Abstract
Objective: To conduct a systematic review of the available evidence on hydroxychloroquine (HCQ)-induced psychiatric side effects and their management.
Data sources: A literature search was conducted in PubMed, MEDLINE, PsycINFO, and Cochrane collaboration databases from 2000 to 2024 using the keywords “hydroxychloroquine” AND “psychiatry” OR “psychosis” OR “depression” OR “anxiety” OR “bipolar disorder” OR “delirium” OR “psychotic disorders” OR “psychiatric side effects” OR “psychiatric disorders.”
Study selection: English-language articles with studies reporting HCQ-induced psychiatric/neuropsychiatric side effects were included. Duplicate records and studies reporting only chloroquine side effects were excluded.
Results: The review included 16 case reports, 8 original articles, and 3 review articles. HCQ was found to trigger symptoms of psychosis, depression, suicidal ideation, mania/hypomania, anxiety, sleep disturbances, and cognitive impairments. The onset of these psychiatric side effects varied, appearing shortly after starting the medication to a more extended period.
Conclusion: Based on the literature, HCQ may be associated with short-term psychiatric adverse effects. A psychiatric consultation for a thorough clinical and risk factor evaluation to differentiate a primary psychiatric disorder from a drug induced adverse effect would help guide the management. Dosage adjustments, discontinuing HCQ if feasible, and psychotropic medications like olanzapine or risperidone may be necessary when psychiatric side effects are secondary to HCQ. Further studies are needed to validate these findings.
Prim Care Companion CNS Disord 2025;27(3):24r03857
Author affiliations are listed at the end of this article.
Hydroxychloroquine (HCQ) is a hydroxide derivative of chloroquine (CQ). It is approved by the US Food and Drug Administration (FDA) for treating malaria, systemic lupus erythematosus (SLE), discoid lupus erythematosus, and rheumatoid arthritis. The off-label uses include Q-fever, porphyria cutanea tarda, primary Sjogren disease, and sarcoidosis.1–4
Since CQ and HCQ exhibit similar physiochemical properties, many pharmacokinetic features of HCQ are often inferred from CQ studies,5 namely, high oral bioavailability, high tissue penetrance, partial hepatic metabolism, and high volumes of distribution (as they diffuse into adipose tissue). However, CQ has diminished recently due to widespread malarial parasite drug resistance and drug toxicity. It is now used mainly for malaria prophylaxis in combination with proguanil.6,7
Research shows that in addition to anti-inflammatory and immunomodulatory action, HCQ can improve vascular risk by acting directly on the lipid profile and has antithrombotic properties.8,9 It has also been proven to have an in-vitro antiviral effect on various RNA viruses.9 Its antiviral action is exerted through the drug’s accumulation and alkalinization of the endosome, lysosome, and Golgi vesicles, leading to an increase in the pH resulting in enzyme dysfunction, thus making it impossible for vesicles containing a virus to enter the cell.9
Owing to these properties, the year 2020 saw an emergence in the use of HCQ as an emergency-use drug to treat COVID-19, although no proper randomized controlled trial had been completed at that time to support the drugs’ safety and efficacy in this COVID-19 population.9 Its use declined due to directives from the FDA indicating no evidence that HCQ is effective against COVID-19.9
Adding a hydroxide derivative to CQ allowed HCQ to have fewer adverse events.10 However, adverse reactions are seen, the most common being gastrointestinal disturbances, intravascular hemolysis, retinal toxicity, rash, and bone marrow suppression. Neuropsychiatric side effects are not rare with HCQ.6 Historically, these were reported dating back to the 1970s. Neuropsychiatric adverse reactions include agitation, insomnia, confusion, mania, hallucinations, paranoia, depression, catatonia, psychosis, and suicidal ideation.11,12 These adverse effect profiles have suggested caution when HCQ is prescribed.13,14 Hence, our review’s primary focus is to summarize the available evidence about HCQ-induced psychiatric side effects and their management to support physicians in clinical settings.
METHODS
A literature search of English-language articles was conducted in PubMed, MEDLINE, PsycINFO, and Cochrane Collaboration databases from 2000 to 2024. Keywords included “hydroxychloroquine” AND “psychiatry,” “psychosis,” “depression,” “anxiety,” “bipolar disorder,” “delirium” OR “psychotic disorders,” “psychiatric side effects,” “psychiatric disorders.” A study was included if it reported psychiatric side effects/adverse reactions due to HCQ use. Some studies reported neuropsychiatric side effects and did not provide a clear distinction between neurological and psychiatric side effects. They were also included in this study.
The authors (M.J.S., R.K.K., P.S., N.P.) assessed the studies for relevance to inclusion in the study, and the data were entered into an Excel sheet. Duplicate records and studies reporting only CQ side effects were excluded from the study. Studies not reporting HCQ psychiatric/ neuropsychiatric side effects were also excluded.
