Lessons Learned at the Interface of Medicine and Psychiatry
The Psychiatric Consultation Service at Massachusetts General Hospital sees medical and surgical inpatients with comorbid psychiatric symptoms and conditions. During their twice-weekly rounds, Dr Stern and other members of the Consultation Service discuss diagnosis and management of hospitalized patients with complex medical or surgical problems who also demonstrate psychiatric symptoms or conditions. These discussions have given rise to rounds reports that will prove useful for clinicians practicing at the interface of medicine and psychiatry.
Prim Care Companion CNS Disord 2025;27(5):25f03968
Author affiliations are listed at the end of this article.
Have you been unsure about what you should discuss with your patients who might undergo hematopoietic stem cell transplantation (HSCT)? Have you been uncertain about what HSCT involves and how your patients should prepare for the procedure and their recovery? Have you wondered whether and how their fertility might be affected by HSCT? If you have, the following case vignette and discussion should prove useful.
CASE VIGNETTE
Mr A, a 32-year-old man, presented to his primary care physician with new-onset fatigue, unexplained weight loss, and frequent infections over the past month. His physical examination was notable for pallor and mild splenomegaly, but no lymphadenopathy. His blood tests revealed an elevated white blood cell (WBC) count with an increased percentage of blasts, and a subsequent bone marrow biopsy confirmed the diagnosis of acute lymphoblastic leukemia (ALL).
Given the aggressive nature of his ALL, its high risk of relapse, and his relatively young age, Mr A’s oncologist recommended an initial chemotherapy regimen that would induce remission and HSCT after remission to maximize Mr A’s chances for long-term survival (as chemotherapy alone would not prevent relapse).
DISCUSSION
What Is HSCT?
HSCT, also referred to as a bone marrow transplant (BMT), is a medical procedure used to treat a variety of blood and immune system disorders (eg, leukemia, lymphoma) and certain genetic conditions. It involves administering healthy hematopoietic stem cells to individuals with dysfunctional or depleted bone marrow, an organ that is responsible for producing blood cells. This approach was based on observational studies conducted in mouse models, which demonstrated that the introduction of healthy bone marrow components into a myelosuppressed bone marrow could induce functional recovery in the recipient.1 HSCT was then explored for use in humans starting in the 1950s.2
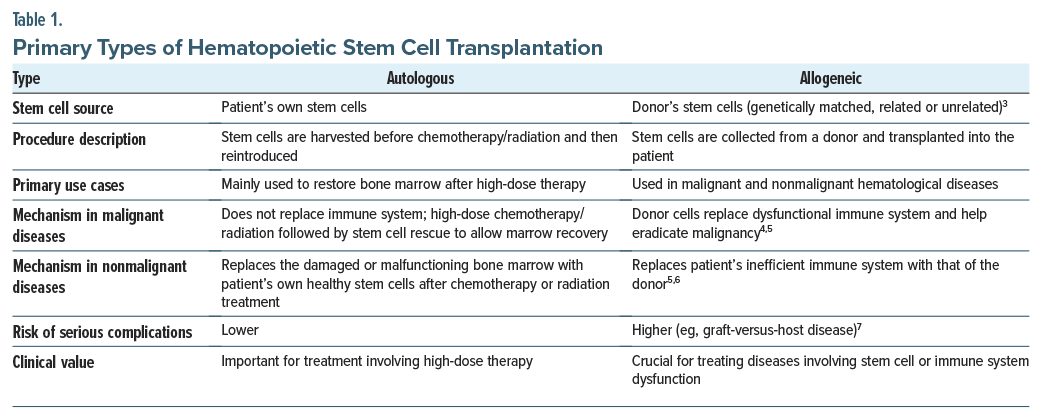
Deciding which type of transplantation to use involves complex decision-making, based on factors that include the underlying disease, age, the overall health of the patient, and the availability of suitable donors7 (Table 1). Despite challenges, HSCT remains an invaluable treatment option for many individuals with life-threatening hematologic diseases.
What Are the Indications for HSCT?
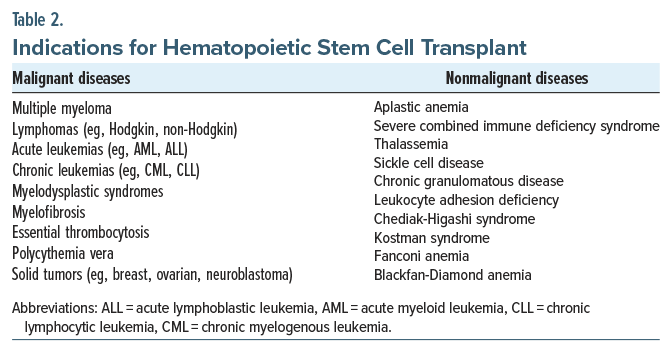
HSCT is typically indicated for those with certain hematologic disorders, particularly those that are refractory to other treatments or in cases where a patient has relapsed after initial therapy. It is indicated for malignant conditions (eg, multiple myeloma, acute and chronic leukemias, lymphomas, myelodysplastic syndrome [MDS], myelofibrosis, essential thrombocytosis, polycythemia vera, and some solid tumors [eg, testicular germ cell tumors and medulloblastoma]) and nonmalignant conditions (eg, aplastic anemia, severe combined immune deficiency syndrome, thalassemia, sickle cell disease, chronic granulomatous disease, leukocyte adhesion deficiency) (Table 2).3 In general, the allogeneic type of HSCT has been used predominantly for the treatment of leukemias and myeloblastic syndromes, while the autologous type of HSCT has been used more often for solid tumors, lymphoma, and myeloma.8
The procedure is most beneficial when the disease is aggressive, has a high potential for relapse, or when no other viable treatments are left. However, its efficacy is significantly limited by a non-negligible morbidity and mortality associated with the procedure.9 Health care professionals often struggle when determining whether HSCT should be offered as a treatment option.10–14 Since a definitive answer on the best treatment approach is lacking, it is important to consider the opinions of patients and their families during the decision-making process.14
How Quickly Do Decisions About Undergoing HSCT Need to Be Made?
The decision to undergo HSCT often needs to be made relatively quickly, particularly in cases in which the disease is progressing rapidly or relapsing after prior treatments. Timing is crucial, as delays can impact the procedure’s success and the overall posttransplant survival.15 Optimal outcomes are achieved when the transplant is performed early in the disease course, while the malignancy remains responsive to chemoradiotherapy, and when the tumor burden is minimal.8 In certain cases (eg, acute leukemia), the decision is likely to be made within several weeks of remission or relapse to prevent disease progression. Chronic conditions (eg, MDS or chronic leukemia) also require prompt evaluation to prevent disease worsening. For instance, in the case of MDS, several studies have demonstrated that advanced disease stage at the time of transplantation is associated with a worse overall survival rate.16,17 The urgency of the decision is also influenced by other factors (eg, the availability of a suitable donor, the patient’s overall health, and the feasibility of other treatments). Coordination among a multidisciplinary team (including hematologists, transplant specialists, and supportive care providers)14 is essential for making the most informed and timely decision.
What Is the Process for Selecting a Donor?
