Abstract
Importance: Alcohol use disorder (AUD) is a critical health condition that increases the risk of a variety of social and physical health impairments. Glucagon-like peptide-1 (GLP-1) receptor agonists are potentially effective in reward system–related disorders. The use of GLP-1 receptor agonists has been shown to decrease overall consumption of alcohol in AUD in addition to managing obesity and weight loss. The main objective of this narrative review was to examine the potential benefits, dosages, and mechanisms of GLP-1 receptor agonists on alcohol consumption and how they can potentially modify alcohol-seeking behavior.
Observations: The principal observation included the effect of GLP-1 receptor agonists on the mesolimbic pathways in the central nervous system, the central amygdala, and the GABAergic neurons in the central nervous system. Current research also focuses on the use of GLP-1 receptor agonists in improving glycemic control and reduction of obesity.
Conclusions and Relevance: Clinically, GLP-1 receptor agonists can be potentially used as an adjunct to the treatment of AUD in patients with a body mass index >30 kg/m2 and in those with AUD who have coexisting diabetes mellitus. As decreasing glucose levels and alcohol-seeking behavior are 2 dual effects of the GLP-1 receptor agonists, the dosage can be adjusted accordingly to achieve the desired benefits while reducing the potential side effects of the drug class.
Prim Care Companion CNS Disord 2025;27(3):24nr03855
Author affiliations are listed at the end of this article.
Alcohol use disorder (AUD) is one of the most common mental health disorders globally and affects people of various ages, sexes, and socioeconomic backgrounds. The DSM-5 describes it as problematic patterns of alcohol use that cause clinically significant impairment or distress. AUD is associated with widespread consequences, including health implications like comorbid chronic diseases and reduced life expectancy, social impairments like family disruption and relationship conflicts, and occupational impairments like underperformance, potential job loss, or unemployment.1
The drugs approved for this purpose include disulfiram (aldehyde dehydrogenase inhibitor), naltrexone (opioid receptor antagonist), acamprosate (various sites of action), and nalmefene (opioid receptor modulator). Naltrexone has been found to reduce cravings and heavy drinking, while acamprosate helps with abstinence. Despite the various treatments available for AUD, new medications still must be developed due to the high risk of relapse and varying response rates to treatment observed among different patients.2
Recent research has shown that glucagon-like peptide-1 (GLP-1), an intestinal hormone secreted in response to food consumption that provokes increased insulin secretion and reduced glucagon secretion from the pancreas, might play a role in alcohol-related behaviors. Recent reports have shown that GLP-1 receptor agonists such as liraglutide and semaglutide, which are currently approved for treating type 2 diabetes, decrease alcohol-seeking behaviors by inhibiting the mesolimbic reward system of the brain.1 In this review, we describe how these GLP-1 agonists influence alcohol consumption and alcohol-seeking behaviors.
METHODS
An electronic search was conducted from June 2024 to July 2024, with an updated search in August 2024, using databases PubMed, PubMed Central, Clinicaltrials.gov, Ovid Medline, and CINAHL. The Medical Subject Headings terms included were GLP-1 agonists, glucagon like peptide OR glutides OR semaglutide OR glucose dependent insulinotropic peptides AND alcohol OR alcohol use OR alcohol use disorder OR ethanol OR alcoholism OR alcoholics. Overall, 60 articles were retrieved, and articles older than 10 years and those specific to diabetic patients were removed. Seventeen studies including observational studies, experimental studies, and narrative reviews were included in the review.