The following data were extracted from the studies wherever possible: sociodemographic data, HCQ use, psychiatric/neuropsychiatric side effects/adverse reactions, frequency and severity of symptoms, and management of such effects. Study Quality Assessment Tools by NIH (National Heart, Lung, and Blood Institute)15 was used to assess the quality of the included studies. This systematic review adhered to the PRISMA 2020 guidelines.16
RESULTS
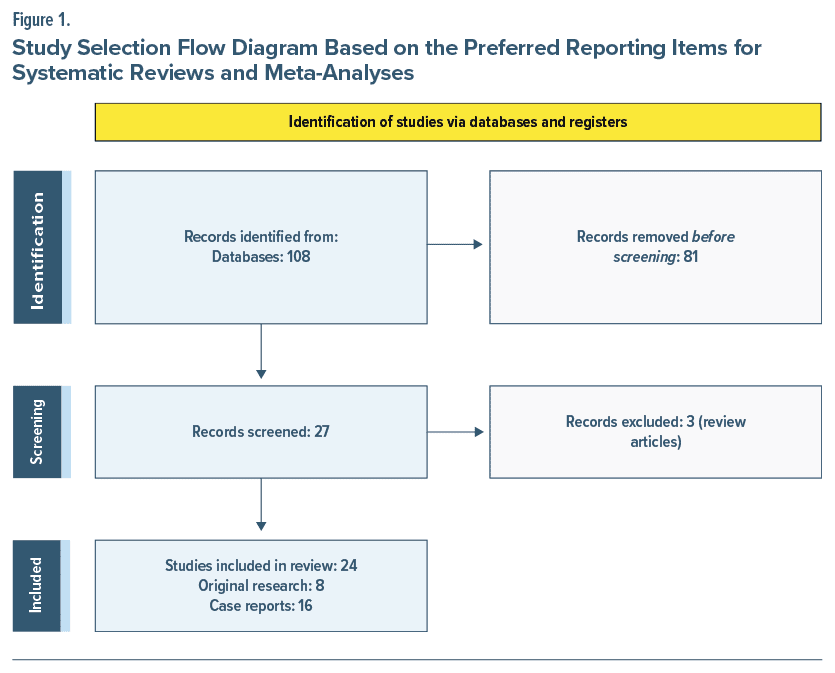
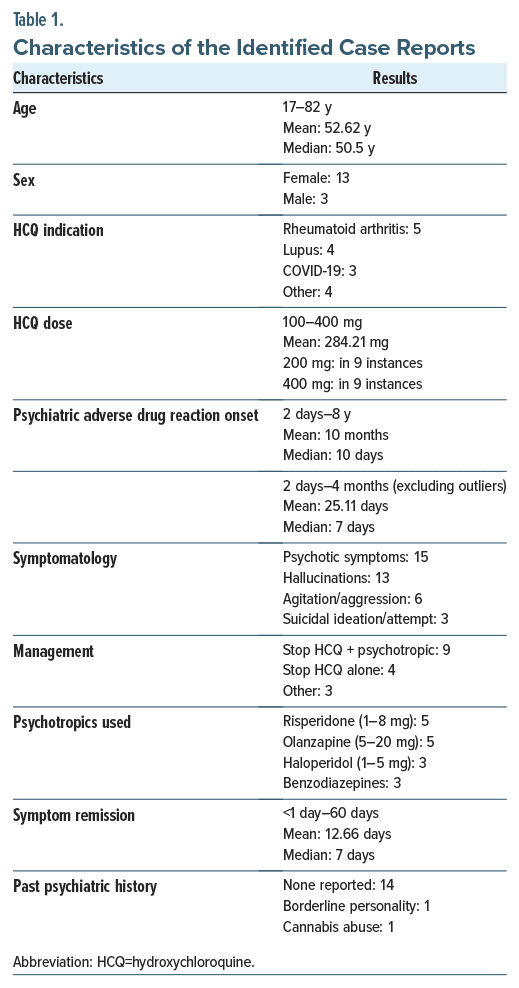
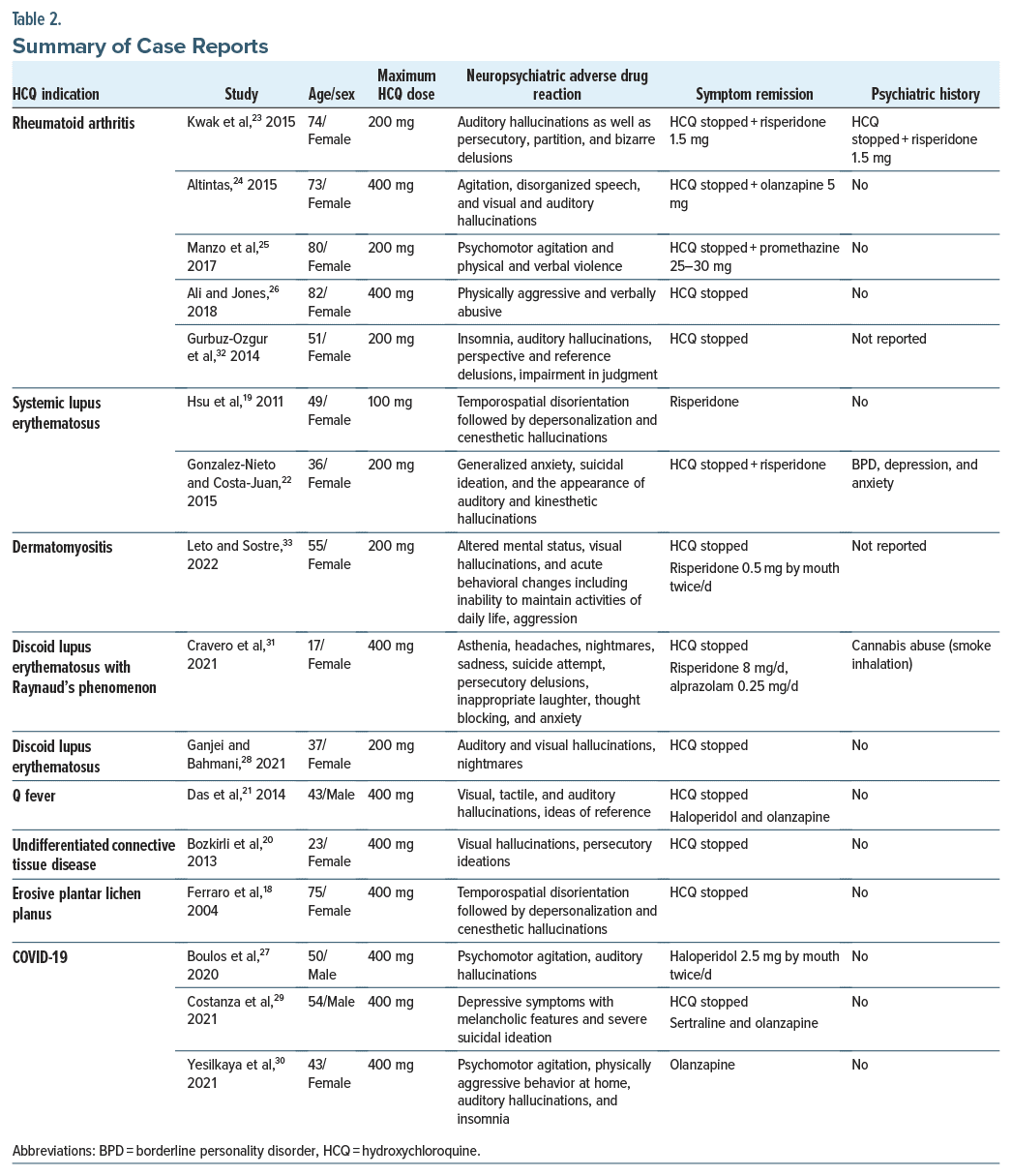
A total of 108 articles was found using the inclusion criteria. After applying exclusion criteria, 27 articles were identified. There