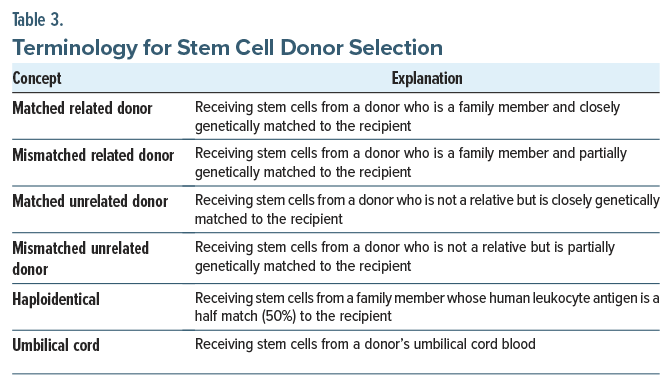
Stem cells for HSCT can be obtained either from the patient receiving the stem cells (in autologous HSCT) or from a donor (in allogeneic HSCT). Allogeneic stem cell donors are selected based on the compatibility of human leukocyte antigen (HLA) genes. HLA genes distinguish between self and nonself cells in major histocompatibility complexes, which code for certain proteins or genetic markers. Through HLA matching, a process in which 8–12 different markers in a stem cell donor’s deoxyribonucleic acid are compared to the recipient’s, a donor is determined to be a match for the recipient.18 Common HLA genes include HLA-A, HLA-B, HLA-C, and HLA-DRB1.18 Although identical sibling donors are the “gold standard” for allogeneic HSCT, only 30% of patients have that option.19 Most patients who need allogeneic HSCT must receive stem cells from other sources, including matched or mismatched related donors, matched or mismatched unrelated donors, haploidentical donors, or umbilical cord donors (Table 3 provides additional details).20 Over the past 3 decades, most stem cells for allogeneic HSCT have been mobilized from the peripheral blood, as opposed to bone marrow, which was the initial source of stem cell grafts. Clinicians make decisions about the appropriateness of a donor for allogeneic HSCT based on patient preference, demographic factors, and clinical factors (eg, patient and donor age and the disease indication).18
How Should One Prepare for HSCT?
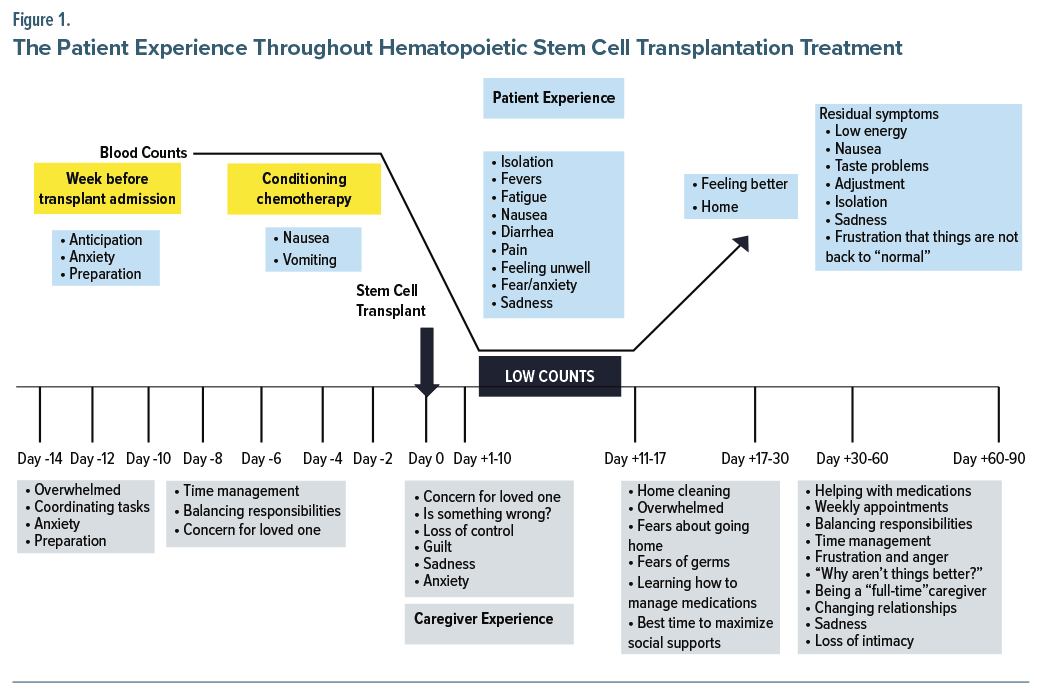
Preparation for HSCT may take several weeks, and these days are known as “minus days.” Figure 1 outlines the patient’s journey, including preparation for transplant, the stem cell infusion, and recovery. Preparation for HSCT begins with collection of stem cells from the donor (allogeneic HSCT) or the patient (autologous HSCT), obtained from peripheral blood, bone marrow, or a newborn’s umbilical cord.21 Peripheral blood stem cells are collected after the donor/patient has received daily injections of granulocyte-colony- stimulating factor or other factors to promote the release of stem cells from the bone marrow into the peripheral blood.21 Once the number of stem cells peak in the peripheral blood, donors/patients undergo an apheresis to extract and separate the stem cells from other blood components (ie, plasma, platelets, WBCs, red blood cells) so that the stem cells can be reinfused in the donor/ patient via a central venous catheter.22 Apheresis usually takes 3–5 hours, and it is performed in an outpatient facility. On occasion, apheresis may need to be repeated over several days until enough stem cells have been collected. Apheresis is usually well-tolerated; however, some donors/patients experience dizziness, fatigue, tingling of lips and fingers, low or high blood pressure, or bruising.23 In contrast, stem cells harvested from bone marrow are collected in an operating room while the donor/patient is anesthetized. Stem cells are usually collected from the anterior or iliac crest. Donors/ patients remain in the hospital until they have recovered from the procedure, and they are typically discharged to their home that same day.24 Finally, stem cells obtained from an umbilical cord are collected upon the baby’s birth and then are frozen until needed. This procedure results in no side effects for the newborn or the mother.25 These first few weeks are characterized by patient anxiety and preparing for the transplant (Figure 1).
Once the stem cells have been collected, patients are typically hospitalized while they are receiving the conditioning regimen (which often includes administration of high-dose chemotherapy, total body irradiation [to destroy any remaining malignant cells and suppress the patients’ immune system to decrease the likelihood of rejection of the transplanted stem cells]).26 In allogeneic HSCT, conditioning regimens can be myeloablative or of reduced intensity. In myeloablative regimens, patients receive high doses of chemotherapy, with or without total body irradiation, to eliminate the illness.26 Nevertheless, due to its intensity, myeloablative regimens are restricted to younger patients and to those without comorbidities. In contrast, reduced intensity or nonablative regimens aim to suppress the host’s hemopoiesis via an immune-mediated effect, which acts against any underlying malignancies. Reduced intensity regimens are usually better tolerated by older individuals and by those who have comorbid conditions.
Both conditioning regimens lead to similar survival rates since myeloablative regimens are associated with greater toxicity and nonrelapse mortality, whereas reduced intensity regimens are linked with higher relapse rates. Most chemotherapies used in conditioning regimens include intense immunosuppressive agents, such as high- dose cyclophosphamide, alkylating agents (eg, busulfan), nucleoside analogs (eg, fludarabine), or others (melphalan, antithymocyte globulin, rituximab, gemcitabine).3 Total body irradiation is usually administered in fractionated doses to mitigate toxicity. Side effects of the conditioning regimen include nausea and vomiting, although fatigue, alopecia, and infertility are also common (Figure 1). Although most adverse effects (eg, fatigue, vomiting, nausea) of the conditioning regimen are reversible, others (eg, infertility) can be permanent.
How Is HSCT Performed?
