aNational Institute of Mental Health and Neuro Sciences (NIMHANS), Bengaluru, India
*Corresponding author: Shayanth Manche Gowda, MD, DNB, National Institute of Mental Health and Neuro Sciences (NIMHANS), Lakkasandra, Hosur Rd, Bengaluru, Karnataka 560029, India ([email protected]).
Prim Care Companion CNS Disord 2021;23(2):20l02654
To cite: Gowda SM, KG VK, Venkatasubramanian G. Retrograde ejaculation with second-generation antipsychotics in schizophrenia: a case series and literature review. Prim Care Companion CNS Disord. 2021;23(2):20l02654.
To share: https://doi.org/10.4088/PCC.20l02654
© Copyright 2021 Physicians Postgraduate Press, Inc.
Sexual adverse effects related to antipsychotic medications are not uncommon in patients with schizophrenia.1,2 However, retrograde ejaculation (RE) is quite often underreported by patients, which could have an influence on treatment adherence, fertility, and quality of life.3–5 Hence, effective identification and appropriate management of RE is essential. In this report, we describe RE with second-generation antipsychotics (SGAs) and its management in 4 patients with schizophrenia and briefly review the literature on antipsychotic-induced RE.
Case Reports
Case 1. A 30-year-old married man diagnosed with schizophrenia for the past 2 years with failed treatment trials of risperidone, aripiprazole, and amisulpride (hyperprolactinemia—serum prolactin level = 114 ng/mL with amisulpride) presented to our clinic. He had significant positive symptoms. Amisulpride was stopped, and he was initiated on treatment with oral olanzapine 10 mg/d. At the end of 4 weeks of olanzapine treatment, his positive symptoms improved significantly and serum prolactin normalized to 3 ng/mL. During the follow-up at the end of 2 weeks of treatment with olanzapine 10 mg/d, the patient reported features suggestive of RE in the form of near-complete absence of seminal fluid after sexual intercourse, and these symptoms persisted even in the third month follow-up. Post masturbatory urine analysis showed multiple motile sperm cells. Thus, the dose of olanzapine was reduced to 7.5 mg/d, and he showed complete improvement in RE in about 3 weeks. Subsequently, in view of sedation and oculogyric crisis, the dose of olanzapine was further reduced to 5 mg/d after 3 months. The patient has been in regular follow-up with us for more than a year while being on olanzapine 5 mg with complete improvement in his psychotic symptoms as well as RE. The patient scored 7 on the Naranjo Adverse Drug Reaction Probability Scale,6 suggesting probable adverse drug reaction (ADR).
Case 2. A 26-year-old unmarried man diagnosed with schizophrenia with no prior exposure to antipsychotic medications was initiated on treatment with tablet risperidone. The dose was gradually titrated based on the clinical improvement up to 8 mg/d over 4 weeks given at night time. After 2 weeks of receiving 8 mg/d of risperidone, the patient reported the absence of seminal fluid after masturbatory practice. The diagnosis of RE was confirmed with post-masturbatory urine analysis, which showed 6–8 spermatozoa/high-power field. Hence, attempts were made to decrease the dose of risperidone by reducing by 2 mg at every second monthly follow-up visit until a dose of 2 mg/d was achieved at the end of the 6-month period. However, the patient continued to have features of RE in the absence of psychotic symptoms. Finally, risperidone was reduced to 1 mg/d with the plan to subsequently stop. Meanwhile, he was started on aripiprazole 10 mg/d with which the patient experienced a complete improvement in RE. Risperidone was stopped at the next follow-up visit. At the time of this writing, the patient has been in follow-up for more than 6 months with aripiprazole 10 mg/d and maintains improvement in psychotic symptoms as well as RE. The patient scored 8 on the Naranjo Adverse Drug Reaction Probability Scale, suggesting probable ADR.
Case 3. A 40-year-old married man known to have treatment-refractory schizophrenia was treated with clozapine 350 mg/d. In view of persistent symptoms, he was treated with add-on risperidone, and the dose was gradually increased to 4 mg/d. Within a week of initiation of risperidone, the patient developed features suggestive of RE in the form of near-complete absence of seminal fluid after sexual intercourse, and these symptoms persisted for 4 months as noted at the clinical follow-up. Post-masturbatory urine analysis showed motile sperm cells. As the patient was distressed secondary to RE, the risperidone dose was reduced to 2 mg/d; nonetheless, the RE persisted. About 2 months after the reduction of the risperidone dose, the patient had a relapse of psychosis. Hence, risperidone was cross-tapered with aripiprazole 10 mg/d, following which he reported complete improvement in psychotic symptoms and RE. The patient has been in follow-up for more than 8 months and maintains improvement in RE. He scored 8 on the Naranjo Adverse Drug Reaction Probability Scale suggesting probable ADR.