were 16 case reports, 8 original articles, and 3 review articles. The 3 review articles were excluded, leaving a final total of 24 articles.17–40 The PRISMA flow diagram in Figure 1 outlines this process and breaks down the reasons for the exclusion of ineligible studies. The characteristics of the case reports are included in Table 1, and the reports are summarized in Table 2.
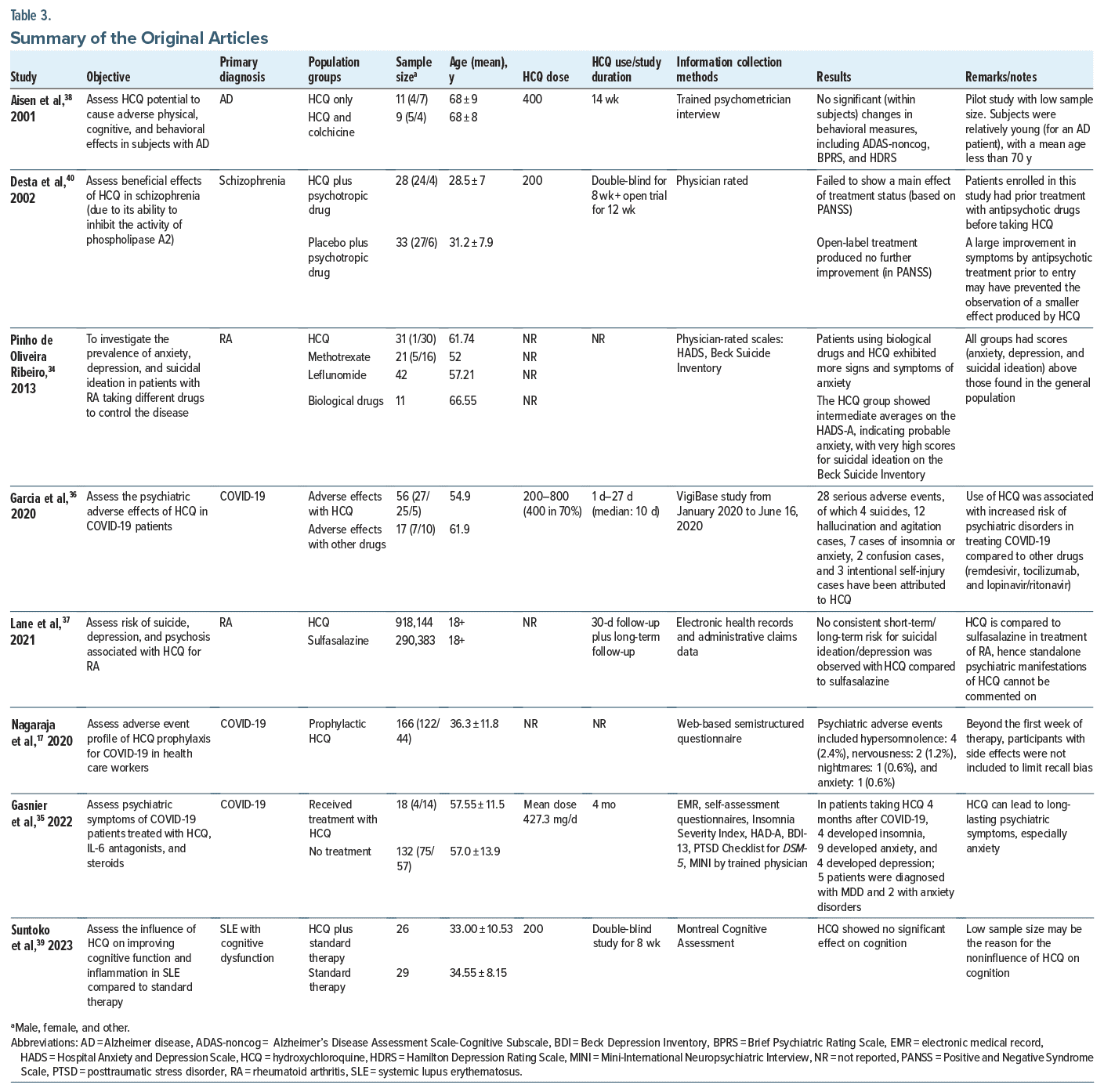
Summary of the Original Articles
Two original articles reported significant psychiatric side effects with HCQ, and 3 articles sought to assess psychiatric adverse effects (PAEs) by analyzing existing electronic health records or adverse event reporting data (Table 3).
