ABSTRACT
Objective: To study brain atrophy and cognitive decline in elderly patients with first-episode psychosis (FEP).
Methods: Elderly patients aged ≥ 60 years with FEP and onset of psychotic symptoms of ≤ 1 year remitted to the Helsinki University Hospital from December 2009 to December 2011 were included in the study. Diagnoses were made using DSM-IV-TR criteria. All patients had a brain scan, magnetic resonance imaging, or computed tomography. Cognitive performance was assessed using the Consortium to Establish a Registry for Alzheimer`s Disease cognitive test battery.
Results: Of the 85 patients with FEP, 18 had very late-onset schizophrenia–like psychosis (VLOSP), 20 had delusional psychosis, 12 had depressive psychosis, and 35 had psychosis due to a general medical condition. Fifteen of the patients had an earlier history of schizophrenia not known at the time of admittance. These patients were analyzed separately. A vast majority of the FEP patients in all diagnostic groups exhibited signs of cortical atrophy, which was associated with early stage cognitive decline. Multivariate analyses showed that brain atrophy was associated with a decline in Mini-Mental State Examination, Clock Drawing Test, and verbal fluency scores in FEP patients.
Conclusions: Brain atrophy is a frequent finding in elderly FEP patients and is associated with cognitive decline. Elderly patients with FEP should always undergo brain atrophy and cognition screening, as they may constitute an etiologic factor in such patients.
Prim Care Companion CNS Disord 2021;23(6):20m02865
To cite: Louhija U-M, Martola J, Juva K, et al. Brain atrophy in first-episode psychosis of the elderly is associated with cognitive decline. Prim Care Companion CNS Disord. 2021;23(6):20m02865.
To share: https://doi.org/10.4088/PCC.20m02865
© Copyright 2021 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry, Helsinki University Hospital, Helsinki, Finland
bDepartment of Radiology, Helsinki University Hospital, Helsinki, Finland
*Corresponding author: Ulla-Marja Louhija, MD, Helsinki University Hospital, Jorvi Hospital, Psychiatry, Administration, Turuntie 150, 02740 ESPOO, Finland ([email protected]).
Although the etiology of first-episode psychosis (FEP) of the elderly is unknown, it has been suggested that an underlying neurodegenerative disorder may be an important background factor.1,2 If this is true, one would expect to find signs of degenerative brain changes as well as cognitive decline in FEP patients.
Previous studies3 have shown that cortical brain atrophy of the frontal and temporal regions is a common, although not consistent, finding in brain scans of patients with schizophrenia. Imaging studies in patients with very late-onset schizophrenia–like psychosis (VLOSP) have reported enlarged third ventricle volume, lower volume in the left temporal lobe or superior temporal gyrus, and higher ventricle-to-brain ratio compared to age-matched peers as well as increased focal white matter lesions and cortical abnormality.4,5
It has also been reported that in addition to atrophy of the hippocampal cortex, elderly patients with depression may also show thinning of brain cortex in other areas of the brain, including frontal, temporal, and parietal regions.6 In an earlier retrospective study,7 we showed that cortical brain atrophy is a common finding in elderly patients with FEP.
The aim of the study was to prospectively characterize elderly patients with FEP. We were especially interested in the neuroradiologic findings and their relationship to possible early signs of cognitive decline. We chose age ≥ 60 years as a cutoff to distinguish late-onset schizophrenia from VLOSP as proposed by an international consensus panel.5
METHODS
Patients
Elderly patients aged ≥ 60 years with FEP who were referred to 1 of the 4 geropsychiatric wards of the Helsinki University Hospital, Department of Psychiatry with FEP with onset of ≤ 1 year were included in the study on a voluntary basis. The Helsinki University Hospital is owned by the communities in the region, with a catchment area of 1.2 million people.
All patients were examined and evaluated in more detail by a geriatric psychiatrist (U-M.L.) and then received a neurologic assessment by a neurologist (K.J.). Only patients with FEP were included in the study, with the exception of 15 patients admitted to the hospital as FEP patients but found (after interviewing their relatives and studying their earlier—in some cases more than 20 years old—hospital records) to have suffered from ≥ 1 earlier psychotic episode.
Diagnoses were made using DSM-IV-TR criteria. Patients with mania were excluded from the study as were patients with previously documented major neurologic or general medical conditions known to affect the central nervous system.
