objective: Clinicians have access to a variety of formulations of methylphenidate and amphetamine to treat attention-deficit/hyperactivity disorder (ADHD). However, due to new emerging formulations, clinicians may lack up-to-date knowledge about all available stimulant formulations. Presented here is a comprehensive guide to13 formulations of methylphenidate and 10 formulations of amphetamine that have US Food and Drug Administration approval to treat ADHD.
Data Sources: PubMed was searched using the following MeSH terms: attention-deficit/hyperactivity disorder, ADHD, stimulant, amphetamine, and methylphenidate. Inclusion criteria were randomized controlled trials and systematic reviews published through 2017.
Study Selection and Extraction: Forty-eight articles were identified; however, these included analyses using product labels and anecdotal or uncontrolled reports of apparent clinical inequivalence. Thus, 34 articles were included in the final review to provide a thorough evidence-based guide.
Results: Each formulation has a unique pharmacokinetic profile. Clinically, one formulation may not be suitable for all patients. To select the most appropriate formulation, clinicians should consider the individual patient’s preferences such as dosing schedule, time required to reach peak plasma concentration and duration of action, and tolerability.
Conclusion: This review provides clinical guidance to help clinicians prescribe the most suitable treatment for an individual.’ ‹’ ‹’ ‹

CME Background
Articles are selected for credit designation based on an assessment of the educational needs of CME participants, with the purpose of providing readers with a curriculum of CME articles on a variety of topics throughout each volume. Activities are planned using a process that links identified needs with desired results.
To obtain credit, read the article, correctly answer the questions in the Posttest, and complete the Evaluation.
CME Objective
After studying this article, you should be able to:
- Select suitable stimulant formulations for patients with attention-deficit/hyperactivity disorder using the medications’ unique pharmacokinetic profiles
Accreditation Statement
The CME Institute of Physicians Postgraduate Press, Inc., is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Credit Designation
The CME Institute of Physicians Postgraduate Press, Inc., designates this journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Creditâ„¢. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Note: The American Academy of Physician Assistants (AAPA) accepts certificates of participation for educational activities certified for AMA PRA Category 1 Creditâ„¢ from organizations accredited by ACCME or a recognized state medical society. Physician assistants may receive a maximum of 1.0 hour of Category I credit for completing this program.
Release, Expiration, and Review Dates
This educational activity was published in December 2018 and is eligible for AMA PRA Category 1 Creditâ„¢ through December 31, 2020. The latest review of this material was December 2018.
Financial Disclosure
All individuals in a position to influence the content of this activity were asked to complete a statement regarding all relevant personal financial relationships between themselves or their spouse/partner and any commercial interest. The CME Institute has resolved any conflicts of interest that were identified. In the past year, Larry Culpepper, MD, MPH, Editor in Chief, has been a consultant for Alkermes, Jazz, Lundbeck, Merck, and Sunovion; has been a stock shareholder of M-3 Information; and has received royalties from UpToDate and Oxford University Press. No member of the CME Institute staff reported any relevant personal financial relationships. Faculty financial disclosure appears at the end of the article.
ABSTRACT
Objective: Clinicians have access to a variety of formulations of methylphenidate and amphetamine to treat attention-deficit/hyperactivity disorder (ADHD). However, due to new emerging formulations, clinicians may lack up-to-date knowledge about all available stimulant formulations. Presented here is a comprehensive guide to13 formulations of methylphenidate and 10 formulations of amphetamine that have US Food and Drug Administration approval to treat ADHD.
Data Sources: PubMed was searched using the following MeSH terms: attention-deficit/hyperactivity disorder, ADHD, stimulant, amphetamine, and methylphenidate. Inclusion criteria were randomized controlled trials and systematic reviews published through 2017.
Study Selection and Extraction: Forty-eight articles were identified; however, these included analyses using product labels and anecdotal or uncontrolled reports of apparent clinical inequivalence. Thus, 34 articles were included in the final review to provide a thorough evidence-based guide.
Results: Each formulation has a unique pharmacokinetic profile. Clinically, one formulation may not be suitable for all patients. To select the most appropriate formulation, clinicians should consider the individual patient’s preferences such as dosing schedule, time required to reach peak plasma concentration and duration of action, and tolerability.
Conclusion: This review provides clinical guidance to help clinicians prescribe the most suitable treatment for an individual.