In a naturalistic study by Pinho de Oliveira Ribeiro et al,34 patients taking HCQ exhibited more signs and symptoms of anxiety when compared with methotrexate and leflunomide. They also had anxiety, depression, and suicidal ideation scores above those found in the general population. Further, a bivariate analysis by Gasnier et al35 within propensity score–matched cohorts revealed that HCQ was associated with significant anxiety symptoms, observed in 50% of the HCQ group compared to 20.1% in the control group (odds ratio [OR] = 3.8, 95% CI, 1.3−11.3, P = .01). HCQ was also linked to prolonged anxiety symptoms up to 4 months after acute COVID-19 infection. In contrast, PAEs were less common with treatments such as anti-interleukin-6, which were more associated with depressive symptoms, and corticosteroids, which showed no significant link to psychiatric symptoms. Garcia et al36 conducted a pharmacovigilance analysis involving 1,754 COVID-19 patients treated with HCQ, and 56 PAEs were reported. Half of these were classified as serious, including 4 suicides, 3 instances of intentional self-injury, and 12 cases of psychotic disorders characterized by hallucinations, agitation, or aggression. The risk of developing psychiatric disorders with HCQ was significantly higher compared to other COVID-19 treatments like remdesivir, tocilizumab, and lopinavir/ ritonavir, with a reporting OR of 6.27 (95% CI, 2.74–14.35). These adverse effects were more prevalent in men, with a mean age of 54.9 years, and typically appeared within a median of 5 days of starting HCQ treatment. The majority of serious psychiatric cases occurred at a dosage of 400 mg/day. In the observational cohort study by Lane et al,37 no consistent psychiatric side effect risk (such as depression, suicidal ideation or suicide, acute psychosis) was observed in the short term in patients on HCQ compared to sulfasalazine when electronic health records and administrative claims data were analyzed. Also, no consistent long-term risk for suicidal ideation/depression was observed with HCQ compared to sulfasalazine. Nagaraja et al17 conducted a retrospective, cross-sectional study by screening 166 patients taking HCQ prophylaxis for adverse reactions. Psychiatric manifestations were reported in 4.8% of the patients, including hypersomnolence in 4 (2.4%) patients, nervousness in 2 (1.2%), and nightmares and anxiety in 1 (0.6%).
The Effect of HCQ on Cognition
In a pilot feasibility study by Aisen et al,38 comparing HCQ use alone and HCQ plus colchicine use in patients with Alzheimer disease, no significant changes in behavioral measures, including the Alzheimer’s Disease Assessment Scale-Cognitive Subscale, ADAS-noncog, Brief Psychiatric Rating Scale, and Hamilton Depression Rating Scale, were seen on a HCQ dosage of 400 mg for 14 weeks.
Suntoko et al39 conducted a double-blind, randomized controlled trial to assess the influence of HCQ on improving cognitive function and inflammation in patients’ SLE with cognitive dysfunction while comparing HCQ with standard therapy. They found that HCQ showed no significant effect on cognition at a dose of 200 mg for 8 weeks.
Are There Any Beneficial Effects of HCQ in Schizophrenia?
In a study by Desta et al,40 the authors conducted a controlled trial to assess the beneficial effects of HCQ in schizophrenia (due to its ability to inhibit the activity of phospholipase A2). This study failed to show any effect on treatment status (based on the Positive and Negative Syndrome Scale [PANSS]) during the 8-week double-blind period while comparing HCQ plus standard antipsychotic therapy and placebo plus standard antipsychotic therapy. Open-label treatment for the next 12 weeks also produced no further improvement in PANSS.
Although an article by Sato et al41 showed significantly higher reports of delirium, loss of consciousness, amnesia, hallucinations, and depression in the CQ plus HCQ group, it was not included in the study, as it did not differentiate between CQ and HCQ psychiatric adverse drug reactions.
DISCUSSION
This systematic review, to our knowledge, is the first to compare pre- and post–COVID-19 literature about the PAEs of HCQ. We sought to review the studies’ pre– and post–COVID-19 (the year 2020) and summarized our findings. HCQ is associated with psychiatric adverse reactions, primarily insomnia, anxiety, depression, and psychotic disorders, with a median onset time of 5 days from the initiation of medication.3 In the pre–COVID-19 period, HCQ was mainly used to treat conditions such as rheumatoid arthritis (RA) and SLE that require long-term medication use. For a brief duration, HCQ was used for COVID-19 treatment before further evidence emerged that it is ineffective against severe acute respiratory syndrome coronavirus 2.