RESULTS
Pathophysiology
GLP-1 receptor agonists cause a significant decrease in alcohol use by inhibiting and modulating dopaminergic neurons present in the central areas of the mesolimbic reward system, namely, the ventral tegmental area (VTA) and the nucleus accumbens (NAc). Alcohol and other drugs of abuse stimulate dopamine release from the ventral striatum (a part of the NAc) in healthy individuals, with the magnitude and rate of release closely related to the euphoria or drug-seeking behavior seen with alcohol use.2 Research has shown that GLP 1 receptor gene polymorphisms are implicated in AUDs. Therefore, the use of GLP-1 receptor agonists for alcohol addiction by inhibiting the mesolimbic system has yielded a reduction in the motivation to consume alcohol and alcohol-seeking behaviors.1
Another observed mechanism of the GLP-1 receptor agonists was on the central amygdala.3 The receptor expression in this brain structure nullifies the neurotransmitter γ-aminobutyric acid (GABA) transmission, hence decreasing the motivation to seek alcohol in those with AUD.3
Besides modulating GABAergic receptors in the central nervous system (CNS), GLP-1 agonists delay gastric emptying.4 A longer duration of alcohol digestion in the stomach causes an accumulation of acetaldehyde, further clarifying the harmful effects associated with the ingestion of GLP-1 agonists with alcohol.4
Several appetite-regulatory peptides have been associated with alcohol-mediated behaviors. Research has shown that GLP-1, along with other gut-brain peptides like ghrelin, leptin, and galanin, acts on the mesolimbic dopamine system of the brain and influences the activation of this system by alcohol and other addictive drugs. Studies2 have found that ghrelin increases cravings in alcohol-dependent heavy drinkers and that antagonizing this peptide reduces the motivation to consume alcohol, leading to an overall decreased alcohol intake.
Pharmacokinetics
When analyzing the use of GLP-1 receptor agonists and their doses in the management of AUD, providers must consider multiple factors, including age, medical history, current medications, comorbid conditions, metabolic state, response to therapy, side effect profile, and potential risks and benefits for each individual.1,5
Because of minimal gastrointestinal (GI) absorption and less oral bioavailability, these drugs are available in subcutaneous injectable form, except for semaglutide, which is available both in oral and injectable forms.6 A newer drug, tirzepatide, with dual agonist action on glucose-dependent insulinotropic peptide and GLP-1 receptors, is also available in subcutaneous injectable form.7
Since exenatide and liraglutide metabolize through the liver and kidneys, one should exercise caution when using them in AUD patients with liver and kidney problems.6 One should be wary of formulating and dispensing these medicines, as compounding errors in semaglutide administration have caused severe adverse effects, as Lambson and colleagues8 reported in a case series. As these medications are not yet on the list of US Food and Drug Administration–approved drugs for AUD, any prescriptions are through off-label use, requiring providers to exhibit prudence when prescribing and dispensing them.5