Once the conditioning regimen has been completed, patients have stem cells infused (commonly known as day 0) via an intravenous line, usually a central catheter. This infusion may take 1–2 hours, depending on the number of cells that need to be infused. The infusion is typically painless, and caregivers can be present during the infusion. Once the stem cells have been infused, it takes several days for the cells to engraft (called “days plus”). Specifically, 2–3 weeks are needed for engraftment of peripheral blood cells and bone marrow cells, whereas umbilical cord blood stem cells often take 3–5 weeks to engraft. The period before engraftment, also known as the patient’s nadir, is typically the most symptomatic and difficult time for patients (Figure 1). Successful engraftment of the transplanted cells helps to maintain long-term functional hematopoiesis (involving red blood cells, WBCs, and platelets). While patients are hospitalized during engraftment (due to the risk of infections and to monitor side effects), some individuals can be managed as outpatients when this transplant modality is available. Similarly, it is common for patients to have blood and/or platelets transfused until engraftment has occurred. Patients are also monitored closely for signs of infection and discomfort; they may also need analgesics as well as fluid and nutritional support due to chemotherapy-related side effects that may impede the ability to ingest food or drink fluids.
What Complications Can Result From HSCT?
HSCT can be complicated by myriad factors (including the patient’s age, the baseline performance status, the source of the stem cell transplant, and the type and intensity of the preparative regimen), which can vary in their severity and impact on the overall success of the procedure (Table 4).3 One of the most significant risks is graft-versus-host disease (GVHD), particularly following allogeneic HSCT, where the donor’s immune cells attack the recipient’s tissues, leading to inflammation and organ damage (eg, to the skin, liver, and intestines).3,7 Between 10% and 50% of patients who receive an allogeneic transplant develop GVHD, which is divided into acute (within 3 months) and chronic (over 3 months) phases.3 It can also vary from mild to severe and may require treatment with long-term immunosuppressive agents.3 Another potential complication is infection, due to the immunosuppressive therapy given to prevent transplant rejection and to treat the underlying disease.3,27 Such infections can be bacterial, viral, or fungal and may occur shortly after the transplant when the immune system is most compromised. Several prophylactic regimens have been suggested to prevent infections, with each tailored to the patient’s risk stratification.3 Other complications include organ toxicity (eg, of the liver, kidney), bleeding disorders, secondary cancers, fertility issues, and depression.3,7 The transplant process itself rarely leads to graft failure (that arises in approximately 1% of cases), where the transplanted stem cells fail to engraft successfully and do not produce blood cells, which requires additional treatments.7,28
In general, there is a lower risk for disease recurrence following allogeneic transplants compared to autologous transplants; however, allogeneic HSCTs may contribute to a more potentially fatal complication (including regimen-related organ toxicity, graft failure, and GVHD).8 Managing these complications involves close monitoring and supportive care to improve outcomes and the quality of posttransplant life.
How Might Fertility Be Affected, and How Can It Be Addressed?
Issues related to sexual dysfunction and fertility are significant late effects associated with allogeneic HSCT, and they have a considerable impact on the patient’s quality of life (QOL).29,30 As the probability of long-term survival reaches 80% in those who have survived 2 years after allogeneic HSCT, the management of these late effects is becoming a major concern.29,31 The risk of permanent infertility depends on the treatments used (eg, high-dose chemotherapy vs total body irradiation, ablative vs nonablative regimens) and the dosages administered.7,32 These treatments can damage the reproductive organs, leading to either temporary or permanent infertility. In men, HSCT can cause azoospermia due to damage to the testes, while women may experience ovarian failure, leading to the loss of menstrual function and to potential infertility.30
Enhancing care requires an increased awareness of these issues by clinicians, along with the need for specialized gynecologic follow-up and the option for sexology consultations to improve early screening and management.29 It is important to discuss concerns regarding fertility with patients before proceeding with HSCT to facilitate informed decision-making and planning (Table 5).
What Might the Recovery From HSCT Involve?
Recovery from HSCT can be arduous and last several weeks. Patients undergoing an autologous transplant often remain hospitalized for 2–3 weeks, while those undergoing allogenic transplantation are hospitalized for 3–4 weeks. During this time, patients experience side effects from the conditioning regimen, including mucositis (which may manifest as intense pain, difficulty maintaining adequate oral intake), fever, fatigue, nausea, diarrhea, and pain as well as anxiety and sadness (Figure 1).37 Although side effects tend to improve with successful engraftment, recovery and hospital discharge may be delayed by unsuccessful engraftment or early complications, including acute GVHD.38,39 During this period, patients often stay in protected environments (due to the high risk of infection associated with severe immunosuppression); they must also adhere to strict hygiene protocols, eat a low microbial diet, and be monitored closely for infectious complications. Visits from friends and relatives are also restricted and everyone who enters their room must wear protective clothing (eg, masks and gowns). As a result, patients are at risk for psychiatric problems during their hospitalization.40 In fact, 6%–15% of patients develop depression before their transplant, and this rate soars to 38% at day 8 following their stem cell infusion.41 In addition, more than three-fourths (ie, up to 82%) of patients experience moderate-to-severe insomnia,42 while roughly one-third (35%) develop delirium,43 and nearly one-fourth (22%) have clinically significant anxiety.40 Since psychological distress is associated with worse treatment outcomes (including worse WBC recovery, prolonged hospital stays, an increased risk of GVHD, and worse adherence to medical interventions), screening for and treating distress is crucial.44–46
How Much Does HSCT Cost, and How Can It Be Paid For?
HSCT is costly and resource intensive. The cost associated with HSCT depends on multiple factors, including the type of HSCT (autologous vs allogeneic) and the potential complications that might require hospitalization. Current literature describing the cost of HSCT uses heterogeneous cost measures, which makes it difficult for patients, clinicians, and policymakers to define the resources needed to support patients undergoing HSCT (Table 6).
Notably, more than 75% of the cost of HSCT flows from the initial transplant hospitalization, regardless of transplant type.48 In the United States, public and private insurance covers the cost associated with the HSCT hospitalization. Beyond hospital-related costs, patients and their families must contend with significant financial burdens from copays for medications and physician visits, transportation costs, and relocation expenses for patients who need to stay near the transplant center during the acute recovery phase.49,50 Unfortunately, the financial burden following transplant can accumulate for years, particularly due to intensive follow-up care, late effects, medical complications, and chronic conditions (eg, GVHD, which may require inpatient hospitalizations and chronic medication management).51 Although the financial burden is high for those receiving a HSCT, it is rarely discussed in clinical encounters. Targeted empirical interventions to address financial toxicity—the financial hardship and psychological distress associated with the financial burden of HSCT52—are limited. The Comprehensive Score of Financial Toxicity measure has been used reliably to measure financial toxicity in this population. In addition, financial navigation programs, which educate patients about resources to manage the costs associated with treatment and recovery, as well as provide direct assistance to patients in applying for programs and managing their needs longitudinally, show promise as an intervention to alleviate financial toxicity in recipients of HSCT.53,54
Which Psychosocial Considerations of HSCT Are Important for Patients?