This study was performed in a clinical hospital setting, meaning that the brain images were available to the authors (U-M.L., K.J., B.G.A.) who participated in the diagnostic process. However, the neuroradiologist (J.M.) who later scored and rated the brain images was blinded to the psychiatric diagnoses, as were his radiologic diagnoses and scores to those participating in the diagnostic process. The patients with schizophrenia–not FEP were analyzed as a separate control group.
Cognitive Measurements
We used the Consortium to Establish a Registry for Alzheimer`s Disease (CERAD) cognitive test battery8 and Mini-Mental State Examination (MMSE),9 verbal fluency,10,11 Clock Drawing Test,12 and 15-item Boston Naming Test13 subscores to examine the cognitive performance of the patients.
Neuroimaging
Brain imaging, magnetic resonance imaging (MRI), or computed tomography (CT) scans were performed as part of the routine clinical management. All imaging scans were later evaluated and assessed by the same neuroradiologist (J.M.) who scored the signs of vascular degeneration and atrophy in the hippocampus, frontal area, temporal region, parietal area, and central area using the following scales:
- The Fazekas Scale14,15 is used for the neuroradiologic assessment of white matter lesions of the brain, which are indicative of vascular dementia. It is scored from 0 (no pathology) to 3 (maximum pathology). In elderly patients, a Fazekas score ≤ 1 is usually considered to be within normal limits.
- The Scheltens Scale15,16 (medial temporal atrophy) is used for the neuroradiologic assessment of atrophy of the hippocampus. It is scored from 0 (no pathology) to 4 (maximum pathology). Scores ≥ 2 for those aged < 75 years and ≥ 3 for those aged ≥ 75 years are considered abnormal.
- The Pasquir Scale17,18 is used for the neuroradiologic assessment of global cortical atrophy. It is scored from 0 (no pathology) to 3 (maximum pathology).
The neuroradiologic assessment of atrophy of the temporal lobe and cingulate gyrus and central atrophy was also done by scoring the pathology from 0 (no pathology) to 3 (maximum pathology).
Statistical Analysis
Mean values and standard deviations were calculated. Neuroradiologic findings in the different diagnostic groups were compared with those of the rest of the pooled patients using Kruskal-Wallis 1-way analysis of variance.19 Furthermore, the neuroradiologic findings of patients with VLOSP were compared to those of patients with schizophrenia–not FEP.
We used Spearman rank order correlation20 to screen the associations between cognitive rating scales and the measures of brain atrophy. Multivariate general linear modeling (MGLM) analysis, using the Spearman correlation matrices19 as input, was then used to further study the correlation between cognitive measures (MMSE, verbal fluency, Clock Drawing Test, naming) and the measures of brain atrophy.
An MGLM model with the cognitive measure variable to be tested (MMSE, verbal fluency, Clock Drawing Test, naming) separately as the dependent variable and the atrophy scores and age as possible independent variables, with stepwise exclusion of independent variables not adding to the explanatory power of the model, was tested until an explanatory model of the relationship (if any) was found.
Ethical Aspects
The study was approved by the Ethics Committee of Helsinki University Hospital. Patients were recruited into the study after signing informed consent.
RESULTS
Patients
One hundred patients were admitted to the psychiatric hospital from December 2009 to December 2011 with FEP and included in the study. Further examination revealed that 1 male and 14 female patients had an earlier history of at least 1 psychotic episode. These patients, not having FEP but fulfilling the diagnostic criteria of schizophrenia, were analyzed as a separate group.
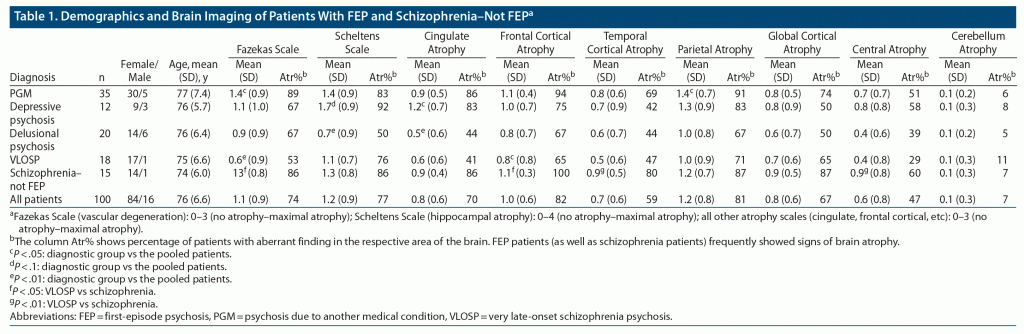
The remaining patients were categorized into 4 diagnostic groups according to DSM-IV-TR criteria and criteria for VLOSP5: (1) patients with VLOSP, (2) patients with delusional psychosis, (3) patients with depressive psychosis, and (4) patients with psychosis due to another medical condition (PGM). The mean age of all patients was 76 years (Table 1).