Some common GLP-1 and mixed GLP-1 receptor agonists that are in trials for AUD include exenatide (Byetta and Bydureon), liraglutide (Victoza and Saxenda), dulaglutide (Trulicity), and semaglutide (Ozempic) and tirzepatide (Mounjaro).4,5,9 In trials for AUD, the dosage of GLP-1 receptor agonists varied, but they were mainly similar to the doses standardized for treating type 2 diabetes mellitus and obesity. These doses were already clinically tested for safety and efficacy.4 Exenatide (Bydureon) is administered at a dose of 2 mg subcutaneously once weekly in an extended-release form for AUD.9 Liraglutide is initially dosed at 0.6 mg and injected subcutaneously once daily. The dose of exenatide (Bydureon) is eventually titrated in uniform increments of 0.6 mg every quarter or half month to a maximum dose of 3.0 mg once daily. Similarly, dulaglutide is administered at a starting dose of 0.75 mg subcutaneously once weekly and increased to double the amount in a weekly period based on the tolerability and dose response.10 Semaglutide is dosed initially at 0.25 mg subcutaneously once weekly and incrementally escalated weekly until a dose of 1.0 mg once weekly is reached.4 Tirzepatide is administered subcutaneously at a medium dose of 7.5 mg with a range of 0.5–15 mg.4 Most trials adopt a multimodal approach, combining the GLP-1 receptor agonist therapy with behavioral therapies. A randomized clinical trial mixed 26 weeks of exenatide treatment for AUD with cognitive-behavioral therapy and showed optimistic results related to alcohol abstinence.9
Preclinical and Clinical Studies
A study conducted in alcoholic trait-induced mice showed liraglutide can decrease alcohol intake and preference.11 Similarly, this easing effect in Wistar rats was more pronounced in high baseline consumption groups with subcutaneous administration of liraglutide.12 Exendin (9-39), a GLP-1 receptor antagonist, and blockade of dipeptidyl peptidase-4 (DPP4), an enzyme responsible for GLP-1 degradation, did not nullify the decreased alcohol consumption and alcohol-seeking behaviors induced by liraglutide and semaglutide, signifying the lasting impact of the GLP-1 receptor agonists despite antagonistic influences.13
Also, GLP-1 receptor agonists influence AUD through their potentiating effect on neuroprotective influences. Liraglutide potentiated learning and memory performance, as well as eased anxiety levels in alcohol withdrawal mice.11 The reduced craving and the relapsed drinking in the alcohol withdrawal mice might be from the neurobiological impacts of liraglutide related to replenishing the dendritic spine density in the medial prefrontal cortex and the hippocampus as well as from enhancing the synaptic proteins.11
Research in rodents and humans has substantiated that GLP-1 receptor agonists have shown promising results in reducing alcohol consumption and preference through neurobiological impact on the mesolimbic reward pathways in the VTA and NAc by modulating neurotransmitters involved in addiction such as dopamine and glutamine.1,5 A randomized clinical trial using exenatide demonstrated a reduction in the alcohol cue reactivity in these notable neural reward zones on functional magnetic resonance images and a decrease in dopamine transporter availability.9