HSCT is a physically demanding procedure that significantly impacts a patient’s mental health. Many patients experience psychological challenges, including depression, anxiety, and distress, which can adversely affect their QOL. In fact, among survivors of HSCT, 12%–30% experience clinically significant depressive symptoms.55 Risk factors include younger age, female sex, lower socioeconomic status,55 the stress of the transplant process, prolonged hospitalizations, and concerns about treatment outcomes.56 Following HSCT, patients undergo a prolonged recovery phase characterized by significant prognostic uncertainty.41,57–65 Since under-detection and under-treatment of depression and anxiety can lead to poorer health outcomes and a diminished QOL, addressing psychosocial aspects is crucial. Furthermore, declines in QOL and increased depressive symptoms during hospitalizations for HSCT predict long-term symptoms of posttraumatic stress disorder (PTSD).66 Interventions (eg, cognitive-behavioral therapy [CBT], mindfulness practices, palliative care, routine distress screenings) help to manage these psychological concerns.67–69 In addition, providing patients with adequate support and resources can help them navigate the emotional complexities associated with HSCT.70,71
Dysregulated mood often persists even after the transplant for HSCT survivors, often triggered by GVHD, which affects approximately 40%–60% of HSCT survivors.72 Moreover, chronic GVHD is a major contributor to morbidity and nonrelapse mortality.72,73 Patients with chronic GVHD often experience substantial limitations and a diminished QOL, with up to 40% reporting symptoms of depression and 18% experiencing anxiety.74–77
Which Psychosocial Considerations of HSCT Are Important for Family Members and Friend Caregivers?
Caregivers (ie, family and friends) play a crucial role surrounding HSCT, as patients with a designated caregiver tend to experience better outcomes, including increased survival rates.78 However, caregivers of HSCT recipients endure a tremendous strain, which impacts their QOL and mood.41,79 Specifically, caregivers experience considerable emotional distress as they prepare for the patient’s hospitalization and navigate uncertainties related to the illness and HSCT.41,58–60,80–83 During HSCT, caregivers’ QOL declines, and symptoms of depression intensify as they witness the patient developing multiple treatment-related toxicities throughout their hospital stay.58–60,80,81 In the months following HSCT, caregivers continue to support the patient by managing persistent physical symptoms, handling complex medication regimens, attending frequent outpatient appointments, and coping with the uncertainty that surrounds the patient’s health.82,83 Beyond handling daily caregiving responsibilities, caregivers must also cope with their own fears regarding their loved one’s health and prognosis. As a result, caregivers face significant disruptions to their employment, household responsibilities, and personal lives during this period.84–86 Consequently, caregivers of those receiving HSCT experience a substantial and prolonged caregiving burden, which leads to profound distress.
A growing body of research has highlighted the heightened psychological distress experienced by this population,60–63 showing that 40% of caregivers of HSCT recipients report clinically significant depression and anxiety.41,87 In addition to the distress experienced by the patient and caregiver, the dyad experiences distress.88 Taken together, the decrements in patient and caregiver mental health, as well as the interface of patient QOL and caregiver well-being, warrant psychosocial interventions that protect against precipitous declines and buffer the effect of stress on physical and emotional health.89
How Can the Coping of Patients and Their Family Members Be Enhanced After HSCT?
Coping is an active process through which patients navigate, adjust to, and adapt to stressful situations.90 This process may involve taking steps to manage their condition (task-oriented coping) or distancing themselves from the stressor (avoidance-oriented coping).91 The way patients cope with HSCT significantly impacts their QOL and is closely linked to symptom burden, mood disturbances (eg, depression and anxiety), physical functioning, and social support as they adjust to the limitations imposed by a chronic disease.92,93 Patients who rely on negative emotion- oriented coping may be more likely to experience anxiety and depression, as well as a decline in QOL. In contrast, those who employ task-oriented coping often report fewer symptoms of depression and an improved QOL.94 Since one’s coping style is a modifiable factor, shifting toward more adaptive coping strategies in response to chronic medical stressors can improve the long-term QOL trajectory for patients post-HSCT.94
Psychosocial and behavioral interventions can reduce psychological distress in patients undergoing HSCT; however, more randomized controlled trials are needed to confirm their efficacy.40 CBT is widely recognized for its ability to address distress by teaching coping skills and restructuring maladaptive thoughts.95 Variants (eg, telephone-based CBT and stress management with relaxation techniques) have been used following HSCT.69,70 Enhancing patients’ ability to manage stress is a goal of these interventions, which may involve several techniques (eg, relaxation, cognitive restructuring, and assertive communication). Coping effectiveness, in accordance with Lazarus and Folkman’s Stress Coping Theory, teaches the ability to choose an effective coping technique that accounts for the controllability of the stressor.96 Mindfulness-based psychological interventions that allow patients to share their experiences also foster emotional resilience, reduce uncertainty, and enhance preparedness.67 Palliative care interventions have shown promise in improving QOL, with studies demonstrating reductions in depression and symptoms of PTSD post-HSCT.68,97
Pharmacotherapy is widely used to manage psychiatric symptoms following HSCT, although research on this has lagged behind clinical practice.40 Various psychopharmacologic agents (eg, antidepressants, antipsychotics, anxiolytics, and mood stabilizers) have demonstrated efficacy but require careful selection due to the potential for drug interactions and adverse effects.98 For example, antidepressants (eg, selective serotonin reuptake inhibitors [SSRIs] and serotonin-norepinephrine reuptake inhibitors) effectively treat depression and anxiety but may increase bleeding risks,99 while mirtazapine offers additional benefits, eg, appetite stimulation, but it carries a rare risk of neutropenia.100 Some SSRIs, such as sertraline, citalopram, escitalopram, and paroxetine, may be preferred due to lower interaction risk with the anticipated transplant medications. Benzodiazepines can mitigate acute anxiety, although some are linked to blood cell abnormalities. Antipsychotics manage delirium and related behavioral issues but may cause neutropenia and agranulocytosis.100 Mood stabilizers and antiepileptics, used for conditions like bipolar disorder, can also adversely impact blood cell counts, thereby necessitating careful monitoring following HSCT.101
Building from patient-centered intervention research, psychosocial interventions have also been designed to reduce the negative impact of cancer caregiving and have shown improvements in caregivers’ distress, mood, QOL, coping abilities, and self-efficacy.102 Evidence suggests that mastering coping skills (eg, practicing mindful awareness and self-compassion) may reduce distress among caregivers in other cancer populations.103 For example, a multimodal psychosocial intervention, BMT-CARE, has improved caregivers’ coping skills and boosted their confidence in providing care for their loved ones.104 Developing coping abilities can enhance QOL and mood among caregivers.104 Similarly, a problem-solving education intervention for caregivers of patients undergoing allogeneic transplantation significantly improved self-efficacy and reduced caregiver distress.77 Testing of positive psychology interventions is also underway for caregivers of HSCT survivors and appears to have potential benefits.105
How Can Primary Care Physicians Continue To Be Involved as a Patient Goes Through HSCT?
Primary care providers (PCPs) can play a vital and often under-recognized role in the continuum of care for patients undergoing HSCT. Their involvement spans both the acute and long-term phases of treatment, beginning with pretransplant assessments and extending well into survivorship. Effective communication between the PCP and the transplant team is essential to ensure coordinated care. When the patient and their family are geographically distant from the transplant center, PCPs can consider maintaining continuity through telehealth and other digital communication tools. They are also well-positioned to support the patient and family by addressing psychosocial and psychiatric concerns, monitoring mental health symptoms, and providing valuable insights into the patient’s and family’s premorbid psychiatric considerations. Additionally, the longstanding relationship that many PCPs have with their patients enables them to offer valuable emotional support and help manage expectations throughout the HSCT journey, enhancing the overall quality of care.
What Happened to Mr A?