Among the various adverse effects reported from HCQ, suicide was cited as the leading cause of death before the year 2020. Given the neurotropic impact of CQ/HCQ, all studies recommend informing patients and their relatives about this possible increase in suicide risk.29 In our review, we found that after 2020, anxiety was the most commonly reported PAE of HCQ. Before 2020, there was a higher propensity for psychiatric adverse reactions like insomnia, anxiety, and depression in women. In the case reports, women (n = 13) more than men (n = 3) were reported to have PAEs. This may be because of the use of HCQ in women for SLE and RA.36 After 2020, given the brief increase in the use of HCQ in males for COVID-19 management/prophylaxis, an increasing number of adverse effects is also being reported in men.36 Of the 3 case reporting adverse drug reactions while on HCQ for COVID-19, 2 of the patients were male, and 1 was female. Self-harm and suicide risk stayed comparable pre– and post–COVID-19. This could suggest that PAEs associated with HCQ may be consistent in both genders.
We further looked into risk factors for developing psychiatric side effects. One such factor could be a higher dosage of HCQ, and the use of concomitant cytochrome P3A4 (CYP3A4) inhibitors may increase the risk of psychiatric side effects.18,36,42 Another possible risk factor is old age. The mean age of patients in the case reports is 52.62 years. The mean age of patients on HCQ in most articles included in the study ranges from 33 to 68 years, reflecting the probability of HCQ use in a slightly older population owing to the later onset of rheumatoid arthritis and lupus erythematosus. This can also be explained by possibly diminished neuronal reserve and the presence of mixed neurodegenerative and vascular brain injuries in the elderly, leading to PAEs.29
Mechanism of Action of HCQ and How Psychiatric Side Effects May Develop
Multiple mechanisms have been postulated to explain the occurrence of PAEs from HCQ. Further research is needed to understand the definitive causation. Inhibition of acetylcholinesterase, inhibition of prostaglandin synthesis, prostaglandin E antagonism, and imbalance in the dopaminergic pathway have been reported.14,21 HCQ also causes neurotoxicity,19,43–45 N-methyl-D-aspartate excitotoxicity, and γ-aminobutyric acid inhibition.19,45,46 The enhanced dopaminergic activity causes symptoms of mania, psychosis,21,47 and bipolar-like symptoms.48 HCQ affects not only the dopaminergic pathway but also the cholinergic pathway by inhibiting acetylcholinesterase12,44 and muscarinic receptors.19 The net result is increased serotonin levels in the synapse, resulting in mania and psychosis.49 Its accumulation in the brain can disrupt neurotransmitter signaling circuits (neurochemical interference with calcium signaling in neural cells as well as disruption of dopamine and acetylcholine homeostasis) involved in the pathogenesis of suicide. Its metabolic and cardiovascular impacts can result in abnormal cortisol release and arrhythmia, both of which have been linked to increased suicide risk.29 Additionally, there is a downregulation of glycoprotein-P in the blood-brain barrier. Glycoprotein-P is responsible for clearing various substances from neurons and clearing antidepressants along the blood-brain barrier.49
Therefore, a patient on antidepressants and having mental health disorders could have a variation in their mental health status when on HCQ. HCQ decreases seizure threshold when used along with psychotropics like clozapine and chlorpromazine. It increases the electroencephalogram frequencies acting as a cerebrocortical stimulant50 but decreases voltage, which may trigger seizures.51
Role of Pharmacokinetics and Drug Interactions in PAEs of HCQ
HCQ and CQ have a long half-life in the blood, approximately a month.12,40,41,52 Although considered relatively safer drugs, they have a narrow therapeutic index.13,53,54 CQ/HCQ exhibits neurotropism, as its level in the CNS was shown to be 10–30 times higher than its serum concentration after dosing.13,55 It is completely absorbed in the system within 2–4 hours after oral administration,54 which can lead to various psychiatric adverse reactions like acute psychosis, mania, depression, anxiety, and suicidal ideations.53
Variations in absorption, degradation, and consequent differences in the drug’s steady-state concentration may lead to variability in the time window between drug intake and PAE manifestation.24,25,54,56 Concomitant corticosteroid use and a family history of psychiatric disorder can increase the risk of developing PAEs.
When CQ or its analogs are used with psychotropics with CYP3A4 inhibitory potential, side effects will ensue due to raised levels of CQ. CYP3A4 inducers could decrease levels of CQ or HCQ. The combined cardiac side effects of CQ analogs must be kept in mind when prescribed in conjunction with psychiatric medications that prolong QTc interval with the resulting cumulative cardiotoxicity.52,57 The risk of PAEs was particularly signalized among patients in cotreatment with metformin.29,58
There are many areas in psychiatric treatment where caution must be exerted.20 In particular, when HCQ is prescribed for chronic medical conditions, a psychiatric review of symptoms and monitoring for any appearance of PAEs is recommended. Psychiatry consultants must be aware of these side effects and guide the management. When side effects occur, stopping HCQ and the addition of psychotropics such as olanzapine, risperidone, and haloperidol were reported as successful interventions for the resolution of symptoms.21,59 Psychotropic drugs must be used, making sure to avoid SLE-inducing drugs, such as chlorpromazine, carbamazepine, and lithium carbonate.59
Limitations of the Study
The first limitations of our study are publication bias, selection bias, attrition bias, inadequate blinding, and selective outcome reporting. Duplicate references, transcription errors, and accidental inclusions or exclusions can skew or invalidate the results of our review, which we have minimized to the best of our ability. Second, it is not uncommon for patients to develop mental health disorders such as depression and anxiety as a long-term sequela of the chronic disease. Recent studies indicate a higher prevalence (13%–47%) of depression and anxiety disorders in patients with RA compared to the general population, with symptoms sometimes leading to panic attacks, low self-esteem, and suicidal trends.19,45,46 The chronic nature of RA and SLE can worsen the prognosis of comorbid psychiatric disorders.19,47 Conditions such as rheumatoid arthritis require long-term medications, including drugs like HCQ, which can confound HCQ-induced PAEs.