Their role in improving metabolic parameters such as glycemic control and weight reduction can also be associated with alleviating alcohol intake and cravings. Research has identified a significant connection between GLP-1 receptor agonists, metabolic parameters, and AUD. Targeted GLP-1 receptor agonist therapy for weight reduction or type 2 diabetes mellitus has shown substantial improvement in symptoms of AUD.4 A case series conducted through a retrospective chart review showed a 100% improvement in AUD conditions assessed by Alcohol Use Disorder Identification Test (AUDIT) scores, following the utilization of semaglutide for weight reduction.14 A semaglutide and tirzepatide study in obese individuals yielded similar results, showing a reduction in alcohol intake, binge episodes, average drinks, and AUDIT scores.4 This mitigation is envisioned to be from the dual mechanistic mode of action on the central reward pathways and influence on the gut via delayed gastric emptying, leading to enhanced blood alcohol content and a spike in aversive acetaldehyde levels.4 Dulaglutide reduced alcohol consumption in patients treated for smoking cessation and showed increased abstinence from smoking in an alcoholic group compared to the nonalcoholic group.10
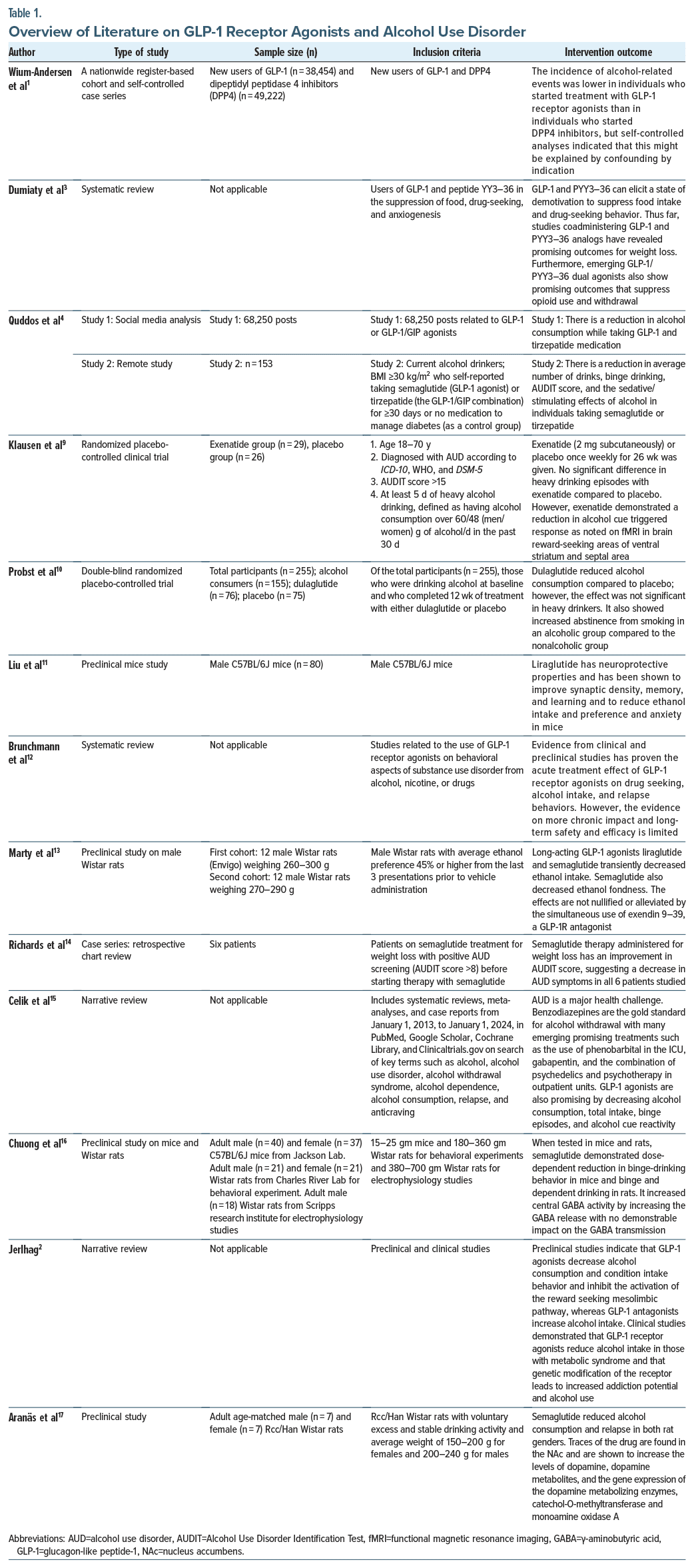
In comparison of acute vs chronic impact of GLP-1 receptor agonists, a nationwide register-based study found a profound impact of GLP-1 receptor agonists on AUD as evaluated through alcohol-related and benzodiazepine withdrawal treatments in the initial 3 months when compared with DPP4 inhibitors. However, the result faded after 3 months, which made the researchers debate whether the impact could be from the initial frequent provider visits, motivated lifestyle choices, or the excessive nausea and malaise experienced on initiation of GLP-1 receptor agonists, which could have reduced the alcohol intake during the first few months.1 Table 1 provides an overview of the key findings on the role of GLP-1 receptor agonists in AUD.
DISCUSSION
This review examines how GLP-1 receptor agonists have various effects on neurochemical and physiologic pathways in the brain to mitigate alcohol-seeking behavior. Literature has shown that GLP-1 agonists have a beneficial effect when changing impulsive behavior in AUD. This narrative review builds on the evidence that semaglutide (a GLP-1 receptor agonist) and other drugs in the same class can have a favorable effect in controlling alcohol-seeking actions.
GLP-1 receptor agonists have been shown to decrease binge drinking and lessen overall alcohol consumption.15 Celik et al15 noted suppression in intake of a variety of drinks besides alcohol, which suggests that appetite suppression and lowering of palatability can cause a lower motivation to consume alcohol in those who used GLP-1 receptor agonists. Here, a question also arises as to whether this effect of GLP-1 receptor agonists is associated with an individual’s genetic makeup and if a genetic tendency to gain weight affects the intensity of response of the drugs in alcoholics.
Those with a body mass index >30 kg/m2 experienced an enhanced observed effect of GLP-1 agonists in reducing motivation to seek alcohol.16 The appetite suppressing effect of GLP-1 agonists explains why they were effective in reducing alcohol-seeking behavior in obese individuals.16 However, semaglutide can cause nausea and malaise in patients,16 suggesting that it can reduce alcohol-seeking behavior by both physiologic and neurologic mechanisms.