Mr A was counseled on the risks and benefits of HSCT, as well as the challenges of posttransplant recovery, including the need for close monitoring in a sterile isolation room to prevent infections. His oncologist also discussed the risk of infertility as a complication of intensive chemotherapy and radiation therapy that were required before HSCT. Recognizing that he might want to have children, Mr A was advised to consider sperm cryopreservation before beginning treatment. After careful consideration and discussion with his partner, Mr A proceeded with sperm cryopreservation.
Mr A underwent myeloablative conditioning with high-dose chemotherapy to eliminate leukemia cells and suppress his immune system; he achieved remission within several months. Although Mr A’s siblings were not a donor match, a 10/10 HLA match was found in an unrelated donor, and stem cells were collected for transplantation.
Following his HSCT, Mr A was monitored in the hospital for complications. Unfortunately, Mr A developed an acute GVHD (manifest by a skin rash, diarrhea, and elevated liver enzymes, which was controlled with immunosuppressive medications) and a bacterial infection (that was managed successfully with antibiotics).
Although Mr A’s health insurance covered most of his transplant-related expenses (which were considerable), he incurred out-of-pocket expenses for medications and postdischarge care. The hospital’s social worker helped Mr A apply for financial assistance programs, which provided additional support for travel and lodging during his recovery.
Since the psychological toll of HSCT was significant for Mr A and his family, Mr A was connected to a support group comprised by others who had undergone HSCT. Family counseling sessions with a social worker also helped his relatives cope with the HSCT-induced stress, and his team maintained regular communication with them throughout his hospitalization. Support services were helpful in navigating the difficult journey of transplant and recovery.
After several months in the hospital and in follow-up care, Mr A’s condition improved. His bone marrow began producing healthy blood cells. Although he experienced mild chronic GVHD, his symptoms were manageable with medication. One year after his transplant, Mr A was in complete remission, without signs of a leukemia recurrence. Although his long-term prognosis was considered favorable, he was scheduled for ongoing monitoring for potential late complications, including the development of secondary cancers and chronic GVHD.
Mr A returned to work, and he resumed a relatively normal life. He and his family expressed gratitude for the comprehensive care they received throughout the process. His decision to preserve his fertility through sperm cryopreservation provided him with peace of mind regarding his plans for having children.
CONCLUSION
HSCT (ie, BMT) is a procedure that involves administering healthy hematopoietic stem cells to individuals with dysfunctional or depleted bone marrow to treat a variety of blood and immune system disorders (eg, leukemia, lymphoma) and certain genetic conditions, especially those that are refractory to other treatments or in cases where a patient has relapsed after initial therapy (eg, multiple myeloma, acute and chronic leukemias, lymphomas, MDS, aplastic anemia, and severe combined immune deficiency syndrome).
HSCT can be complicated by the source of the stem cell transplant and the type and intensity of the preparative regimen. Side effects of the conditioning regimen include nausea, vomiting, fatigue, alopecia, and infertility. Some of the most significant risks of HSCT are GVHD (in which the donor’s immune cells attack the recipient’s tissues, leading to inflammation and organ damage) and infection (due to the immunosuppressive regimen used to prevent transplant rejection and to treat the underlying disease) as well as organ toxicity, bleeding disorders, secondary cancers, fertility issues, and depression. Fertility preservation strategies can be considered prior to HSCT to provide the possibility of having future biological children.
Article Information
Published Online: September 30, 2025. https://doi.org/10.4088/PCC.25f03968
© 2025 Physicians Postgraduate Press, Inc.
Submitted: March 18, 2025; accepted June 12, 2025.
To Cite: Lee JH, Amonoo HL, Barata A, et al. Talking to your patients about hematopoietic stem cell transplantation: a guide for primary care providers. Prim Care Companion CNS Disord 2025;27(5):25f03968.
Author Affiliations: Harvard Medical School, Boston, Massachusetts (Lee, Amonoo, Barata, Jacobs, Stern); Department of Psychiatry, Brigham and Women’s Faulkner Hospital, Boston, Massachusetts (Lee); Department of Psychiatry, Brigham and Women’s Hospital and Dana-Farber Cancer Institute, Boston, Massachusetts (Amonoo); Department of Psychiatry, Massachusetts General Hospital, Boston, Massachusetts (Barata, Stern); Center for Psychiatric Oncology and Behavioral Sciences, Massachusetts General Hospital, Boston, Massachusetts (Jacobs).
Lee, Amonoo, Barata, and Jacobs are co-first authors; Stern is senior author.
Corresponding Author: Jeong Hoo Lee, MD, Department of Psychiatry, Brigham and Women’s Faulkner Hospital, Harvard Medical School, 1153 Centre St, Jamaica Plain, MA 02130 ([email protected]). Dr Lee is a consultation-liaison psychiatrist at Brigham and Women’s Faulkner Hospital and an instructor and associate clerkship site director for psychiatry at Harvard Medical School.
Relevant Financial Relationships: Dr Jacobs is a consultant for OncoveryCare, Inc and Reunion Neuroscience, Inc and serves on the board of directors for the WeGotThis.org Foundation. Dr Stern has received royalties from Elsevier for editing textbooks on psychiatry. Drs Lee, Amonoo, and Barata report no disclosures.
Funding/Support: None.
Clinical Points
- In autologous hematopoietic stem cell transplantation (HSCT), stem cells are harvested from the patient before they undergo high-dose chemotherapy or radiation therapy, after which the stem cells are reintroduced.
- In allogeneic HSCT, stem cells are collected from a genetically matched (related or unrelated) donor and then infused into the patient; donor stem cells replace those of the dysfunctional immune system. Allogeneic stem cell donors are selected based on the compatibility of human leukocyte antigen genes that distinguish between self and nonself cells in major histocompatibility complexes.
- The decision to undergo HSCT often needs to be made relatively quickly, as delays can impact the procedure’s success and the overall posttransplant survival.
- Patients undergoing HSCT often remain in protected environments (due to the high risk of infection associated with severe immunosuppression), and they must also adhere to strict hygiene protocols, eat a low microbial diet, and be monitored closely for infectious complications.
References (105)

- Barnes DW, Corp MJ, Loutit JF, et al. Treatment of murine leukaemia with X rays and homologous bone marrow; preliminary communication. Br Med J. 1956;2(4993):626–627. PubMed CrossRef
- Thomas ED, Lochte HL, Lu WC, et al. Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N Engl J Med. 1957;257(11):491–496. PubMed CrossRef
- Khaddour K, Hana CK, Mewawalla P. Hematopoietic stem cell transplantation. In: StatPearls [Internet]. StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK536951/. Accessed February 20, 2025
- Maziarz RT, Slater SS, eds. Blood and Marrow Transplant Handbook: Comprehensive Guide for Patient care. Springer International Publishing; 2015.
- Michel RP, Berry G. Pathology Of Transplantation, A Practical Diagnostic Approach. 2016:483.
- Hatzimichael E, Tuthill M. Hematopoietic stem cell transplantation. Stem Cells Cloning. 2010;3:105–117. PubMed CrossRef
- Léger CS, Nevill TJ. Hematopoietic stem cell transplantation: a primer for the primary care physician. CMAJ Can Med Assoc J. 2004;170(10):1569–1577.
- Champlin R. Selection of autologous or allogeneic transplantation. In: Holland-Frei Cancer Medicine. 6th ed. BC Decker; 2003. https://www.ncbi.nlm.gov/books/NBK12844/. Accessed February 27, 2025.