Case 4. A 25-year-old unmarried man diagnosed with paranoid schizophrenia was initiated on olanzapine treatment, and the dose was gradually titrated to 20 mg/d over a period of 2 weeks. He was maintained on 20 mg for about 3 weeks; subsequently, he was concurrently initiated on 210 mg of olanzapine depot intramuscular injection with an intention to completely switch over to depot injections given prior history of poor adherence. The patient was continued on both oral as well as depot olanzapine as he was not willing to receive frequent injection doses in the initiation phase. On day 4 after initiation of depot olanzapine injection, the patient reported complete absence of seminal fluid after masturbation suggestive of RE. These symptoms persisted for the next month, correlating with duration of action of depot olanzapine injection, following which he reported intermittent improvement in RE. After developing RE, the patient withdrew his consent for depot injection, and, hence, a higher dose of oral olanzapine 30 mg/d was considered, as the patient continued excessive use of tobacco smoking. For logistic reasons, urine analysis for RE could not be done in this case. This case further strengthens our prior findings that RE induced by olanzapine could be a dose-dependent effect. The patient scored 7 on the Naranjo Adverse Drug Reaction Probability Scale.
Discussion
The RE associated with antipsychotics is an important, underrecognized adverse effect that may have bearing on treatment adherence. Our report highlights the features of RE associated with SGAs such as onset, dose-dependent nature, and management. None of the these 4 patients had any prior history of RE. To the best of our knowledge, this is the first report to describe RE associated with olanzapine.
Although the exact onset of RE with the SGAs in our report is not known with the exception of case 4, it is evident from all 4 cases that RE occurred within 2 weeks after initiation of SGAs. Similarly, other case reports have also reported the onset of RE with SGAs to be around 2 weeks.7–9 All 4 of our patients scored ≥ 7 on the Naranjo Adverse Drug Reaction Probability Scale,6 which suggests the probable etiologic role of SGAs in the occurrence of RE. In our study, only olanzapine showed a dose-dependent effect on RE wherein improvement in RE was noted on dose reduction. However, with respect to risperidone, it is difficult to comment about the dose dependence of RE since in one patient, RE improved after decreasing the dose to 1 mg/d, and in another, RE did not improve even after decreasing the risperidone dose. The earlier reports have suggested a dose-dependent effect of risperidone on RE.9,10
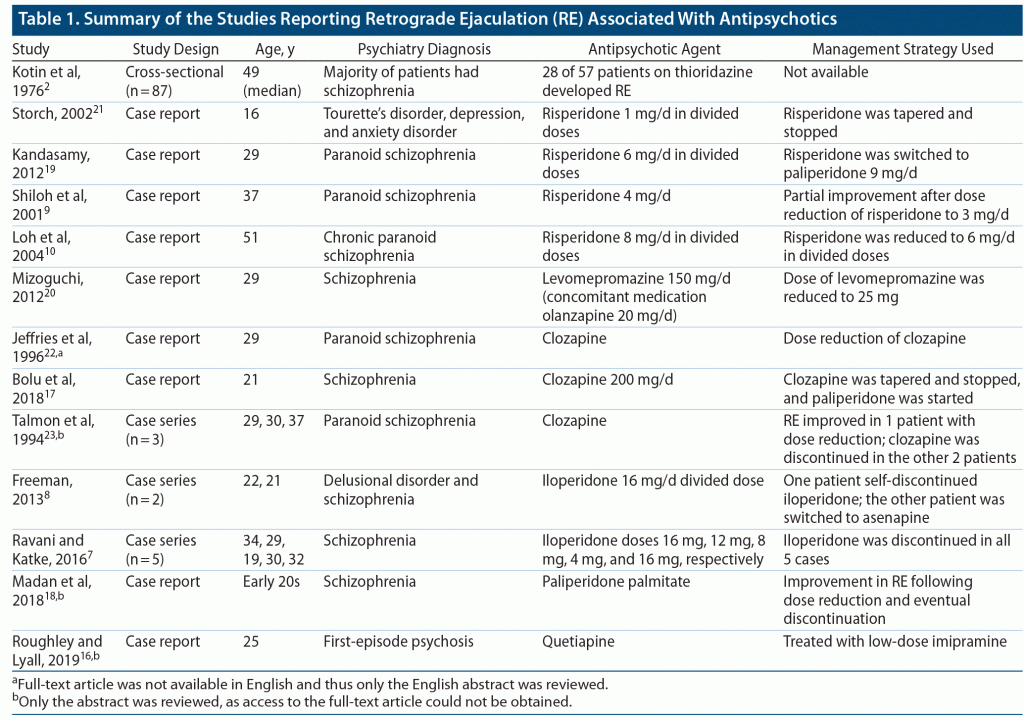
We reviewed the available literature on antipsychotic-induced RE through a search in the PubMed database. We found 14 articles2,7–11,16–23 related to antipsychotic medication–induced RE. Of these 14 articles, 132,7–10,16–23 were related to SGA dose-dependent RE and 111 was discarded from review as either the abstract or full article could not be found.
