In the 1820s, Cruveilhier was the first to report unusual cases of rectal lesions among a group of patients.1 Over a century later, the term solitary rectal ulcer syndrome (SRUS) was introduced into the medical literature.1 It is a rare rectal condition with an unclear etiology, frequently associated with chronic constipation. This disorder does not exhibit any specific preference for age or sex and is characterized by symptoms such as difficulty during bowel movements, rectal bleeding, a sensation of incomplete evacuation, tenesmus, mucus discharge from the rectum, rectal discomfort, and a feeling of incomplete stool passage.1,2 Prolonged straining during bowel movements, direct trauma from instrumentation or digital manipulation by patients, disorganized contractions of the puborectalis muscle, and hormonal factors are considered potential etiologic contributors to this condition.1 Although no medications have been definitively identified as causative factors for SRUS, a recent report suggested that paroxetine may be a probable adverse drug reaction associated with its development.3
This report presents the case of a previously healthy man who was taking fluoxetine and sought medical attention for rectal bleeding. After evaluation, he was diagnosed with solitary ulcer of the rectum, and his treatment course is outlined in detail.
Case Report
Mr A is a 23-year-old man who was admitted to the hospital gastroenterology department with complaints of recurrent hematochezia and rectal pain that had persisted for 5 months. He also reported experiencing constipation for the past 8 months, during which he occasionally needed to manually remove stools. Importantly, he denied any episodes of tenesmus, melena, hematemesis, or diarrhea. His family history revealed no gastrointestinal disorders or malignancies, and there was no significant surgical history.
Mr A had been taking fluoxetine at a dosage of 80 mg/d for 8 months to manage obsessive-compulsive disorder (OCD) with major depressive disorder. At that time, he was not taking any other psychotropic medications. He noted that his constipation began after starting fluoxetine. Upon examination, his abdomen was soft and nondistended, with no signs of guarding or rebound tenderness. A thorough review of systems, along with a complete blood count and inflammatory markers, showed no abnormalities.
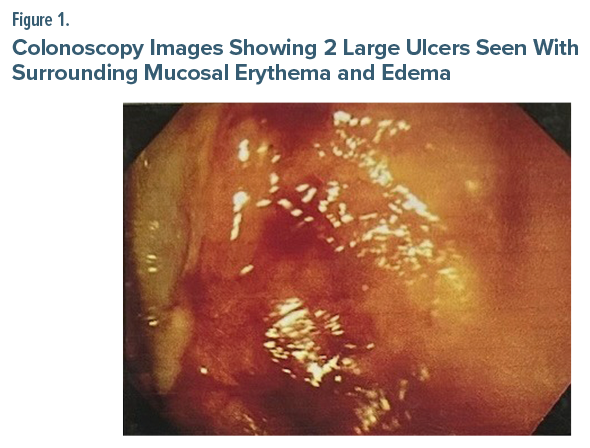
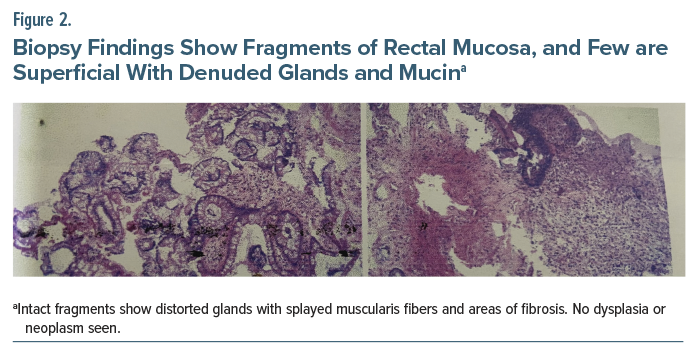
A colonoscopy was performed, which revealed 2 large ulcers with luminal blood in the rectum (Figure 1). Biopsy findings were consistent with the diagnosis of SRUS (Figure 2). Following this diagnosis, Mr A was treated with an injection of tranexamic acid and pantoprazole and was advised to make lifestyle and dietary changes. Fluoxetine was gradually tapered off, and he was started on oral venlafaxine at a dose of 37.5 mg/d, which was increased to 75 mg within 2 weeks. The patient reported complete resolution of his symptoms and showed no signs of recurrence during follow-up visits.