Prim Care Companion CNS Disord 2018;20(6):18r02345
To cite: Gautam M, Prabhakar D. Stimulant formulations for the treatment of attention-deficit/hyperactivity disorder. Prim Care Companion CNS Disord. 2018;20(6):18r02345.
To share: https://doi.org/10.4088/PCC.18r02345
© Copyright 2018 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry, Henry Ford Hospital, Wayne State University, Detroit, Michigan
bDepartment of Psychiatry, Henry Ford Health System, Detroit, Michigan
*Corresponding author: Mohan Gautam, DO, MS, 2799 West Grand Blvd, Henry Ford Hospital, Wayne State University, Detroit, MI 48202 ([email protected]).
The US Food and Drug Administration (FDA) has approved 13 formulations of methylphenidate and 10 formulations of amphetamine for the treatment of attention-deficit/hyperactivity disorder (ADHD). Immediate-release (IR) formulations of methylphenidate have been a gold standard of treatment since 1955.1,2 While there is a historical use of amphetamines even prior to that date, the first extant IR formulation of amphetamine was not marketed until 1996. As a treatment paradigm, IR formulations generally require multiple dosing throughout the day. To address this limitation, many additional formulations have emerged that attempt to decrease the frequency of dosing using extended-release technology. Furthermore, formulations have been developed to provide additional therapeutic controls. These formulations include those that afford a more gradual increase in peak plasma concentration, liquid suspensions, chewable tablets, orally disintegrating tablets, utilization of transdermal drug delivery, enantiomers, and a prodrug that must be metabolized before the active compound is activated.
The drug delivery mechanisms of these compounds create unique parameters that differ considerably among the available formulations. We review all currently available methylphenidate and amphetamine formulations to provide a comprehensive, in-depth guide for prescribing clinicians. Each formulation is described considering individual design and pharmacokinetic parameters.