Another finding was that some GLP-1 agonists crossed the blood-brain barrier more quickly than others, supporting the idea that a centrally acting mechanism is involved in the effect of GLP-1 agonists on alcohol seeking behavior.2
A decrease in alcohol-seeking behavior persisted after the drugs were discontinued in male mice and mice with some genetic alleles but not others.15 This finding suggests that besides exploring the neural pathways associated with AUD’s reward and pleasure mechanism, other physiologic pathways should be explored in future studies. Research should also focus on identifying the therapeutic effects of the drug class on both males and females, different body types, and a broader sample of genotypes.
Current evidence best supports the potential use of GLP-1 agonists in patients where suppression of reward-seeking behavior related to food and alcohol is desired.17 GLP-1 agonists can reduce motivation to seek alcohol and prevent a relapse of AUD2 in a dose dependent manner. This effect results from a broad range of effects on appetite, weight, palatability, and the reward-seeking pathway in the CNS.15
Some strategies to help physicians improve patient care are as follows. Since a high dose of semaglutide (0.1 mg/kg) was examined17 when using the drug in animals to reduce alcohol-seeking behavior, adjusting the dosage to obtain the desired effect while decreasing the potentially harmful effects is recommended. Data also suggest the possible use of GLP-1 agonists in AUD coexisting with type 2 diabetes mellitus or obesity.4 Also, anxiety levels should be clinically monitored when using GLP-1 agonists in patients.
Some barriers to successful treatment include the concern that medications that suppress appetite and can also affect mood may lead to suicidal ideation3 and determining the potential dosage. However, some studies have claimed no effect on axiogenesis when used acutely3 in both males and females except a slight increase in anxiety in female rats.17 Since the dosage of GLP-1 agonists across multiple dosages and in patients with different body mass indices has not been examined, alcohol reduction across medication levels and in various populations would need to be studied for successful incorporation of their use in a diverse population.
CONCLUSION
In summary, this review highlights the key effects of GLP-1 agonists on modifying alcohol-seeking behavior, providing support for their potential use as an adjunct to treatment while raising ideas for future research, such as their effect on AUD in association with different sets of alleles, in various clinical settings and a wider set of the population. Preclinical and initial human studies suggest that GLP-1 receptor agonists may be potential treatment for AUD. Nevertheless, it is advisable to utilize established approved treatments until clinical trials establish their safety and effectiveness.
Article Information
Published Online: May 15, 2025. https://doi.org/10.4088/PCC.24nr03855
© 2025 Physicians Postgraduate Press, Inc.
Submitted: September 17, 2024; accepted January 9, 2025.
To Cite: Khan S, Sayana P, Waseem S, et al. The role of glucagon-like peptide 1 receptor agonists in alcohol use disorder. Prim Care Companion CNS Disord 2025;27(3):24nr03855
Author Affiliations: PPD, Part of Thermo Fisher, Cypress, Texas (Khan); University of Arizona, Integrative Psychiatry Program, Tucson, Arizona (Sayana); McMaster University of Health Sciences, Hamilton, Ontario (Waseem); University of Illinois at Chicago Medical Center, Neuropsychology Service, Chicago, Illinois (Olu-Lawal Oluwanifesimi); Texas Tech University Health Science Center at Permian Basin, Midland, Texas (Yadav, Jain); Yale New Haven School of Medicine, New Haven, Connecticut (Mansuri).
Corresponding Author: Sadaf Khan, MBBS, PPD, Part of Thermo Fisher, 16603 Cypress Marsh CT, Cypress, TX 77429 ([email protected]).
Relevant Financial Relationships: None.
Funding/Support: None.
Clinical Points
- Glucagon-like peptide-1 (GLP-1) agonists can reduce motivation to seek alcohol and prevent a relapse of alcohol use disorder in a dose-dependent manner; this effect results from a broad range of effects on appetite, weight, palatability, and the reward-seeking pathway in the central nervous system.
- As decreasing glucose levels and alcohol-seeking behavior are 2 dual effects of GLP-1 receptor agonists, the dosage can be adjusted accordingly to achieve the desired benefits while reducing the potential side effects of the drug class.