- de Witte T, Bowen D, Robin M, et al. Allogeneic hematopoietic stem cell transplantation for MDS and CMML: recommendations from an international expert panel. Blood. 2017;129(13):1753–1762. PubMed CrossRef
- Nickel RS, Hendrickson JE, Haight AE. The ethics of a proposed study of hematopoietic stem cell transplant for children with “less severe” sickle cell disease. Blood. 2014;124(6):861–866. PubMed CrossRef
- Walters MC, Patience M, Leisenring W, et al. Bone marrow transplantation for sickle cell disease. N Engl J Med. 1996;335(6):369–376. PubMed CrossRef
- Angelucci E, Matthes-Martin S, Baronciani D, et al. Hematopoietic stem cell transplantation in thalassemia major and sickle cell disease: indications and management recommendations from an international expert panel. Haematologica. 2014;99(5):811–820. PubMed CrossRef
- Nickel RS, Kamani NR. Ethical challenges in hematopoietic cell transplantation for sickle cell disease. Biol Blood Marrow Transpl. 2018;24(2):219–227. PubMed CrossRef
- Mekelenkamp H, van Zanten H, de Vries M, et al. How to facilitate decision- making for hematopoietic stem cell transplantation in patients with hemoglobinopathies. The perspectives of healthcare professionals. Front Pediatr. 2021;9:690309. PubMed CrossRef
- Silva TS, Horvath JDC, Pereira MP, et al. Impact of waitlist time on post-HSCT survival: a cohort study at a hospital in southern Brazil. Hematol Transfus Cell Ther. 2024;46(3):242–249. PubMed CrossRef
- Alessandrino EP, Della Porta MG, Bacigalupo A, et al. WHO classification and WPSS predict posttransplantation outcome in patients with myelodysplastic syndrome: a study from the Gruppo Italiano Trapianto di Midollo Osseo (GITMO). Blood. 2008;112(3):895–902. PubMed CrossRef
- Lim Z, Brand R, Martino R, et al. Allogeneic hematopoietic stem-cell transplantation for patients 50 years or older with myelodysplastic syndromes or secondary acute myeloid leukemia. J Clin Oncol. 2010;28(3):405–411. PubMed CrossRef
- Amonoo HL, Stefanski HE, Brewer B. Allogeneic stem cell donation. JAMA. 2025;333(1):81–82. PubMed CrossRef
- Dehn J, Spellman S, Hurley CK, et al. Selection of unrelated donors and cord blood units for hematopoietic cell transplantation: guidelines from the NMDP/ CIBMTR. Blood. 2019;134(12):924–934. PubMed CrossRef
- Cusatis R, Litovich C, Feng Z, et al. Current trends and outcomes in cellular therapy activity in the United States, including prospective patient-reported outcomes data collection in the Center for International Blood and Marrow Transplant Research Registry. Transpl Cell Ther. 2024;30(9):917 e1–917 e12. PubMed CrossRef
- Kiene S, Albrecht M, Theurich S, et al. Bone marrow versus peripheral blood allogeneic haematopoietic stem cell transplantation for haematological malignancies in adults. Cochrane Database Syst Rev. 2024;11(11):Cd010189. PubMed CrossRef
- Balogun RAA N, Alicia G, Pham Huy P, et al. Principles of Apheresis Technology. Technical Principles of Apheresis Medicine. 7th ed. American Society for Apheresis; 2020.
- Marlow SD, House M. Managing apheresis complications during the hematopoietic stem cell collection. Methods Mol Biol. 2012;904:93–96. PubMed CrossRef
- Gorin NC, Carreras E, Fernández-Sojo J, et al. Bone marrow harvesting for HCT. In: Sureda A, Corbacioglu S, Greco R, et al, eds. The EBMT Handbook: Hematopoietic Cell Transplantation and Cellular Therapies. Springer Copyright 2024, The Author(s). 2024:143-149.
- Ballen KK, Gluckman E, Broxmeyer HE. Umbilical cord blood transplantation: the first 25 years and beyond. Blood. 2013;122(4):491–498. PubMed CrossRef
- Gagelmann N, Kröger N. Dose intensity for conditioning in allogeneic hematopoietic cell transplantation: can we recommend “when and for whom” in 2021? Haematologica. 2021;106(7):1794–1804. PubMed CrossRef
- Young JAH, Weisdorf DJ. Infections in recipients of hematopoietic stem cell transplants. Mand Douglas Bennetts Princ Pract Infect Dis. 2015:3425–3439.e5.
- Wolff SN. Second hematopoietic stem cell transplantation for the treatment of graft failure, graft rejection or relapse after allogeneic transplantation. Bone Marrow Transpl. 2002;29(7):545–552. PubMed CrossRef
- Forgeard N, Jestin M, Vexiau D, et al. Sexuality- and fertility-related issues in women after allogeneic hematopoietic stem cell transplantation. Transpl Cell Ther. 2021;27(5):432.e1–432.e6.
- Tichelli A, Rovó A. Fertility issues following hematopoietic stem cell transplantation. Expert Rev Hematol. 2013;6(4):375–388. PubMed CrossRef
- Bhatia S, Francisco L, Carter A, et al. Late mortality after allogeneic hematopoietic cell transplantation and functional status of long-term survivors: report from the Bone Marrow Transplant Survivor Study. Blood. 2007;110(10):3784–3792. PubMed CrossRef
- Meirow D, Nugent D. The effects of radiotherapy and chemotherapy on female reproduction. Hum Reprod Update. 2001;7(6):535–543. PubMed CrossRef
- Gracia CR, Ginsberg JP. Fertility risk in pediatric and adolescent cancers. Cancer Treat Res. 2007;138:57–72. PubMed CrossRef
- Jeruss JS, Woodruff TK. Preservation of fertility in patients with cancer. N Engl J Med. 2009;360(9):902–911. PubMed CrossRef
- Wyns C, Curaba M, Petit S, et al. Management of fertility preservation in prepubertal patients: 5 years’ experience at the Catholic University of Louvain. Hum Reprod. 2011;26(4):737–747. PubMed CrossRef
- Donnez J, Silber S, Andersen CY, et al. Children born after autotransplantation of cryopreserved ovarian tissue. a review of 13 live births. Ann Med. 2011;43(6):437–450. PubMed CrossRef
- Sawyer J, Elliott T, Orton L, et al. Prevention and management of acute toxicities from conditioning regimens during hematopoietic stem cell transplantation. Clin Hematol Int. 2024;6(2):1–10. PubMed CrossRef
- Holler E, Geinix H, Zeiseer R, et al. Acute graft-versus-host disease. In: Carreras E, Dufour C, Mohty M, et al eds. The EBMT Handbook: Hematopoietic Stem Cell Transplantation and Cellular Therapies. Springer Copyright 2019, The Editor(s) (if applicable) and The Author(s). 2019.