METHODS
PubMed was searched for English-language articles using the following medical subject heading (MeSH) terms: attention-deficit/hyperactivity disorder, ADHD, stimulant, amphetamine, and methylphenidate. Inclusion criteria were randomized controlled trials and systematic reviews published through 2017. Initial versions of the review included 48 articles. However, these articles included analyses using product labels and anecdotal or uncontrolled reports of apparent clinical inequivalence. We ultimately decided to exclude these references to provide a thorough evidence-based guide and, therefore, utilized 34 articles.1-34
CLINICAL POINTS
- Many stimulant formulations are available for the treatment of attention-deficit/hyperactivity disorder (ADHD).
- Stimulant formulations for ADHD have unique properties such as type of formulation, time to reach peak plasma concentration, duration of action, and dosing schedule.
- Currently available formulations of methylphenidate and amphetamine are highly customizable for many unique patient factors.
RESULTS
Methylphenidate
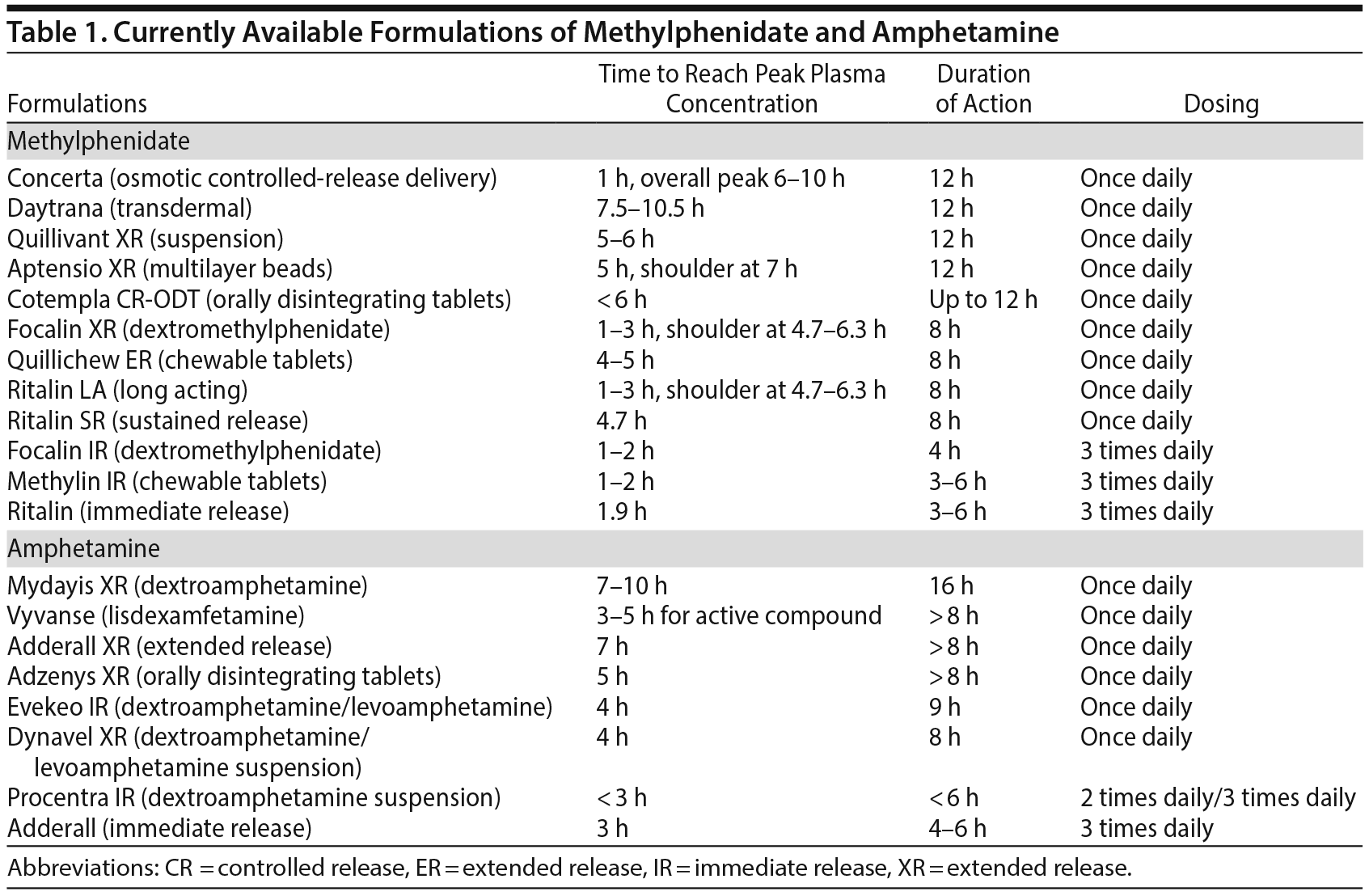
Immediate release. Conventional IR methylphenidate formulation has been a standard treatment for ADHD since 1955, when the disorder was still referred to as “minimal brain dysfunction.”1,2 Brand-name IR methylphenidate is Ritalin. In the multimodality treatment study of ADHD, the gold-standard treatment was chosen as IR methylphenidate administered 3 times per day.3 The peak serum concentration is achieved in about 1.9 hours, and the duration of action ranges from at least 3 hours to 6 hours. Clinically, IR formulations of methylphenidate can be used as monotherapy dosed 3 times daily or as adjunct therapy to extend the duration of other formulations that may not provide an adequate duration of action.
