Depression with significant anhedonia can contribute to the development and complicate the treatment of opioid use disorder (OUD).1–3 Pramipexole, a dopamine receptor agonist with high affinity for D3 receptors and with possible additional anti-inflammatory properties, has been shown to be effective in treating anhedonic depression.4–7 We report a case in which pramipexole rapidly improved depression and anhedonia symptoms in a patient on buprenorphine and an antidepressant.
Case Report
Mr A is a 38-year-old man with a ≥ 10-year history of OUD, major depressive disorder, post-traumatic stress disorder (PTSD), and chronic pain, who was admitted to a residential rehabilitation program for treatment of OUD and PTSD. His opioid use started with prescribed hydrocodone for pain, then transitioned to heroin and fentanyl.
Previously, he was treated while in legal custody with buprenorphine 24 mg/d in dosing 3 times/d. After his release, he quickly relapsed back to high-dose fentanyl use. He had not taken buprenorphine or any of his other medications for the 3 weeks prior to admission.
After buprenorphine was reinitiated and titrated up to previous dosing, his Montgomery-Asberg Depression Rating Scale (MADRS)8 scores were 16 and 17 one week apart, indicating mild depression. Duloxetine was started and titrated up to 60 mg. Despite antidepressant treatment, he again scored 17 on the MADRS. He continued to endorse cravings, pain, anhedonia, amotivation, and poor sleep.
Three weeks after duloxetine was initiated (6 weeks after admission), pramipexole was started at 0.25 mg dosed at night and titrated up to 1.0 mg over the course of a week. After a week on pramipexole, he reported improved mood and motivation, as well as increased ability to participate in the milieu. He reengaged in hobbies and stated, “I don’t feel like I have to force a smile anymore.”
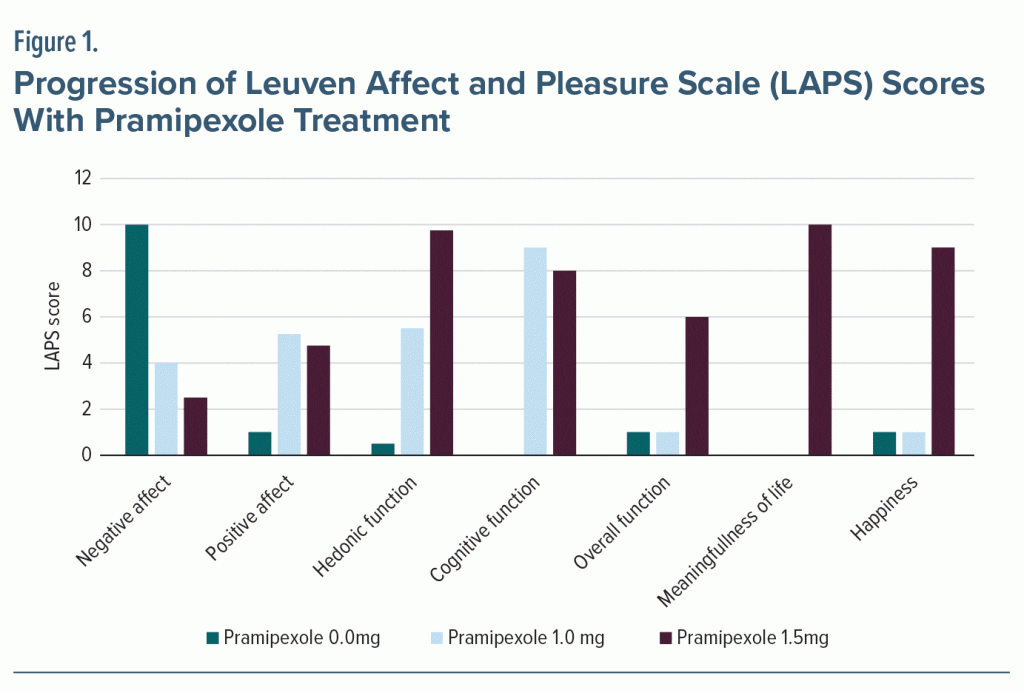
Pramipexole was further increased to 1.5 mg/night in an attempt to reduce pain.7,9 We monitored pain severity via the Brief Pain Inventory,10 and pramipexole effect was modest. However, overall functioning and participation in daily life improved dramatically, as shown by an 18-point improvement in the 10-item Recovering Quality of Life questionnaire (ReQoL-10).11 Approximately 6 weeks after reaching 1.5 mg of pramipexole, his MADRS score was 9. We also used the Leuven Affect and Pleasure Scale (LAPS)12 to measure changes in negative and positive affect, hedonic function, cognitive and overall function, meaningfulness, and happiness (Figure 1). Mr A tolerated pramipexole well and only reported occasional morning nausea during the rapid initial titration. He denied subjective experience of disinhibition and impulsivity, which was confirmed by clinician observation. He endorsed improved sleep and cravings.