Neuroimaging
All patients had brain scans (MRI: n = 95 and CT: n = 5). Eighteen patients had minor cerebral infarcts not previously known. Nine of these patients were diagnosed with PGM, 4 with delusional psychosis, 4 with VLOSP, and 1 with schizophrenia–not FEP (see Table 1).
Four of the patients showed neuroradiologic findings indicative of frontotemporal dementia according to the neuroradiologist. Three were patients with PGM and 1 was a patient with schizophrenia–not FEP according to DSM IV-TR criteria.
Only 1 brain scan (CT) revealed no pathologic findings, which was from a patient in the PGM group. Additionally, 2 patients (1 with PGM and 1 with delusional psychosis) had radiologic normal pressure hydrocephalus not previously known from the rest of the pooled diagnostic groups.
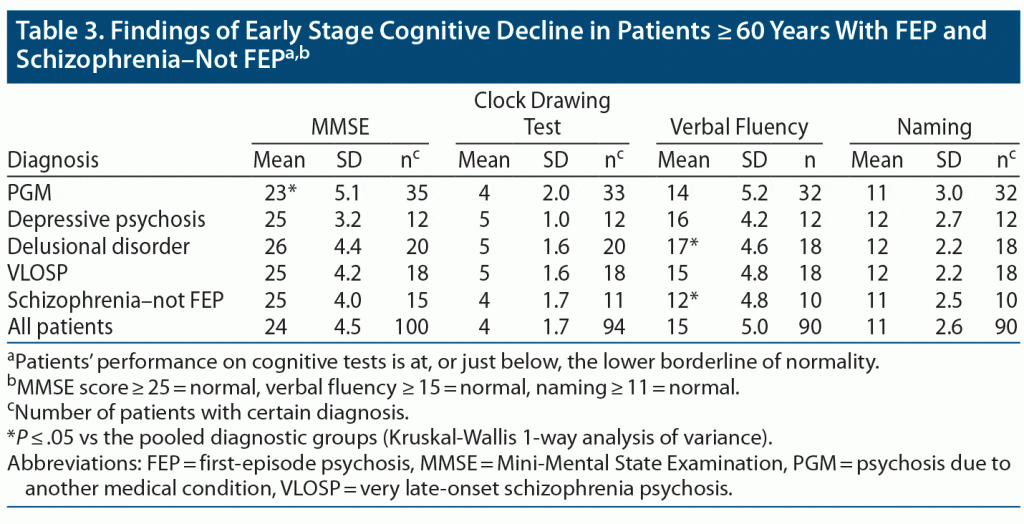
The results of the cognitive tests revealed that mean values at or just below the lower normal borderline were very common findings in patients with FEP and in those with schizophrenia–not FEP (see Table 1).
Patients with PGM exhibited more vascular degeneration than the other pooled patients in the study (Fazekas Scale score: 1.4 vs 1.1, P = .018). They also had more parietal atrophy (1.4 vs 1.2, P = .038) than the rest of the pooled patients.
Patients with psychotic depression showed more cingulate gyrus atrophy (score: 1.2 vs 0.8, P = .043) than the rest of the pooled patients. They also exhibited a tendency toward more hippocampal atrophy (score: 1.7 vs 1.2, P = .053).
Patients with delusional psychosis exhibited less hippocampal atrophy (score: 0.7 vs 1.2, P = .008) and cingulate gyrus atrophy (score: 0.5 vs 0.8, P = .01) than the rest of the pooled patients.
Patients with VLOSP exhibited less vascular changes (Fazekas Scale score: 0.7 vs 1.1, P = .009) and frontal atrophy (score: 0.8 vs 1.0, P = .049) compared to the rest of the pooled patients.
Patients with schizophrenia–not FEP did not significantly differ from the rest of the pooled diagnostic groups (Table 2).