Sustained release. Although IR formulations of methylphenidate were (and continue to be) clearly efficacious, they require multiple daily dosing. To address this challenge, longer-acting preparations began to enter the market. The first generation was methylphenidate sustained release (SR), which utilizes a wax-matrix to provide slow, continual release of methylphenidate (Ritalin SR).4 Unfortunately, this delivery mechanism lacks an IR component. Thus, plasma methylphenidate concentrations may not reach the adequate level for therapeutic action until later in the day, which may be problematic for school or work. The peak plasma concentration is achieved in about 4.7 hours, and the duration of action is up to 8 hours.5
Long acting. Ritalin long acting (LA) delivers methylphenidate using spheroidal oral drug absorption system (SODAS) technology. This formulation causes 50% of the methylphenidate to be released immediately and 50% over a more extended period in a conspicuously pulsatile manner.5 This release is accomplished with polymer-coated beads; a capsule contains 50% IR beads and 50% extended-release (ER) beads. Due to this distinct dissolution property of the beads, there are 2 distinct plasma methylphenidate concentration peaks approximately 4 hours apart. The initial peak concentration is reached within 1-3 hours, and the shoulder (secondary peak) is reached within 4.7-6.3 hours.6 The onset of action of the LA formulation is similar to the onset of action of the IR formulation, but the duration of action is extended to 8 hours.7
The delivery mechanism of Ritalin LA is distinct to controlled-delivery (CD) methylphenidate (Metadate CD). CD methylphenidate also consists of a capsule composed of beads; however, the composition of the beads is 30% IR and 70% ER methylphenidate. The beads were designed to begin to release methylphenidate approximately 1 hour after administration.8 The pharmacokinetic profile of CD methylphenidate, however, tends to resemble the SR formulation, albeit a more rapid rise to peak plasma concentration followed by another secondary peak. Specifically, the early peak plasma concentration is reached within 1.5 hours, and the shoulder is reached in approximately 4.5 hours for a total duration of approximately 8 hours.7,8
Osmotic controlled-release delivery. Concerta utilizes osmotic controlled-release delivery system (OROS) to release 22% of its total methylphenidate content immediately, followed by a gradual release of the remaining content throughout the day. This release mechanism allows for a significantly extended duration of action with once-daily dosing of up to 12 hours.5
Following administration, peak plasma concentration is reached in approximately 1 hour, followed by a gradually increasing plasma concentration over the next 5-9 hours. Mean time to reach peak plasma concentrations in this phase varies between 6 and 10 hours. A unique advantage to this slowly ascending pharmacokinetic profile is the argument that it can theoretically combat the development of acute tolerance.9-11
Transdermal system. Daytrana is a unique methylphenidate transdermal system that provides continuous delivery via diffusion. Daytrana is the only LA formulation to reach peak plasma methylphenidate concentration later than Concerta in head-to-head studies.12 There is only a single peak plasma concentration, reached approximately 7.5-10.5 hours after administration.5 The advantage to this transdermal system is once-daily dosing and a slowly ascending pharmacokinetic profile. To some patients, however, the very slow ascension of peak plasma concentration of methylphenidate may be reached too late into the school or work day for adequate symptom control. This formulation has several other limitations and complications including adhesive backing recalls, incidences of dermatitis, and inconsistent delivery of methylphenidate into the systemic circulation.
Chewable tablets. Methylphenidate chewable tablet, eg, Methylin chewable tablets, is an IR formulation that is readily absorbed after oral administration. The peak plasma concentration of methylphenidate following administration of IR chewable is reached within 1-2 hours.13 In 2015, Pfizer received FDA approval to release Quillichew ER, a longer-acting preparation of this delivery system consisting of flavored tablets composed of 15% IR and the remainder as ER methylphenidate.14 The peak plasma methylphenidate concentration of the ER formulation is reached within 4-5 hours after administration. Whereas twice-daily dosage of an IR chewable tablet reaches a higher peak plasma concentration, a single dose of ER chewable tablet entails a more gradual increase of plasma concentration followed by a broader peak and fewer fluctuations in plasma concentration of methylphenidate.13
Dexmethylphenidate. Methylphenidate consists of a racemic mixture of d– and l-enantiomers. The major mechanism of action of methylphenidate in the treatment of ADHD symptoms is accomplished by the reuptake inhibition of dopamine in the synaptic cleft. Specifically, this reuptake inhibition is primarily accomplished by the d-enantiomer. The l-enantiomer, on the other hand, is a largely inactive isomeric ballast.15-18 Therefore, in an effort to minimize systemic toxicity, the d-enantiomer was isolated and marketed as Focalin (IR formulation) and Focalin XR.
The pharmacokinetic profile of dexmethylphenidate is similar to dl-methylphenidate. Dexmethylphenidate is formulated as a tablet; upon administration, peak plasma dexmethylphenidate concentration is reached within 1-2 hours for a total duration of approximately 4 hours.19 Dexmethylphenidate ER uses the SODAS technology as described previously for LA formulations, which allows for an immediate release of 50% of the dexmethylphenidate and, therefore, an initial peak followed by a shoulder more than 4 hours later.16
Suspension solution. Quillivant XR is a powder that forms an extended-release formulation consisting of 20% IR and 80% ER after reconstitution with water.14 This formulation allows for an oral solution that can be easily administered to individuals who cannot tolerate tablets or capsules. The peak plasma methylphenidate concentration after administration of this formulation is reached within 5-6 hours for a total duration of approximately 12 hours.14,20 Clinically, using a reconstitution solution will require more extensive patient education and more close monitoring for safe preparation and administration.