Discussion
The initial results of our case suggest that pramipexole can be a rapid and effective adjunctive treatment in patients with anhedonic depression, chronic pain, and OUD who are receiving buprenorphine therapy and are not responding well to an antidepressant.
Improvement in MADRS, ReQoL-10, and LAPS scores show not only decreased depressive symptoms, but also increased overall enjoyment of life and increased functionality. Additionally, this increase in quality of life occurred despite no significant improvement in pain via Brief Pain Inventory scores. These scores reflect the subjective improvement stated by the patient and observed by the providers. Furthermore, since anhedonia is associated with increased risk of relapse and dropping out of treatment, we believe the addition of pramipexole contributed to Mr A’s successful stay in residential treatment and ongoing sobriety, despite multiple risk factors such as a recent relapse, PTSD, and criminal charges.
Article Information
Published Online: October 26, 2023. https://doi.org/10.4088/PCC.22cr03535
© 2023 Physicians Postgraduate Press, Inc.
Prim Care Companion CNS Disord 2023;25(5):22cr03535
Submitted: March 31, 2023; accepted June 2, 2023.
To Cite: Laubenthal CE, Escalona R, Welch JE. Rapid treatment of anhedonia with pramipexole as adjunct to buprenorphine in opioid use disorder. Prim Care Companion CNS Disord. 2023;25(5):22cr03535.
Author Affiliations: New Mexico VA Health Care System, Albuquerque, New Mexico (Laubenthal, Escalona, Welch); University of New Mexico School of Medicine, Department of Psychiatry and Behavioral Sciences, Albuquerque, New Mexico (Escalona).
Corresponding Author: Clare E. Laubenthal, PA-C, Department of Psychiatry, Mayo Clinic Health System, 1025 Marsh St, Mankato, MN 56001-4752 ([email protected]).
Relevant Financial Relationships: Dr Escalona has received consulting and speaking fees from Alkermes and is named co-inventor on patent application no. US 2020/0121651 A1: “Pramipexole for Use in the Treatment of Pain”; inventors: Milligan ED, Escalona PR. Clare E. Laubenthal, PA-C and Jaclyn E. Welch, PMHNP-BC declare no conflicts of interest.
Funding/Support: None.
Patient Consent: Written consent was obtained from the patient to publish the case report, and information has been de-identified to protect anonymity.
References (12)

- Sullivan MD. Depression effects on long-term prescription opioid use, abuse, and addiction. Clin J Pain. 2018;34(9):878–884. PubMed CrossRef
- Hudson TJ, Painter JT, Gressler LE, et al. Factors associated with opioid initiation in OEF/OIF/OND veterans with traumatic brain injury. Pain Med. 2018;19(4):774–787. PubMed CrossRef
- Martinotti G, Cloninger CR, Janiri L. Temperament and character inventory dimensions and anhedonia in detoxified substance-dependent subjects. Am J Drug Alcohol Abuse. 2008;34(2):177–183. PubMed CrossRef
- Fujiwara S, Kimura F, Hosokawa T, et al. Anhedonia in Japanese patients with Parkinson’s disease. Geriatr Gerontol Int. 2011;11(3):275–281. PubMed CrossRef
- Lemke MR, Brecht HM, Koester J, et al. Anhedonia, depression, and motor functioning in Parkinson’s disease during treatment with pramipexole. J Neuropsychiatry Clin Neurosci. 2005;17(2):214–220. PubMed CrossRef
- Willner P, Lappas S, Cheeta S, et al. Reversal of stress-induced anhedonia by the dopamine receptor agonist, pramipexole. Psychopharmacology (Berl). 1994;115(4):454–462. PubMed CrossRef
- Escalona R, Fawcett J, Rush AJ. Pramipexole augmentation of buprenorphine improves pain and depression in opioid use disorder: a case report. Prim Care Companion CNS Disord. 2020;22(5):20l02598. PubMed CrossRef
- Williams JBW, Kobak KA. Development and reliability of a structured interview guide for the Montgomery-Asberg Depression Rating Scale (SIGMA). Br J Psychiatry. 2008;192(1):52–58. PubMed CrossRef
- Holman AJ, Myers RR. A randomized, double-blind, placebo-controlled trial of pramipexole, a dopamine agonist, in patients with fibromyalgia receiving concomitant medications. Arthritis Rheum. 2005;52(8):2495–2505. PubMed CrossRef
- Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singap. 1994;23(2):129–138. PubMed
- Keetharuth AD, Brazier J, Connell J, et al. Recovering Quality of Life (ReQoL): a new generic self-reported outcome measure for use with people experiencing mental health difficulties. Br J Psychiatry. 2018;212(1):42–49. PubMed CrossRef
- Demyttenaere K, Mortier P, Kiekens G, et al. Is there enough “interest in and pleasure in” the concept of depression? the development of the Leuven Affect and Pleasure Scale (LAPS). CNS Spectr. 2019;24(2):265–274. PubMed CrossRef
Enjoy this premium PDF as part of your membership benefits!