Ejaculation is a complex process predominantly comprising 2 phases, emission and expulsion, which is subserved by the sympathetic nervous system. During the expulsion phase, failure of closure of the bladder neck results in varying degrees of retrograde ejaculation characterized by the absence of seminal emission, reduced ejaculate volume, and reduced ejaculation force.5,12 Dopaminergic blockade and α1-adrenoreceptor blockade with antipsychotics are thought to be the key factors responsible for retrograde ejaculation.10,13,14 On the basis of these above-mentioned mechanisms, α-receptor agonists such as pseudoephedrine, midodrine, ephedrine, and the tricyclic antidepressant imipramine and antihistaminic brompheniramine have shown some promise in the pharmacologic management of RE.15 The exact mechanism of retrograde ejaculation induced by antipsychotic medications still remains elusive; however, there are many reports that have implicated few of the most commonly used atypical antipsychotic medications such as risperidone, iloperidone, clozapine, quetiapine, and paliperidone as presented in Table 1.2,7–11,16–23 Among these atypical antipsychotic medications, there are more reports of RE associated with risperidone use.10,19 Even typical antipsychotic medications such as thioridazine2 and levomepromazine20 have been implicated for causing retrograde ejaculation. It appears that antipsychotic-induced RE is long lasting, as is evident from our patient presented in case 2 wherein the effects have lasted nearly 6 months while on antipsychotic medications, which is similar to a case from the retrospective chart review2 in which RE symptoms persisted for 2.5 years.
In general, the strategies to handle antipsychotic-induced RE are as follows: dose reduction of the offending drug, drug holidays wherein the offending drug will be discontinued 2 to 3 days before the anticipated sexual activity, adjunctive treatment with other medications, and switching to another antipsychotic drug. Most of the case reports of antipsychotic-induced RE have employed all 4 of these strategies. In our case series, dose reduction with olanzapine and complete switchover of risperidone resulted in the improvement of RE. The possible dose-dependent effect of olanzapine versus dose-independent adverse effect of risperidone on RE needs further evaluation in a large-sample systematic study.
To summarize, RE is not uncommon among patients taking SGAs, it is generally evident by 2 weeks after its initiation, and certain SGAs could have a dose-dependent effect on RE.
Clinical Recommendations
The treating physician should be aware of the possibility of retrograde ejaculation, especially with antipsychotic medications which are known to cause RE (see Table 1), and actively inquire about possible sexual dysfunction, as patients might not be forthcoming in reporting sexual dysfunction unless it is too distressing for them. The general strategies discussed previously can be tailored to patients to balance improvement in both RE and psychotic symptoms. Although it is difficult to clinically recommend aripiprazole as an alternative antipsychotic medication for patients with antipsychotic-induced RE, it is worthwhile to further evaluate its role as our patients 2 and 3 showed improvement in RE after switching to aripiprazole and due to its favorable receptor profile, including partial dopamine agonistic and relatively lesser α1-adrenergic antagonistic properties. Furthermore, there is no literature available to implicate aripiprazole in causation of RE other than a report of spontaneous ejaculation.24
Published online: April 8, 2021.
Potential conflicts of interest: None.
Funding/support: None.
Informed consent: Consent was received from the patient and their family members to publish this case series, and information has been de-identified to protect anonymity.