Orally disintegrating tablets. In 2017, another novel mechanism for methylphenidate delivery received FDA approval for market entry: ER orally disintegrating tablets (Cotempla XR-ODT). This formulation is placed on the tongue, where it undergoes rapid absorption. Randomized controlled studies14,21 on this formulation have showed symptom control of up to 12 hours.
Multilayer-release beads. Another newly developed delivery of methylphenidate is a multilayer-release (MLR) bead formulation contained in a hard gelatin capsule (Aptensio XR). This medication received FDA approval for market entry in 2016. The formulation consists of 37% IR beads and 63% controlled-release beads with a layer of coating that slowly releases the drug over a more extended time period. Pharmacokinetic information on this formulation, available from 2 randomized controlled trials, demonstrates a rapid initial peak in plasma methylphenidate concentration followed by a decline in plasma methylphenidate concentration over the subsequent 5 hours and a gradual increase in concentration in the subsequent 2 hours to reach a shoulder at approximately 7 hours after initial administration. Total duration of action of this formulation is approximately 12 hours, regardless whether the capsule is administered whole or the contents of the capsule are sprinkled on a food item.14,22,23
Amphetamines
Mixed amphetamine salts. Although amphetamines have been used since 1935, the first extant formulation of IR mixed amphetamine salts was marketed in 1996 as Adderall.24 The IR formulation contains both enantiomers, d-amphetamine and l-amphetamine, in a ratio of 3:1. The peak plasma amphetamine concentration is reached approximately 3 hours after administration, and the duration of action of this formulation is approximately 4-6 hours.24,25
The success of Adderall led Shire Pharmaceuticals to introduce the ER formulation in 2001 (Adderall XR). Like the IR formulation, the XR formulation also consists of d-amphetamine and l-amphetamine in a ratio of 3:1. The XR formulation is composed of a capsule with 50% IR beads and 50% ER beads.24 The XR formulation reaches a peak plasma concentration approximately 7 hours after administration, which allows for once-daily dosing. Therapeutic efficacy does not appear to be altered if the XR capsule is ingested whole or the contents of the capsule are sprinkled over a food item.26-28
Dextroamphetamine immediate release and sustained release. During the 1930s, Smith, Kline, and French manufactured racemic amphetamine as Benzedrine tablets. In 1937, the firm began marketing only the d-isomer of amphetamine (dextroamphetamine) under the trade name Dexedrine. The use of Benzedrine to treat ADHD declined dramatically after 1976, when it was reported that Dexedrine was more effective.29 In 1975, Shire Pharmaceuticals received FDA approval for Dextrostat tablets (dextroamphetamine IR). In 1976, GlaxoSmithKline received FDA approval for Dexedrine tablets (dextroamphetamine IR), elixir (dextroamphetamine IR oral solution), and spansule (dextroamphetamine SR). Currently, all of these trade formulations have been discontinued. The generic formulation of Dexedrine spansule (dextroamphetamine SR) is available, and dextroamphetamine IR became available under the brand name Zenzedi in 2015.
Lisdexamfetamine: expanded analysis of a prodrug. Shire Pharmaceuticals received FDA approval for lisdexamfetamine in 2007 as Vyvanse. This approval filled a yet uninhabited niche in the stimulant market as the first and only prodrug formulation. A prodrug is a biologically inactive compound that must be metabolized prior to becoming a drug that can induce effects. Furthermore, there is significant potential for the application of this prodrug if there is concern for substance abuse, as it is not possible to insufflate or inject this formulation. Lisdexamfetamine consists of the pharmacologically inactive parent compound dextroamphetamine linked to L-lysine; this inactive compound is absorbed in the small intestine and transported into the blood. Finally, when it is in the blood, lisdexamfetamine is hydrolyzed by a hydrolase into the pharmacologically active dextroamphetamine.30
Although the time to reach peak plasma concentration for lisdexamfetamine is 1 to 2 hours, the time needed for the active compound dextroamphetamine is 3-5 hours because of the necessary process of conversion of the prodrug (lisdexamfetamine) to this active compound (dextroamphetamine). The time to reach peak plasma concentration of dextroamphetamine following lisdexamfetamine administration (3-5 hours) is approximately 1 hour later than administration of dextroamphetamine (2-3 hours).31 Therapeutic efficacy of lisdexamfetamine has been demonstrated up to 14 hours after administration, and, as is the case for OROS-methylphenidate, it can be argued that the slowly ascending plasma concentration of lisdexamfetamine would theoretically combat the development of acute tolerance.30-32
Dextroamphetamine extended release. In June 2017, Shire Pharmaceuticals announced the release of its latest amphetamine formulation, dextroamphetamine ER, Mydayis. Currently available, the formulation contains dextroamphetamine and levoamphetamine in a ratio of 3:1. There is a paucity of clinical research available on this formulation to confidently delineate its unique pharmacokinetic properties. One distinguishing feature, however, is a delayed time course to reach a single peak plasma concentration approximately 7-10 hours after administration and a duration of action of up to 16 hours.