- Maqbool S, Nadeem M, Shahroz A, et al. Engraftment syndrome following Hematopoietic stem cell transplantation: a systematic approach toward diagnosis and management. Med Oncol. 2022;40(1):36. PubMed CrossRef
- Amonoo HL, Massey CN, Freedman ME, et al. Psychological considerations in hematopoietic stem cell transplantation. Psychosomatics. 2019;60(4):331–342. PubMed CrossRef
- El-Jawahri AR, Traeger LN, Kuzmuk K, et al. Quality of life and mood of patients and family caregivers during hospitalization for hematopoietic stem cell transplantation. Cancer. 2015;121(6):951–959. PubMed CrossRef
- Jim HS, Evans B, Jeong JM, et al. Sleep disruption in hematopoietic cell transplantation recipients: prevalence, severity, and clinical management. Biol Blood Marrow Transpl. 2014;20(10):1465–1484. PubMed CrossRef
- Rueda-Lara M, Lopez-Patton MR. Psychiatric and psychosocial challenges in patients undergoing haematopoietic stem cell transplants. Int Rev Psychiatry. 2014;26(1):74–86. PubMed CrossRef
- El-Jawahri A, Chen YB, Brazauskas R, et al. Impact of pre-transplant depression on outcomes of allogeneic and autologous hematopoietic stem cell transplantation. Cancer. 2017;123(10):1828–1838. PubMed CrossRef
- Prieto JM, Blanch J, Atala J, et al. Psychiatric morbidity and impact on hospital length of stay among hematologic cancer patients receiving stem-cell transplantation. J Clin Oncol. 2002;20(7):1907–1917. PubMed CrossRef
- McGregor BA, Syrjala KL, Dolan ED, et al. The effect of pre-transplant distress on immune reconstitution among adult autologous hematopoietic cell transplantation patients. Brain Behav Immun. 2013;30(suppl):S142–S148. PubMed CrossRef
- Kim NV, McErlean G, Yu S, et al. Healthcare resource utilization and cost associated with allogeneic hematopoietic stem cell transplantation: a scoping review. Transpl Cell Ther. 2024;30(5):542.e1–542.e29.
- Majhail NS, Mau LW, Denzen EM, et al. Costs of autologous and allogeneic hematopoietic cell transplantation in the United States: a study using a large national private claims database. Bone Marrow Transpl. 2013;48(2):294–300. PubMed CrossRef
- Khera N, Chang YH, Hashmi S, et al. Financial burden in recipients of allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transpl. 2014;20(9):1375–1381. PubMed CrossRef
- Majhail NS, Rizzo JD, Hahn T, et al. Pilot study of patient and caregiver out-of- pocket costs of allogeneic hematopoietic cell transplantation. Bone Marrow Transpl. 2013;48(6):865–871. PubMed CrossRef
- Waters AR, Biddell CB, Killela M, et al. Financial burden and recommended multilevel solutions among caregivers of pediatric hematopoietic stem cell transplant recipients. Pediatr Blood Cancer. 2023;70(12):e30700. PubMed CrossRef
- de Souza JA, Yap BJ, Wroblewski K, et al. Measuring financial toxicity as a clinically relevant patient-reported outcome: the validation of the COmprehensive Score for financial Toxicity (COST). Cancer. 2017;123(3):476–484. PubMed CrossRef
- Edward JS, McLouth LE, Rayens MK, et al. Coverage and cost-of-care links: addressing financial toxicity among patients with hematologic cancer and their caregivers. JCO Oncol Pract. 2023;19(5):e696–e705. PubMed CrossRef
- Wheeler SB, Birken SA, Wagi CR, et al. Core functions of a financial navigation intervention: an in-depth assessment of the Lessening the Impact of Financial Toxicity (LIFT) intervention to inform adaptation and scale-up in diverse oncology care settings. Front Health Serv. 2022;2:958831. PubMed CrossRef
- Bevans M, El-Jawahri A, Tierney DK, et al. National Institutes of Health Hematopoietic Cell Transplantation Late Effects Initiative: The Patient-Centered Outcomes Working Group Report. Biol Blood Marrow Transpl. 2017;23(4):538–551. PubMed CrossRef
- Di Giuseppe G, Thacker N, Schechter T, et al. Anxiety, depression, and mental health-related quality of life in survivors of pediatric allogeneic hematopoietic stem cell transplantation: a systematic review. Bone Marrow Transpl. 2020;55(7):1240–1254. PubMed CrossRef
- Braamse AM, Gerrits MM, van Meijel B, et al. Predictors of health-related quality of life in patients treated with auto- and allo-SCT for hematological malignancies. Bone Marrow Transpl. 2012;47(6):757–769. PubMed CrossRef
- Curtis RE, Rowlings PA, Deeg HJ, et al. Solid cancers after bone marrow transplantation. N Engl J Med. 1997;336(13):897–904. PubMed CrossRef
- Duell T, van Lint MT, Ljungman P, et al. Health and functional status of long-term survivors of bone marrow transplantation. EBMT Working Party on Late Effects and EULEP Study Group on Late Effects. European Group for Blood and Marrow Transplantation. Ann Int Med. 1997;126(3):184–192. PubMed CrossRef
- Gratwohl A, Baldomero H, Frauendorfer K, et al. EBMT activity survey 2004 and changes in disease indication over the past 15 years. Bone Marrow Transpl. 2006;37(12):1069–1085. PubMed CrossRef
- Pidala J, Anasetti C, Jim H. Health-related quality of life following haematopoietic cell transplantation: patient education, evaluation and intervention. Br J Haematol. 2010;148(3):373–385. PubMed CrossRef
- Pidala J, Anasetti C, Jim H. Quality of life after allogeneic hematopoietic cell transplantation. Blood. 2009;114(1):7–19. PubMed CrossRef
- McQuellon RP, Russell GB, Rambo TD, et al. Quality of life and psychological distress of bone marrow transplant recipients: the “time trajectory” to recovery over the first year. Bone Marrow Transpl. 1998;21(5):477–486. PubMed CrossRef
- Bevans MF, Marden S, Leidy NK, et al. Health-related quality of life in patients receiving reduced-intensity conditioning allogeneic hematopoietic stem cell transplantation. Bone Marrow Transpl. 2006;38(2):101–109. PubMed CrossRef
- Fife BL, Huster GA, Cornetta KG, et al. Longitudinal study of adaptation to the stress of bone marrow transplantation. J Clin Oncol. 2000;18(7):1539–1549. PubMed CrossRef
- El-Jawahri AR, Vandusen HB, Traeger LN, et al. Quality of life and mood predict posttraumatic stress disorder after hematopoietic stem cell transplantation. Cancer. 2016;122(5):806–812. PubMed CrossRef
- Grossman P, Zwahlen D, Halter JP, et al. A mindfulness-based program for improving quality of life among hematopoietic stem cell transplantation survivors: feasibility and preliminary findings. Support Care Cancer. 2015;23(4):1105–1112. PubMed CrossRef
- El-Jawahri A, LeBlanc T, VanDusen H, et al. Effect of inpatient palliative care on quality of life 2 weeks after hematopoietic stem cell transplantation: a randomized clinical trial. JAMA. 2016;316(20):2094–2103. PubMed CrossRef
- DuHamel KN, Mosher CE, Winkel G, et al. Randomized clinical trial of telephone- administered cognitive-behavioral therapy to reduce post-traumatic stress disorder and distress symptoms after hematopoietic stem-cell transplantation. J Clin Oncol. 2010;28(23):3754–3761. PubMed CrossRef
- Baliousis M, Rennoldson M, Snowden JA. Psychological interventions for distress in adults undergoing haematopoietic stem cell transplantation: a systematic review with meta-analysis. Psychooncology. 2016;25(4):400–411. PubMed CrossRef