The third limitation is that symptoms of a neuropsychiatric nature in patients with COVID-19 may not always be drug induced and instead caused by the huge cytokine storm due to the disease process and neuroinflammatory mechanisms.60 Some evidence points towards external stressors as the reason for the rise of PAEs in patients who took HCQ for COVID 19.25 This would skew our data for the causation-effect relationship of HCQ-induced PAEs in COVID-19 patients.
Strengths of the Study
First, we included literature up to year 2024, which combined the reporting of side effects during COVID 19 treatment. Second, we sought to explore various presentations of the PAEs and how they were managed.
CONCLUSION
HCQ is a commonly prescribed medication for chronic medical conditions like RA and SLE. HCQ has been associated with a few psychiatric side effects such as depression, mania and hypomania, anxiety disorders, suicidal ideation, psychosis, insomnia, cognitive impairments like confusion, disorientation, memory problems, and other behavioral changes like irritability and restlessness. The onset of these psychiatric side effects can vary in duration. In our review, we found a propensity in the usage of second-generation antipsychotics, particularly olanzapine and risperidone, for the treatment of these PAEs. Haloperidol was also helpful in some cases. Further investigative studies and clinical trials are needed to understand the mechanisms behind the causation of these side effects from HCQ.
Article Information
Published Online: May 29, 2025. https://doi.org/10.4088/PCC.24r03857
© 2025 Physicians Postgraduate Press, Inc.
Submitted: September 24, 2024; accepted January 31, 2025.
To Cite: Setty MJ, Kavi RK, Sulthana P, et al. Psychiatric adverse effects from hydroxychloroquine use: a systematic review. Prim Care Companion CNS Disord 2025;27(3):24r03857.
Author Affiliations: Teas Tech University Health Sciences Center-Permian Basin, Odessa, Texas (Setty); Sanjeevini Neuropsychiatric Care, Visakhapatnam, Andhra Pradesh, India (Kavi); Adichunchanagiri Institute of Medical Sciences, Nagamangala, Karnataka, India (Sulthana); All India Institute of Medical Sciences, Bhubaneswar, Odisha, India (Prabhakaran).
Corresponding Author: Madhuri Jakkam Setty, MD, Texas Tech Physicians-Permian Basin, Texas Tech University Health Sciences Center-Permian Basin, 701 W 5th St, Odessa, TX ([email protected]).
Funding/Support: None.
Relevant Financial Relationships: None.
Previous Presentation: Parts of the data were presented as a scientific poster at the Academy of Consultation Liaison Psychiatry 2020.
ORCID: Madhuri Jakkam Setty, https://orcid.org/0009-0000-5695-4557; Ramananda Kishore Kavi, https://orcid.org/0009-0000-8331-9145; Nishanth Prabhakaran, https://orcid.org/0009-0006-2275-3996
Clinical Points
- Hydroxychloroquine (HCQ) is used in the treatment of chronic diseases like rheumatoid arthritis and systemic lupus erythematosus.
- HCQ is associated with neuropsychiatric adverse effects like depression, mania and hypomania, anxiety disorders, suicidal ideation, psychosis, sleep disturbances, cognitive impairments such as confusion, disorientation, memory problems, and other behavioral changes like irritability and restlessness.
- Clinicians can familiarize themselves with these psychiatric side effects in patients and collaborate with psychiatrists for further evaluation and treatment.
References (60)

- WHO Guidelines for malaria. Accessed May 26, 2024. https://www.who.int/publications-detail-redirect/guidelines-for-malaria
- Hydroxychloroquine: Drug information - UpToDate. Accessed May 26, 2024. https://www.uptodate.com/contents/hydroxychloroquine-druginformation?search=hydroxychloroquine&source=panel_search_result&selectedTitle=1%7E149&usage_type=panel&kp_tab=drug_general&display_rank=1
- Chloroquine. Drug information - UpToDate. Accessed May 28, 2024. https://www.uptodate.com/contents/chloroquine-drug-information?topicRef=5704&source=see_link
- Goel P, Gerriets V. Chloroquine. In: StatPearls. StatPearls Publishing; 2024. Accessed May 28, 2024. http://www.ncbi.nlm.nih.gov/books/NBK551512/
- Rainsford KD, Parke AL, Clifford-Rashotte M, et al. Therapy and pharmacological properties of hydroxychloroquine and chloroquine in treatment of systemic lupus erythematosus, rheumatoid arthritis and related diseases. Inflammopharmacology. 2015;23(5):231–269. PubMed CrossRef
- Aronson JK, ed. Chloroquine and hydroxychloroquine Meyler’s Side Effects of Drugs (Sixteenth Edition). Elsevier;2016:253–267. https://doi.org/10.1016/B978-0-444-53717-1.00478-9
- Wani WA, Jameel E, Baig U, et al. Ferroquine and its derivatives: new generation of antimalarial agents. Eur J Med Chem. 2015;101:534–551. PubMed CrossRef
- Frimpong A, Thiam LG, Arko-Boham B, et al. Safety and effectiveness of antimalarial therapy in sickle cell disease: a systematic review and network meta analysis. BMC Infect Dis. 2018;18(1):650. PubMed CrossRef
- Emmanuel S, Östlundh L. Psychiatric adverse events with hydroxychloroquine during COVID-19 pandemic. Asian J Psychiatr. 2020;54:102203. PubMed CrossRef
- Liu J, Cao R, Xu M, et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:16. PubMed CrossRef