Amphetamine extended-release orally disintegrating tablet. Adzenys received FDA approval in 2016. It is the first formulation of amphetamine ER orally disintegrating tablet. This formulation is a tablet that is placed on the tongue where it disintegrates rapidly. There is a paucity of clinical research available on this formulation. Amphetamine ER orally disintegrating tablets offer an alternative route of administration for patients who cannot swallow tablets or capsules. The peak plasma concentration of this formulation is reached approximately 5 hours after administration.33
Amphetamine immediate release. Unlike mixed amphetamine salts, the formulation of Evekeo is 1:1 dextroamphetamine and levoamphetamine. This formulation received FDA approval in 2012. Purportedly, the onset of action is debated to be 45 minutes after administration, with greatest efficacy observed at 4 hours, and a duration of action of at least 9 hours.34 Hence, the duration of action of amphetamine IR is suggested to be significantly longer than the duration of action of mixed amphetamine salts IR (4-6 hours).
Amphetamine oral solution. Dynavel XR contains IR and ER components of dextroamphetamine and levoamphetamine in a ratio of 3.2:1. This formulation is suitable for patients who are unable to swallow tablets and allows for once-daily dosing. The peak plasma concentration is reached in approximately 4 hours, with at least an 8-hour duration of action. This formulation does not have FDA approval for children younger than 6 years.
On the other hand, ProCentra is a dextroamphetamine oral solution with FDA approval for children as young as 3 years. Because there are no XR components to this formulation, it requires multiple dosing throughout the day.
CONCLUSION
Currently available stimulant formulations for the treatment of ADHD offer similar efficacy with customizable differences. For example, most LA formulations are considerably more expensive than short-acting formulations. If affordability is a significant concern, short-acting formulations administered 2 or 3 times daily may be a more appropriate treatment approach. If the development of tolerance or abuse potential is a concern, then LA methylphenidate, OROS-methylphenidate, mixed amphetamine salts-XR, or more expensive newer formulations such as lisdexamfetamine may be the most appropriate approach. Table 1 provides a list of currently available formulations, along with guidelines concerning time to reach peak plasma concentration, duration of action, and dosing.
To select the most appropriate formulation for each patient, we recommend taking into consideration the individual patient’s preferences such as dosing schedule, time required to reach peak plasma concentration and duration of action, and tolerability. Evidence suggests that currently available formulations of methylphenidate and amphetamine are highly customizable for many unique patient factors.
Submitted: June 28, 2018; accepted September 18, 2018.
Published online: December 27, 2018.
Financial disclosure: Drs Gautam and Prabhakar have no personal affiliations or financial relationships with any commercial interest to disclose relative to this article.
Funding/support: None.
Disclosure of off-label usage: The authors have determined that, to the best of their knowledge, no investigational information about pharmaceutical agents that is outside US Food and Drug Administration-approved labeling has been presented in this article.
This CME activity is expired. For more CME activities, visit cme.psychiatrist.com.