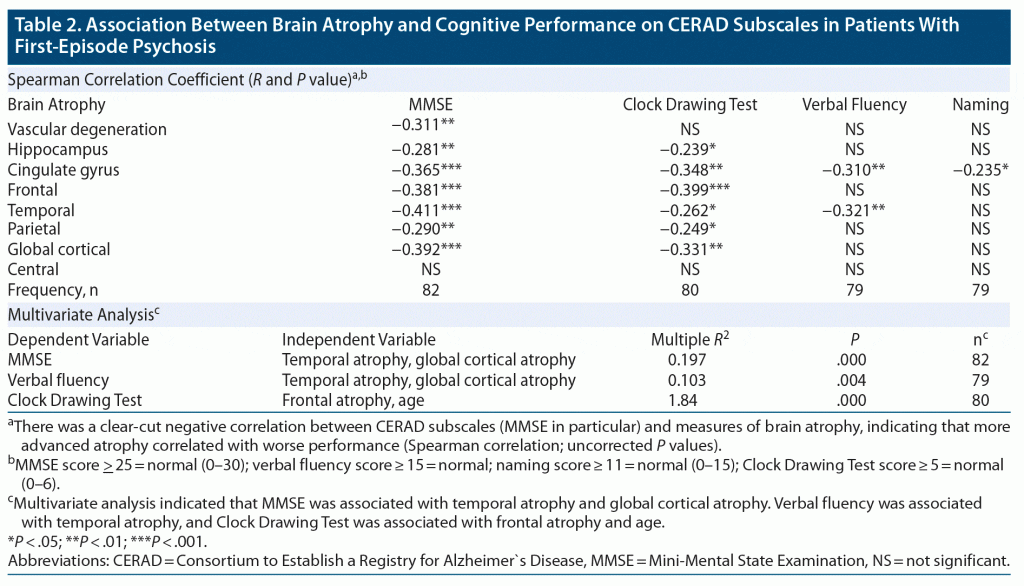
The cognitive test measures showed a clear-cut negative correlation with the neuroradiologic parameter scores indicative of degenerative brain changes. This was especially true for the MMSE (see Table 2).
These associations were further analyzed using multivariate analyses with MMSE, Clock-Drawing Test, verbal fluency, and naming as dependent variables and the neuroradiologic scores (Fazekas Scale, Scheltens Scale, cingulate gyrus, frontal, temporal, parietal lobes atrophy, and global cortical atrophy) and age as independent variables. Automatic stepwise exclusion of variables was then applied.
This analysis revealed the following variations:
(1) MMSE (as dependent variable) was best explained by the variation in temporal atrophy and global cortical atrophy (squared multiple R = 0.197, P = .000, n = 82).
(2) Verbal fluency (as dependent variable) was best explained by the variation in temporal atrophy (squared multiple R = 0.103, P = .004, n = 79).
(3) Clock Drawing Test (as dependent variable) was best explained by the variation in frontal atrophy and age (squared multiple R = 0.184, P = .000, n = 80).
No statistically significant model was found to explain the variation of the dependent variable naming.
DISCUSSION
The findings of the present study strongly support the conclusion of our earlier retrospective study7 that brain atrophy is a common feature in elderly patients with FEP. It is interesting to note that brain changes of patients with VLOSP were less prominent than those of patients with schizophrenia–not FEP, although patients with VLOSP also showed signs of atrophic brain changes. This finding may support the theory that schizophrenia is a progressive disorder or that schizophrenia beginning at an earlier age is associated with more pathology of the frontal brain.21–23
The difference in white matter pathology between VLOSP and schizophrenia patients–not FEP is also interesting but may be explained by the known tendency of more chronic schizophrenia patients to possess unhealthy habits (eg, smoking) associated with white matter pathology of the brain. Patients with PGM showed more vascular atrophy than the other groups, while interestingly patients with VLOSP and delusional psychosis exhibited less vascular changes than the other groups.
Patients with depressive psychosis showed more atrophy of the cingulate gyrus. They also showed a trend toward more hippocampal atrophy than the rest of the patients. This finding is in line with previous reports.24,25
FEP patients also exhibited signs of mild cognitive decline (MMSE score: 23–26), with only minor differences between the diagnostic groups. Patients with PGM had a statistically significantly lower MMSE score (mean: 23) compared to the rest of the pooled patients, including patients with schizophrenia–not FEP (mean: 25). Patients with delusional psychosis performed better on verbal fluency (mean: 17 vs 15) than the rest of the patients, while patients with schizophrenia–not FEP performed worse on verbal fluency (mean: 12 vs 15) (Table 3).