- Rini C, Lawsin C, Austin J, et al. Peer mentoring and survivors’ stories for cancer patients: positive effects and some cautionary notes. J Clin Oncol. 2007;25(1):163–166. PubMed CrossRef
- Pidala J, Kurland B, Chai X, et al. Patient reported quality of life is associated with severity of chronic graft-versus-host disease as measured by NIH criteria: report on baseline data from the Chronic GVHD Consortium. Blood. 2011;117(17):4651–4657. PubMed CrossRef
- Wingard JR, Majhail NS, Brazauskas R, et al. Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. J Clin Oncol. 2011;29(16):2230–2239. PubMed CrossRef
- Lee SJ, Fairclough D, Parsons SK, et al. Recovery after stem-cell transplantation for hematologic diseases. J Clin Oncol. 2001;19(1):242–252. PubMed CrossRef
- Lee SJ, Loberiza F, Antin J, et al. Routine screening for psychosocial distress following hematopoietic stem cell transplantation. Bone Marrow Transpl. 2005;35(1):77–83. PubMed CrossRef
- Syrjala K, Chapko M, Vitaliano P, et al. Recovery after allogeneic marrow transplantation: prospective study of predictors of long-term physical and psychosocial functioning. Bone Marrow Transpl. 1993;11(4):319–327. PubMed
- Syrjala KL, Langer SL, Abrams JR, et al. Recovery and long-term function after hematopoietic cell transplantation for leukemia or lymphoma. JAMA. 2004;291(19):2335–2343. PubMed CrossRef
- Kelly DL, Syrjala K, Taylor M, et al. Biobehavioral research and hematopoietic stem cell transplantation: expert review from the Biobehavioral research special interest group of the American Society for transplantation and cellular therapy. Transpl Cell Ther. 2021;27(9):747–757. PubMed CrossRef
- Northouse L, Williams AL, Given B, et al. Psychosocial care for family caregivers of patients with cancer. J Clin Oncol. 2012;30(11):1227–1234. PubMed CrossRef
- Lee SJ, Joffe S, Kim HT, et al. Physicians’ attitudes about quality-of-life issues in hematopoietic stem cell transplantation. Blood. 2004;104(7):2194–2200. PubMed CrossRef
- Prieto JM, Atala J, Blanch J, et al. Patient-rated emotional and physical functioning among hematologic cancer patients during hospitalization for stem- cell transplantation. Bone Marrow Transpl. 2005;35(3):307–314. PubMed CrossRef
- Gemmill R, Cooke L, Williams AC, et al. Informal caregivers of hematopoietic cell transplant patients: a review and recommendations for interventions and research. Cancer Nurs. 2011;34(6):E13–E21. PubMed CrossRef
- Beattie S, Lebel S. The experience of caregivers of hematological cancer patients undergoing a hematopoietic stem cell transplant: a comprehensive literature review. Psychooncology. 2011;20(11):1137–1150. PubMed CrossRef
- Fife BL, Monahan PO, Abonour R, et al. Adaptation of family caregivers during the acute phase of adult BMT. Bone Marrow Transpl. 2009;43(12):959–966. PubMed CrossRef
- Siston AK, List MA, Daugherty CK, et al. Psychosocial adjustment of patients and caregivers prior to allogeneic bone marrow transplantation. Bone Marrow Transpl. 2001;27(11):1181–1188. PubMed CrossRef
- Foxall MJ, Gaston-Johansson F. Burden and health outcomes of family caregivers of hospitalized bone marrow transplant patients. J Adv Nurs. 1996;24(5):915–923. PubMed CrossRef
- Jim HS, Quinn GP, Barata A, et al. Caregivers’ quality of life after blood and marrow transplantation: a qualitative study. Bone Marrow Transpl. 2014;49(9):1234–1236. PubMed CrossRef
- Sannes TS, Simoneau TL, Mikulich-Gilbertson SK, et al. Distress and quality of life in patient and caregiver dyads facing stem cell transplant: identifying overlap and unique contributions. Support Care Cancer. 2019;27(6):2329–2337. PubMed CrossRef
- Sannes TS, Ranby KW, Yusufov M, et al. More often than not, we’re in sync: patient and caregiver well-being over time in stem cell transplantation. Health Qual Life Outcomes. 2022;20(1):6. PubMed CrossRef
- Lazarus RS. Psychological stress and coping in adaptation and illness. Int J Psychiatry Med. 1974;5(4):321–333. PubMed CrossRef
- Endler NPD, Parker JDA. Coping Inventory for Stressful Situations (CISS): Manual. 2 ed. Multi-Health Systems, Inc; 1999.
- Paterson C, Jones M, Rattray J, et al. Exploring the relationship between coping, social support and health-related quality of life for prostate cancer survivors: a review of the literature. Eur J Oncol Nurs. 2013;17(6):750–759. PubMed CrossRef
- Nipp RD, El-Jawahri A, Fishbein JN, et al. Factors associated with depression and anxiety symptoms in family caregivers of patients with incurable cancer. Ann Oncol. 2016;27(8):1607–1612. PubMed CrossRef
- Jacobs JM, Fishman S, Sommer R, et al. Coping and modifiable psychosocial factors are associated with mood and quality of life in patients with chronic graft- versus-host disease. Biol Blood Marrow Transpl. 2019;25(11):2234–2242. PubMed CrossRef
- Zhang L, Liu X, Tong F, et al. Cognitive behavioral therapy for anxiety and depression in cancer survivors: a meta-analysis. Sci Rep. 2022;12(1):21466. PubMed CrossRef
- Lazarus RS, Folkman S. Stress, Appraisal, and Coping. Springer; 1984.
- Levine DR, Baker JN, Wolfe J, et al. Strange bedfellows no more: how integrated stem-cell transplantation and palliative care programs can together improve end-of-life care. J Oncol Pract. 2017;13(9):569–577. PubMed CrossRef
- Fann JR. Neurological effects of psychopharmacological agents. Semin Clin Neuropsychiatry. 2002;7(3):196–205. PubMed CrossRef
- de Abajo FJ. Effects of selective serotonin reuptake inhibitors on platelet function: mechanisms, clinical outcomes and implications for use in elderly patients. Drugs Aging. 2011;28(5):345–367. PubMed CrossRef
- Oyesanmi O, Kunkel EJ, Monti DA, et al. Hematologic side effects of psychotropics. Psychosomatics. Sep-Oct 1999;40(5):414–421. PubMed CrossRef
- Sher Y, Miller Cramer AC, Ament A, et al. Valproic acid for treatment of hyperactive or mixed delirium: Rationale and literature review. Psychosomatics. 2015;56(6):615–625. PubMed CrossRef
- Kedia SK, Collins A, Dillon PJ, et al. Psychosocial interventions for informal caregivers of lung cancer patients: a systematic review. Psychooncology. 2020;29(2):251–262. PubMed CrossRef
- Hsieh CC, Yu CJ, Chen HJ, et al. Dispositional mindfulness, self-compassion, and compassion from others as moderators between stress and depression in caregivers of patients with lung cancer. Psychooncology. 07 2019;28(7):1498–1505. PubMed CrossRef
- Jacobs JM, Nelson AM, Traeger L, et al. Enhanced coping and self-efficacy in caregivers of stem cell transplant recipients: identifying mechanisms of a multimodal psychosocial intervention. Cancer. 2020;126(24):5337-5346. PubMed CrossRef
- Amonoo HL, Daskalakis E, Wolfe ED, et al. A positive psychology intervention in allogeneic hematopoietic stem cell transplantation survivors (PATH): a pilot randomized clinical trial. J Natl Compr Canc Netw. 2024;22(2 D):e237117. PubMed CrossRef
Enjoy this premium PDF as part of your membership benefits!