- Juurlink DN. Safety considerations with chloroquine, hydroxychloroquine and azithromycin in the management of SARS-CoV-2 infection. CMAJ. 2020;192(17):E450–E453. PubMed CrossRef
- Mohan D, Mohandas E, Rajat R. Chloroquine psychosis: a chemical psychosis? J Natl Med Assoc. 1981;73(11):1073–1076. PubMed
- Mascolo A, Berrino PM, Gareri P, et al. Neuropsychiatric clinical manifestations in elderly patients treated with hydroxychloroquine: a review article. Inflammopharmacology. 2018;26(5):1141–1149. PubMed CrossRef
- Good MI, Shader RI. Lethality and behavioral side effects of chloroquine. J Clin Psychopharmacol. 1982;2(1):40–47. PubMed CrossRef
- Study Quality Assessment Tools | NHLBI, NIH. Accessed May 27, 2024. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. PubMed CrossRef
- Nagaraja BS, Ramesh KN, Dhar D, et al. HyPE study: hydroxychloroquine prophylaxis-related adverse events’ analysis among healthcare workers during COVID-19 pandemic: a rising public health concern. J Public Health. 2020;42(3):493–503. PubMed CrossRef
- Ferraro V, Mantoux F, Denis K, et al. [Hallucinations during treatment with hydrochloroquine]. Ann Dermatol Venereol. 2004;131(5):471–473. PubMed CrossRef
- Hsu W, Chiu N, Huang S. Hydroxychloroquine-induced acute psychosis in a systemic lupus erythematosus female. Acta Neuropsychiatr. 2011;23(6):318–319. PubMed CrossRef
- Bozkirli EDE, Turan İ, Altintaş E, et al. A case of a rare side effect of hydroxychloroquine: hallucination. Turk Klin J Case Rep. 2013;21(1):6–9.
- Das P, Rai A, Chopra A, et al. Psychosis likely induced by hydroxychloroquine in a patient with chronic Q fever: a case report and clinically relevant review of pharmacology. Psychosomatics. 2014;55(4):409–413. PubMed CrossRef
- Gonzalez-Nieto JA, Costa-Juan E. Psychiatric symptoms induced by hydroxychloroquine. Lupus. 2015;24(3):339–340. PubMed CrossRef
- Kwak YT, Yang Y, Park SY. Chloroquine-associated psychosis mimicking very late-onset schizophrenia: case Report. Geriatr Gerontol Int. 2015;15(8):1096–1097. PubMed CrossRef
- Altintas E. Hydroxychloraquine-induced acute psychotic disorder in a female patient with rheumatoid arthritis: a case report. Dusunen Adam J Psychiatry Neurol Sci. 2015;28(4):369–373. CrossRef
- Manzo C, Gareri P, Castagna A. Psychomotor agitation following treatment with hydroxychloroquine. Drug Saf Case Rep. 2017;4(1):6. PubMed CrossRef
- Ali SS, Jones H. An adverse neuropsychiatric reaction following treatment with hydroxychloroquine: a case report. Rheumatol Adv Pract. 2018;2(suppl 1):rky033.
- Boulos N, Newman B, Newman W. New-onset psychosis while being treated for coronavirus. Curr Psychiatry. 2020;19(7). doi:10.12788/cp.0016. CrossRef
- Ganjei Z, Bahmani K. A case report of hydroxychloroquine-induced auditory and visual hallucination. Int J Clin Pharmacol Ther. 2021;59(3):254–256. PubMed CrossRef
- Costanza A, Placenti V, Amerio A, et al. Chloroquine/hydroxychloroquine use and suicide risk: hypotheses for confluent etiopathogenetic mechanisms? Behav Sci. 2021;11(11):154. PubMed CrossRef
- Yesilkaya UH, Balcioglu YH, Sen M. Psychotic agitation in a patient with COVID-19: pathogenesis or iatrogenesis? Psychiatry Clin Psychopharmacol. 2021;31(2):238–240. PubMed CrossRef
- Cravero C, Hie M, Barete S, et al. Psychotic episode following treatment with hydroxychloroquine in a 17- year-old female adolescent with cutaneous lupus erythematosus: a drug causality supported by a literature review and a worldwide pharmacovigilance database search. Arch Clin Med Case Rep. 2021;05(06). doi:10.26502/acmcr.96550431. CrossRef
- Gurbuz-Ozgur B, Aslan-Kunt D, Sevincok L. Psychotic symptoms related to hydroxychloroquine. Klin Psikofarmakol Bul. 2014;24(suppl 1):S160.
- Leto AE, Sostre S. Hydroxychloroquine and hallucinosis: a case report. J Acad Consult-Liaison Psychiatry. 2022;63:S49. CrossRef
- Pinho de Oliveira Ribeiro N, Rafael de Mello Schier A, Ornelas AC, et al. Anxiety, depression and suicidal ideation in patients with rheumatoid arthritis in use of methotrexate, hydroxychloroquine, leflunomide and biological drugs. Compr Psychiatry. 2013;54(8):1185–1189. PubMed CrossRef
- Gasnier M, Choucha W, Montani D, et al. Hydroxychloroquine, interleukin-6 receptor antagonists and corticoid treatments of acute COVID-19 infection: psychiatric symptoms and mental disorders 4 Months later. Clin Psychopharmacol Neurosci. 2022;20(4):762–767. PubMed CrossRef
- Garcia P, Revet A, Yrondi A, et al. Psychiatric disorders and hydroxychloroquine for coronavirus disease 2019 (COVID-19): a VigiBase study. Drug Saf. 2020;43(12):1315–1322. PubMed CrossRef
- Lane JCE, Weaver J, Kostka K, et al. Risk of depression, suicide and psychosis with hydroxychloroquine treatment for rheumatoid arthritis: a multinational network cohort study. Rheumatology. 2021;60(7):3222–3234. PubMed CrossRef
- Aisen PS, Marin DB, Brickman AM, et al. Pilot tolerability studies of hydroxychloroquine and colchicine in alzheimer disease. Alzheimer Dis Assoc Disord. 2001;15(2):96–101. PubMed CrossRef
- Suntoko B, Hadisaputro S, Kalim H, et al. A double-blind, randomized controlled trial of hydroxychloroquine for cognitive dysfunction and inflammatory biomarkers in systemic lupus erythematosus patients in Indonesia. Indones Biomed J. 2023;15(4):354–361.