Furthermore, brain atrophy was clearly associated with mild cognitive decline as assessed by the CERAD: a decline in MMSE was associated with temporal and global cortical atrophy, reduced verbal fluency was associated with temporal atrophy, and worse performance on the Clock Drawing Test was associated with frontal atrophy and age. It thus seems that brain atrophy in these patients is a clinically significant phenomenon.
As it is known that lowered cognitive abilities are associated with an increased risk of psychosis,26–31 it is therefore tempting to speculate that the brain atrophy in these patients is causally related to the onset of psychosis. However, longitudinal studies are needed to verify this speculation. Patients were included in this prospective study on a voluntary basis after providing informed consent, thus not all those fulfilling inclusion criteria of the study were included. Hence, this study may not be epidemiologically as representative as our earlier retrospective study including all patients with FEP within 1 year.7
Interestingly, the brain imaging of 4 patients (1 with schizophrenia–not FEP and 3 with PGM) revealed atrophy of the frontal and the temporal areas to the extent that a neuroradiologic diagnosis of behavioral variant frontotemporal dementia,32 characterized by progressive impairment in behavior, personality, and social, cognitive, and functional abilities, was implicated.
One limitation in the present study is that the neuroradiologic images were available to those authors responsible for the diagnostics of the patients. However, they were blinded to the neuroradiologic scoring. The neuroradiologist was blinded to the psychiatric diagnoses, as well as to the CERAD scores of the patients. The CERAD was administered by psychiatric nurses or a psychologist, who were blinded to the neuroradiologic scores.
CONCLUSION
Atrophic changes of the brain are a common finding in elderly FEP patients. These atrophic changes are associated with cognitive decline and may constitute a part of the etiology responsible for the psychotic symptoms. Elderly FEP patients should be screened for brain pathology and cognitive symptoms.
Further controlled and longitudinal studies, including healthy controls, are needed to clarify the relationship between the degenerative brain changes and the psychotic symptoms in these patients.
Submitted: November 13, 2020; accepted March 31, 2021.
Published online: November 11, 2021.
Potential conflicts of interest: None.
Funding/support: None.
Clinical Points
- Elderly patients with first-episode psychosis should always be screened for early stage brain changes using neuroradiologic imaging, such as magnetic resonance imaging.
- It is important to carefully assess the cognitive function of these patients, and if possible, avoid medication (such as anticholinergics) that could further impair their cognitive function.
References (32)

- Pose M, Cetkovich M, Gleichgerrcht E, et al. The overlap of symptomatic dimensions between frontotemporal dementia and several psychiatric disorders that appear in late adulthood. Int Rev Psychiatry. 2013;25(2):159–167. PubMed CrossRef
- Block NR, Sha SJ, Karydas AM, et al. Frontotemporal dementia and psychiatric illness: emerging clinical and biological links in gene carriers. Am J Geriatr Psychiatry. 2016;24(2):107–116. PubMed CrossRef
- Behdinan T, Foussias G, Wheeler AL, et al. Neuroimaging predictors of functional outcomes in schizophrenia at baseline and 6-month follow-up. Schizophr Res. 2015;169(1–3):69–75. PubMed CrossRef
- Chen L, Chen X, Liu W, et al. White matter microstructural abnormalities in patients with late-onset schizophrenia identified by a voxel-based diffusion tensor imaging. Psychiatry Res. 2013;212(3):201–207. PubMed CrossRef
- Howard R, Rabins PV, Seeman MV, et al; The International Late-Onset Schizophrenia Group. Late-onset schizophrenia and very-late-onset schizophrenia-like psychosis: an international consensus. Am J Psychiatry. 2000;157(2):172–178. PubMed CrossRef
- Sexton CE, Mackay CE, Ebmeier KP. A systematic review and meta-analysis of magnetic resonance imaging studies in late-life depression. Am J Geriatr Psychiatry. 2013;21(2):184–195. PubMed CrossRef
- Louhija U-M, Saarela T, Juva K, et al. Brain atrophy is a frequent finding in elderly patients with first episode psychosis. Int Psychogeriatr. 2017;29(11):1925–1929. PubMed CrossRef
- Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD), part I: clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39(9):1159–1165. PubMed CrossRef
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. PubMed CrossRef
- Stuss DT, Alexander MP, Hamer L, et al. The effects of focal anterior and posterior brain lesions on verbal fluency. J Int Neuropsychol Soc. 1998;4(3):265–278. PubMed CrossRef
- Whiteside DM, Kealey T, Semla M, et al. Verbal fluency: language or executive function measure? Appl Neuropsychol Adult. 2016;23(1):29–34. PubMed CrossRef
- Bozikas VP, Kosmidis MH, Gamvrula K, et al. Clock Drawing Test in patients with schizophrenia. Psychiatry Res. 2004;121(3):229–238. PubMed CrossRef
- Haanpää RM, Suhonen NM, Hartikainen P, et al. The CERAD Neuropsychological Battery in patients with frontotemporal lobar degeneration. Dement Geriatr Cogn Disord Extra. 2015;5(1):147–154. PubMed CrossRef
- Fazekas F, Kleinert R, Offenbacher H, et al. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology. 1993;43(9):1683–1689. PubMed CrossRef
- Barkhof F, Fox NC, Bastos-Leite AJ, Scheltens PH. Neuroimaging in Dementia. Springer-Verlag Berlin Heidelberg; 2011.