Discussion
This is only the second case reported in the literature, to the best of the author’s knowledge, linking the use of selective serotonin reuptake inhibitors (SSRIs) to SRUS, and it is the first to specifically implicate fluoxetine. Moussallem et al3 recently reported a case involving a 29-year-old man with generalized anxiety disorder who had been on paroxetine for 6 months. He presented with 3 months of hematochezia, abdominal pain, and mucoid discharge. After an unremarkable physical examination, a colonoscopy revealed a suspicious ulcerated lesion in the rectum, which was diagnosed as SRUS via biopsy. Treatment included lifestyle modifications, discontinuation of paroxetine, and a switch to venlafaxine, leading to gradual symptom resolution without recurrence.3
Similarly, Mr A, who had OCD and major depressive disorder and had been taking fluoxetine for 6 months, also presented with hematochezia and abdominal pain and was ultimately diagnosed with SRUS through biopsy. His symptoms significantly improved after discontinuing fluoxetine, and he tolerated venlafaxine well, mirroring the outcomes seen in the previous case.3
The exact mechanism by which fluoxetine leads to SRUS remains unclear. However, it is believed that the inhibition of the serotonin transporter, which is responsible for serotonin uptake by platelets, along with the SSRI-induced increase in gastric acidity, may significantly contribute to reduced platelet aggregation and gastrointestinal ulceration.3 While low-dose fluoxetine has been effective in managing symptoms of constipation-predominant irritable bowel syndrome, several reports also indicate that it can induce constipation, as in the present case.4,5 This constipation may lead to straining during bowel movements, which was a significant contributing factor to the development of SRUS in Mr A. Recent animal studies also suggest that fluoxetine affects intestinal motility through 5-hydroxytryptamine 3 and muscarinic receptors in ex vivo mouse models. Specifically, fluoxetine has been shown to increase the amplitude of contractions in colonic tissue, which may lead to paradoxical contractions of the puborectalis muscle, potentially contributing to the development of SRUS.5 The decision to switch from fluoxetine to venlafaxine, a serotonin-norepinephrine reuptake inhibitor (SNRI), was based on recent evidence indicating that SNRIs are associated with lower rates of upper and lower gastrointestinal bleeding and potential ulcers compared to SSRIs.6 While OCD itself is a potential confounding factor that could theoretically contribute to SRUS, the temporal association between fluoxetine use and symptom onset strengthens the likelihood of the medication’s role.1 Notably, the patient developed SRUS during a period of significantly reduced OCD symptoms, coinciding with fluoxetine initiation and constipation. This temporal association suggests fluoxetine, rather than the underlying OCD, was more likely the causative factor in this case.
In conclusion, this case shows the possible association between fluoxetine use and SRUS, highlighting the importance of careful monitoring for rare gastrointestinal side effects in patients receiving SSRIs, as well as the need for further research to elucidate the underlying mechanisms involved.
Article Information
Published Online: July 15, 2025. https://doi.org/10.4088/PCC.25cr03915
© 2025 Physicians Postgraduate Press, Inc.
Prim Care Companion CNS Disord 2025;27(4):25cr03915
Submitted: January 8, 2025; accepted April 2, 2025.
To Cite: Uvais NA. Solitary rectal ulcer syndrome in a patient taking fluoxetine. Prim Care Companion CNS Disord 2025;27(4):25cr03915.
Author Affiliation: Department of Psychiatry, Iqraa International Hospital and Research Centre, Calicut, Kerala, India.
Corresponding Author: N. A. Uvais, MBBS, DPM, Department of Psychiatry, Iqraa International Hospital and Research Centre, Calicut, Kerala, India ([email protected]).
Relevant Financial Relationships: None.
Funding/Support: None.
Patient Consent: Consent was received from the patient to publish the case report, and information has been de-identified to protect patient anonymity
References (6)

- Sadeghi A, Biglari M, Forootan M, et al. Solitary rectal ulcer syndrome: a narrative review. Middle East J Dig Dis. 2019;11(3):129–134. PubMed CrossRef
- Forootan M, Darvishi M. Solitary rectal ulcer syndrome: a systematic review and meta-analysis study protocol. Medicine. 2018;97(18):e0565. PubMed CrossRef
- Moussallem N, Haddad GC, Sbeih S, et al. Selective serotonin reuptake inhibitors and solitary rectal ulcer syndrome: a bloody relationship. Eur J Case Rep Intern Med. 2024;11(10):004826. PubMed CrossRef
- Vahedi H, Merat S, Rashidioon A, et al. The effect of fluoxetine in patients with pain and constipation-predominant irritable bowel syndrome: a double-blind randomized-controlled study. Aliment Pharmacol Ther. 2005;22(5):381–385. PubMed CrossRef
- Khuituan P, Nhaemchei C, Pradab S, et al. Fluoxetine affects intestinal motility via 5-HT3 and muscarinic receptors in ex vivo mouse model. Sains Malaysiana. 2021;50(12):3647–3657. CrossRef
- Cheng YL, Hu HY, Lin XH, et al. Use of SSRI, but not SNRI, increased upper and lower gastrointestinal bleeding: a nationwide population-based cohort study in Taiwan. Medicine (Baltimore). 2015;94(46):e2022. PubMed CrossRef
Enjoy this premium PDF as part of your membership benefits!