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders
REFERENCES
1. Yang X, Duan J, Fisher J. Application of physiologically based absorption modeling to characterize the pharmacokinetic profiles of oral extended release methylphenidate products in adults. PLoS One. 2016;11(10):e0164641. PubMed CrossRef
2. Clements SD, Peters JE. Minimal brain dysfunctions in the school-age child: diagnosis and treatment. Arch Gen Psychiatry. 1962;6(3):185-197. PubMed CrossRef
3. Greenhill LL, Abikoff HB, Arnold LE, et al. Medication treatment strategies in the MTA Study: relevance to clinicians and researchers. J Am Acad Child Adolesc Psychiatry. 1996;35(10):1304-1313. PubMed CrossRef
4. Punja S, Zorzela L, Hartling L, et al. Long-acting versus short-acting methylphenidate for paediatric ADHD: a systematic review and meta-analysis of comparative efficacy. BMJ Open. 2013;3(3):e002312. PubMed CrossRef
5. Coghill D, Banaschewski T, Zuddas A, et al. Long-acting methylphenidate formulations in the treatment of attention-deficit/hyperactivity disorder: a systematic review of head-to-head studies. BMC Psychiatry. 2013;13:237. PubMed CrossRef
6. Markowitz JS, Straughn AB, Patrick KS, et al. Pharmacokinetics of methylphenidate after oral administration of two modified-release formulations in healthy adults. Clin Pharmacokinet. 2003;42(4):393-401. PubMed CrossRef
7. Gonzסlez MA, Pentikis HS, Anderl N, et al. Methylphenidate bioavailability from two extended-release formulations. Int J Clin Pharmacol Ther. 2002;40(4):175-184. PubMed CrossRef
8. Quinn D, Bode T, Reiz JL, et al. Single-dose pharmacokinetics of multilayer-release methylphenidate and immediate-release methylphenidate in children with attention-deficit/hyperactivity disorder. J Clin Pharmacol. 2007;47(6):760-766. PubMed CrossRef
9. Swanson K, Volkow N. Pharmacokinetic and pharmacodynamic properties of methylphenidate in humans. Stimulant Drugs and ADHD. New York, NY: Oxford University Press; 2001:259-282.
10. Fallu A, Dabouz F, Furtado M, et al. A randomized, double-blind, cross-over, phase IV trial of oros-methylphenidate (CONCERTA) and generic novo-methylphenidate ER-C (NOVO-generic). Ther Adv Psychopharmacol. 2016;6(4):237-251. PubMed CrossRef
11. van Stralen JP. The clinical impact of switching attention deficit hyperactivity disorder patients from OROS-MPH to Novo-MPH ER-C: a paediatric practice review. Paediatr Child Health. 2013;18(2):70-73. PubMed CrossRef
12. Pierce D, Katic A, Buckwalter M, et al. Single- and multiple-dose pharmacokinetics of methylphenidate administered as methylphenidate transdermal system or osmotic-release oral system methylphenidate to children and adolescents with attention deficit hyperactivity disorder. J Clin Psychopharmacol. 2010;30(5):554-564. PubMed CrossRef
13. Abbas R, Palumbo D, Walters F, et al. Single-dose pharmacokinetic properties and relative bioavailability of a novel methylphenidate extended-release chewable tablet compared with immediate-release methylphenidate chewable tablet. Clin Ther. 2016;38(5):1151-1157. PubMed CrossRef
14. Cortese S, D’ Acunto G, Konofal E, et al. New formulations of methylphenidate for the treatment of attention-deficit/hyperactivity disorder: pharmacokinetics, efficacy, and tolerability. CNS Drugs. 2017;31(2):149-160. PubMed CrossRef
15. Teo SK, Scheffler MR, Wu A, et al. A single-dose, two-way crossover, bioequivalence study of dexmethylphenidate HCl with and without food in healthy subjects. J Clin Pharmacol. 2004;44(2):173-178. PubMed CrossRef
16. Spencer TJ, Adler LA, McGough JJ, et al; Adult ADHD Research Group. Efficacy and safety of dexmethylphenidate extended-release capsules in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2007;61(12):1380-1387. PubMed CrossRef
17. Ding YS, Fowler JS, Volkow ND, et al. Chiral drugs: comparison of the pharmacokinetics of [11C]d-threo and L-threo-methylphenidate in the human and baboon brain. Psychopharmacology (Berl). 1997;131(1):71-78. PubMed CrossRef