- Scheltens PH. MRI Practical Evaluation of Dementia: Beyond Exclusion. Presented at the 8th International Conference of Alzheimer`s Disease and Related Disorders; Stockholm, Sweden; July 20–25, 2002.
- Pasquier F, Leys D, Weerts JG, et al. Inter- and intraobserver reproducibility of cerebral atrophy assessment on MRI scans with hemispheric infarcts. Eur Neurol. 1996;36(5):268–272. PubMed CrossRef
- Ferreira D, Cavallin L, Granberg T, et al; AddNeuroMed consortium and for the Alzheimer’s Disease Neuroimaging Initiative*. Quantitative validation of a visual rating scale for frontal atrophy: associations with clinical status, APOE e4, CSF biomarkers and cognition. Eur Radiol. 2016;26(8):2597–2610. PubMed CrossRef
- Kruskal WH, Wallis WA. Use of ranks in one-criterion variance analysis. J Am Stat Assoc. 1952;47(260):583–621. CrossRef
- Spearman C. The proof and measurement of association between two things. By C. Spearman, 1904. Am J Psychol. 1904;15(1):72–101. PubMed CrossRef
- Mubarik A, Tohid H. Frontal lobe alterations in schizophrenia: a review. Trends Psychiatry Psychother. 2016;38(4):198–206. PubMed CrossRef
- Luauté JP, Favel P, Rémy C, et al. Affective disorders and dementia of the frontal lobe type: hypothesis of a pathogenic relationship [in French]. Encephale. 1994;20(1):27–36. PubMed
- Chan D, Fox NC, Jenkins R, et al. Rates of global and regional cerebral atrophy in AD and frontotemporal dementia. Neurology. 2001;57(10):1756–1763. PubMed CrossRef
- Taylor WD, McQuoid DR, Payne ME, et al. Hippocampus atrophy and the longitudinal course of late-life depression. Am J Geriatr Psychiatry. 2014;22(12):1504–1512. PubMed CrossRef
- Ranjan R, Priyamvada R, Jha GK, et al. Neuropsychological deficits in elderly with depression. Ind Psychiatry J. 2017;26(2):178–182. PubMed CrossRef
- Ebert A, Bär K-J. Emil Kraepelin: a pioneer of scientific understanding of psychiatry and psychopharmacology. Indian J Psychiatry. 2010;52(2):191–192. PubMed CrossRef
- Kraepelin E. Dementia Praecox. Barclay RM (trans.). Melbourne, Fla: Robert E. Krieger; 1913.
- Amano N, Iseki E. Introduction: Pick’s disease and frontotemporal dementia. Neuropathology. 1999;19(4):417–421. CrossRef
- Kerssens CJ, Pijnenburg YA, Schouws S, et al. Late-onset schizophrenia: is it a dementia nonpraecox? review article with advice on differential diagnosis [in Dutch]. Tijdschr Psychiatr. 2006;48(9):717–727. PubMed
- Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12(3):426–445. PubMed CrossRef
- Lagodka A, Robert P. Is late-onset schizophrenia related to neurodegenerative processes? a review of literature [in French]. Encephale. 2009;35(4):386–393. PubMed CrossRef
- Cajanus A, Hall A, Koikkalainen J, et al. Automatic MRI quantifying methods in behavioral-variant frontotemporal dementia diagnosis. Dement Geriatr Cogn Disord Extra. 2018;8(1):51–59. PubMed CrossRef
Enjoy this premium PDF as part of your membership benefits!