References (17)

- Wium-Andersen IK, Wium-Andersen MK, Fink-Jensen A, et al. Use of GLP-1 receptor agonists and subsequent risk of alcohol-related events. A nationwide register-based cohort and self-controlled case series study. Basic Clin Pharmacol Toxicol. 2022;131(5):372–379. PubMed CrossRef
- Jerlhag E. GLP-1 signaling and alcohol-mediated behaviors; preclinical and clinical evidence. Neuropharmacology. 2018;136(Pt B):343–349. PubMed CrossRef
- Dumiaty Y, Underwood BM, Phy-Lim J, et al. Neurocircuitry underlying the actions of glucagon-like peptide 1 and peptide YY3-36 in the suppression of food, drug-seeking, and anxiogenesis. Neuropeptides. 2024;105:102427. PubMed CrossRef
- Quddos F, Hubshman Z, Tegge A, et al. Semaglutide and Tirzepatide reduce alcohol consumption in individuals with obesity. Sci Rep. 2023;13(1):20998. PubMed CrossRef
- Klausen MK, Thomsen M, Wortwein G, et al. The role of glucagon-like peptide 1 (GLP-1) in addictive disorders. Br J Pharmacol. 2022;179(4):625–641. PubMed CrossRef
- Collins L, Costello RA. Glucagon-like peptide-1 receptor agonists. [Updated 2024 Feb 29]. In: StatPearls [Internet]. StatPearls Publishing; 2024. https://www.ncbi.nlm.nih.gov/books/NBK551568/
- Clark L. GLP-1 receptor agonists: a review of glycemic benefits and beyond. JAAPA. 2024;37(4):1–4. PubMed CrossRef
- Lambson JE, Flegal SC, Johnson AR. Administration errors of compounded semaglutide reported to a poison control center-Case series. J Am Pharm Assoc. 2023;63(5):1643–1645. PubMed CrossRef
- Klausen MK, Jensen ME, Møller M, et al. Exenatide once weekly for alcohol use disorder investigated in a randomized, placebo-controlled clinical trial. JCI Insight. 2022;7(19):e159863. PubMed CrossRef
- Probst L, Monnerat S, Vogt DR, et al. Effects of dulaglutide on alcohol consumption during smoking cessation. JCI Insight. 2023;8(22):e170419. PubMed CrossRef
- Liu W, Wang Z, Wang W, et al. Liraglutide reduces alcohol consumption, anxiety, memory impairment, and synapse loss in alcohol dependent mice. Neurochem Res. 2024;49(4):1061–1075. PubMed CrossRef
- Brunchmann A, Thomsen M, Fink-Jensen A. The effect of glucagon-like peptide-1 (GLP-1) receptor agonists on substance use disorder (SUD)-related behavioural effects of drugs and alcohol: a systematic review. Physiol Behav. 2019;206:232–242. PubMed CrossRef
- Marty VN, Farokhnia M, Munier JJ, et al. Long-acting glucagon-like peptide-1 receptor agonists suppress voluntary alcohol intake in male wistar rats. Front Neurosci. 2020;14:599646. PubMed CrossRef
- Richards JR, Dorand MF, Royal K, et al. Significant decrease in alcohol use disorder symptoms secondary to semaglutide therapy for weight loss: a case series therapy for weight loss: a case series. J Clin Psychiatry. 2023;85(1):23m15068. PubMed CrossRef
- Celik M, Gold MS, Fuehrlein B. A narrative review of current and emerging trends in the treatment of alcohol use disorder trends in the treatment of alcohol use disorder. Brain Sci. 2024;14(3):294. PubMed CrossRef
- Chuong V, Farokhnia M, Khom S, et al. The glucagon-like peptide-1 (GLP-1) analogue semaglutide reduces alcohol drinking and modulates central GABA neurotransmission (GLP-1) analogue semaglutide reduces alcohol drinking and modulates central GABA neurotransmission. JCI Insight. 2023;8(12):e170671. PubMed CrossRef
- Aranäs C, Edvardsson CE, Shevchouk OT, et al. Semaglutide reduces alcohol intake and relapse-like drinking in male and female rats. EBioMedicine. 2023;93:104642. https://doi.org/10.1016/j.ebiom.2023.104642
Enjoy this premium PDF as part of your membership benefits!