18. Davids E, Zhang K, Tarazi FI, et al. Stereoselective effects of methylphenidate on motor hyperactivity in juvenile rats induced by neonatal 6-hydroxydopamine lesioning. Psychopharmacology (Berl). 2002;160(1):92-98. PubMed CrossRef
19. Patrick KS, Straughn AB. Absorption differences between immediate-release dexmethylphenidate and dl-methylphenidate. Drug Metab Dispos. 2016;44(3):418-421. PubMed CrossRef
20. Childress AC, Berry SA. The single-dose pharmacokinetics of NWP06, a novel extended-release methylphenidate oral suspension. Postgrad Med. 2010;122(5):35-41. PubMed CrossRef
21. Childress A, Kollins S, Culter A, et al. Efficacy, safety, and tolerability of an extended-release orally disintegrating methylphenidate tablet in children 6-12 years of age with attention-deficit/hyperactivity disorder in the laboratory classroom setting. J Child Adolesc Psychopharmacol. 2017;27(1):66-74. PubMed CrossRef
22. Adjei A, Teuscher NS, Kupper RJ, et al. Single-dose pharmacokinetics of methylphenidate extended-release multiple layer beads administered as intact capsule or sprinkles versus methylphenidate immediate-release tablets (Ritalin) in healthy adult volunteers. J Child Adolesc Psychopharmacol. 2014;24(10):570-578. PubMed CrossRef
23. Adjei A, Kupper RJ, Teuscher NS, et al. Steady-state bioavailability of extended-release methylphenidate (MPH-MLR) capsule vs immediate-release methylphenidate tablets in healthy adult volunteers. Clin Drug Investig. 2014;34(11):795-805. PubMed CrossRef
24. Tulloch SJ, Zhang Y, McLean A, et al. SLI381 (Adderall XR), a two-component, extended-release formulation of mixed amphetamine salts: bioavailability of three test formulations and comparison of fasted, fed, and sprinkled administration. Pharmacotherapy. 2002;22(11):1405-1415. PubMed CrossRef
25. Pelham WE, Aronoff HR, Midlam JK, et al. A comparison of Ritalin and Adderall: efficacy and time-course in children with attention-deficit/hyperactivity disorder. Pediatrics. 1999;103(4):e43. PubMed CrossRef
26. Weisler RH. Review of long-acting stimulants in the treatment of attention deficit hyperactivity disorder. Expert Opin Pharmacother. 2007;8(6):745-758. PubMed CrossRef
27. Kramer W, Read S, Tran B. Pharmacokinetics of mixed amphetamine salts extended release in adolescents with ADHD. CNS Spectr. 2005;10(suppl 15):6-13. CrossRef
28. McGough JJ, Biederman J, Greenhill LL, et al. Pharmacokinetics of SLI381 (Adderall XR), an extended-release formulation of Adderall. J Am Acad Child Adolesc Psychiatry. 2003;42(6):684-691. PubMed CrossRef
29. Heal DJ, Smith SL, Gosden J, et al. Amphetamine, past and present—a pharmacological and clinical perspective. J Psychopharmacol. 2013;27(6):479-496. PubMed CrossRef
30. Ermer JC, Pennick M, Frick G. Lisdexamfetamine dimesylate: prodrug delivery, amphetamine exposure and duration of efficacy. Clin Drug Investig. 2016;36(5):341-356. PubMed CrossRef
31. Jasinski D, Krishnan S. Pharmacokinetics of oral lisdexamfetamine dimesylate (LDX; NRP104) versus d-amphetamine sulfate in healthy adults with a history of stimulant abuse (NRP104.A01). http://library.corporate-ir.net/library/17/179/179878/items/202579/6-18-06 Presentation to CPDD on NRP104 A01 and A03.pdf. 2006.
32. Haffey MB, Buckwalter M, Zhang P, et al. Effects of omeprazole on the pharmacokinetic profiles of lisdexamfetamine dimesylate and extended-release mixed amphetamine salts in adults. Postgrad Med. 2009;121(5):11-19. PubMed CrossRef
33. Stark JG, Engelking D, McMahen R, et al. A randomized crossover study to assess the pharmacokinetics of a novel amphetamine extended-release orally disintegrating tablet in healthy adults. Postgrad Med. 2016;128(7):648-655. PubMed CrossRef
34. Childress AC, Brams M, Cutler AJ, et al. The efficacy and safety of evekeo, racemic amphetamine sulfate, for treatment of attention- deficit/hyperactivity disorder Symptoms: a multicenter, dose-optimized, double-blind, randomized, placebo-controlled crossover laboratory classroom study. J Child Adolesc Psychopharmacol. 2015;25(5):402-414. PubMed CrossRef