References (24)

- Cutler AJ. Sexual dysfunction and antipsychotic treatment. Psychoneuroendocrinology. 2003;28(suppl 1):69–82. PubMed CrossRef
- Kotin J, Wilbert DE, Verburg D, et al. Thioridazine and sexual dysfunction. Am J Psychiatry. 1976;133(1):82–85. PubMed CrossRef
- Lambert M, Conus P, Eide P, et al. Impact of present and past antipsychotic side effects on attitude toward typical antipsychotic treatment and adherence. Eur Psychiatry. 2004;19(7):415–422. PubMed CrossRef
- Olfson M, Uttaro T, Carson WH, et al. Male sexual dysfunction and quality of life in schizophrenia. J Clin Psychiatry. 2005;66(3):331–338. PubMed CrossRef
- Revenig L, Leung A, Hsiao W. Ejaculatory physiology and pathophysiology: assessment and treatment in male infertility. Transl Androl Urol. 2014;3(1):41–49. PubMed
- Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239–245. PubMed CrossRef
- Ravani NN, Katke PH. Iloperidone-induced ejaculatory dysfunction: a case series. Indian J Psychiatry. 2016;58(1):87–89. PubMed CrossRef
- Freeman SA. Iloperidone-induced retrograde ejaculation. Int Clin Psychopharmacol. 2013;28(3):156. PubMed CrossRef
- Shiloh R, Weizman A, Weizer N, et al. Risperidone-induced retrograde ejaculation. Am J Psychiatry. 2001;158(4):650. PubMed CrossRef
- Loh C, Leckband SG, Meyer JM, et al. Risperidone-induced retrograde ejaculation: case report and review of the literature. Int Clin Psychopharmacol. 2004;19(2):111–112. PubMed CrossRef
- Raja M. Risperidone-induced absence of ejaculation. Int Clin Psychopharmacol. 1999;14(5):317–319. PubMed CrossRef
- Jefferys A, Siassakos D, Wardle P. The management of retrograde ejaculation: a systematic review and update. Fertil Steril. 2012;97(2):306–312. PubMed CrossRef
- Kaplan SA. Side effects of alpha-blocker use: retrograde ejaculation. Rev Urol. 2009;11(suppl 1):S14–S18. PubMed
- Peeters M, Giuliano F. Central neurophysiology and dopaminergic control of ejaculation. Neurosci Biobehav Rev. 2008;32(3):438–453. PubMed CrossRef
- Kamischke A, Nieschlag E. Update on medical treatment of ejaculatory disorders. Int J Androl. 2002;25(6):333–344. PubMed CrossRef
- Roughley M, Lyall M. Retrograde ejaculation associated with quetiapine and treatment with low-dose imipramine. BMJ Case Rep. 2019;12(8):e228539. PubMed CrossRef
- Bolu A, Akgün A, Öznur T, et al. Low-dose clozapine-induced retrograde ejaculation. Psychiatry Clin Neurosci. 2018;72(7):541–542. PubMed CrossRef
- Madan R, Langenfeld RJ, Ramaswamy S. Paliperidone palmitate-induced retrograde ejaculation. Clin Schizophr Relat Psychoses. 2018;12(2):86–88. PubMed CrossRef
- Kandasamy A. Paliperidone as an alternative for risperidone in a case of schizophrenia with retrograde ejaculation. J Neuropsychiatry Clin Neurosci. 2012;24(3):E17–E18. PubMed CrossRef
- Mizoguchi Y. Levomepromazine-induced retrograde ejaculation. J Neuropsychiatry Clin Neurosci. 2012;24(1):E32. PubMed CrossRef
- Storch DD. Risperidone-induced retrograde ejaculation. J Am Acad Child Adolesc Psychiatry. 2002;41(4):365–366. PubMed CrossRef
- Jeffries JJ, Vanderhaeghe L, Remington GJ, et al. Clozapine-associated retrograde ejaculation. Can J Psychiatry. 1996;41(1):62–63. PubMed
- Talmon Y, Guy M, Eisenkraft S, Guy N. [Retrograde ejaculation as a side-effect of clozapine]. Harefuah. 1994;126(9):509-10, 63.
- Eğilmez O, Çelik M, Kalenderoğlu A. Spontaneous ejaculations associated with aripiprazole. Noro Psikiyatri Arsivi. 2016;53(1):85–86. PubMed CrossRef
Enjoy this premium PDF as part of your membership benefits!