- Desta M, Tadesse A, Gebre N, et al. Controlled trial of hydroxychloroquine in schizophrenia. J Clin Psychopharmacol. 2002;22(5):507–510. PubMed CrossRef
- Sato K, Mano T, Iwata A, et al. Neuropsychiatric adverse events of chloroquine: a real-world pharmacovigilance study using the FDA Adverse Event Reporting System (FAERS) database. Biosci Trends. 2020;14(2):139–143. PubMed CrossRef
- Crescioli G, Brilli V, Lanzi C, et al. Adverse drug reactions in SARS-CoV 2 hospitalised patients: a case-series with a focus on drug–drug interactions. Intern Emerg Med. 2021;16(3):697–710. PubMed CrossRef
- Bhatia MS, Jhanjee A, Oberoi A. A case of chloroquine-induced recurrent mania. Prim Care Companion CNS Disord. 2012;14(3):PCC.11l01302.
- Bogaczewicz A, Sobow T, Bogaczewicz J, et al. Chloroquine-induced subacute paranoid-like disorder as a complication of dermatological treatment. Int J Dermatol. 2016;55(12):1378–1380. PubMed CrossRef
- Sahoo S, Kumar M, Sinha VK. Chloroquine-induced recurrent psychosis. Am J Ther. 2007;14(4):406–407. PubMed CrossRef
- Aneja J, Goya D, Choudhary B. Psychosis consequent to antimalarial drug use in a young child. J Fam Med Prim Care. 2019;8(5):1781–1783. PubMed CrossRef
- Akhtar S, Mukherjee S. Chloroquine induced mania. Int J Psychiatry Med. 1993;23(4):349–356. PubMed CrossRef
- Bogaczewicz J, Sobów T, Bogaczewicz A, et al. Exacerbations of bipolar disorder triggered by chloroquine in systemic lupus erythematosus-a case report. Lupus. 2014;23(2):188–193. PubMed CrossRef
- Alisky JM, Chertkova EL, Iczkowski KA. Drug interactions and pharmacogenetic reactions are the basis for chloroquine and mefloquine-induced psychosis. Med Hypotheses. 2006;67(5):1090–1094. PubMed CrossRef
- Biswas PS, Sen D, Majumdar R. Psychosis following chloroquine ingestion: a 10- year comparative study from a malaria-hyperendemic district of India. Gen Hosp Psychiatry. 2014;36(2):181–186. PubMed CrossRef
- Malcangi G, Fraticelli P, Palmieri C, et al. Hydroxychloroquine-induced seizure in a patient with systemic lupus erythematosus. Rheumatol Int. 2000;20(1):31–33. PubMed CrossRef
- Hamm BS, Rosenthal LJ. Psychiatric aspects of chloroquine and hydroxychloroquine treatment in the wake of coronavirus disease-2019: psychopharmacological interactions and neuropsychiatric sequelae. Psychosomatics. 2020;61(6):597–606. PubMed CrossRef
- Talarico F, Chakravarty S, Liu YS, et al. Systematic review of psychiatric adverse effects induced by chloroquine and hydroxychloroquine: case reports and population studies. Ann Pharmacother. 2023;57(4):463–479. PubMed CrossRef
- Sapp OL III. Toxic psychosis due to quinacrine and chloroquine. JAMA. 1964;187(5):373–375. PubMed CrossRef
- Telgt DS, van der Ven AJ, Schimmer B, et al. Serious psychiatric symptoms after chloroquine treatment following experimental malaria infection. Ann Pharmacother. 2005;39(3):551–554. PubMed CrossRef
- Garg P, Mody P, Lall KB. Toxic psychosis due to chloroquine. Indian J Pediatr. 1990;57(1):133–134. PubMed CrossRef
- Kunal S, Gupta K, Sharma SM, et al. Cardiovascular system and COVID-19: perspectives from a developing country. Monaldi Arch Chest Dis Arch Monaldi Mal Torace. 2020;90(2):1305.
- Montastruc JL, Toutain PL. A new drug–drug interaction between hydroxychloroquine and metformin? A signal detection study. Drug Saf. 2020;43(7):657–660. PubMed CrossRef
- Ampélas J F, Wattiaux MJ, Van Amerongen AP. Psychiatric manifestations of lupus erythematosus systemic and Sjogren’s syndrome. Encephale. 2001;27(6):588–599. Accessed August 14, 2024. https://pubmed.ncbi.nlm.nih.gov/11865567/
- Gupta L, Agarwal V, Ramanan AV. Interleukin-6 and other cytokine blockade in COVID-19 hyperinflammation. Indian J Rheumatol. 2020;15(2):65. CrossRef
This PDF is free for all visitors!