ABSTRACT
Objective: Rhemercise is a novel mindfulness technique used to prevent relapse in opioid use disorder (OUD). Rhemercise is a quantifiable and intentional slow-breathing technique that could increase subjective well-being, which helps to prevent relapse in OUD by reducing craving, negative affect, and visceral reactivity. The objective of this study was to assess the efficacy of rhemercise as an adjunctive therapy in patients with OUD undergoing detoxification.
Methods: This was a hospital-based, open-label, prospective, and exploratory study conducted between June 2018 and June 2019 that included 126 male inpatients admitted for detoxification of OUD. Patients with OUD diagnosed according to ICD-10 criteria who were aged 18–65 years were included in the study. Patients with other psychiatric disorders were excluded. Participants were divided into 2 groups: group A (n = 63) comprised patients receiving treatment as usual + rhemercise, and group B (n = 63) received treatment as usual only. Assessment tools included the Clinical Opiate Withdrawal Scale, Brief Pain Inventory, and Subjective Well-Being Inventory.
Results: Various domains of the Subjective Well-Being Inventory (general well-being–positive affect [P = .02], confidence in coping [P = .007], inadequate mental mastery [P = .002]) improved significantly among OUD patients who received rhemercise treatment compared to treatment as usual.
Conclusion: Rhemercise promoted general well-being and positive affect and decreased the opioid withdrawal symptoms, thereby potentially reducing the overall risk for relapse. Future studies are warranted with rhemercise to validate these promising findings.
Prim Care Companion CNS Disord 2022;24(1):21m03064
To cite: Kundal D, Raj R, Garg R, et al. Therapeutic efficacy of rhemercise: a novel mindfulness technique in patients with opioid use disorder. Prim Care Companion CNS Disord. 2022;24(1):21m03064.
To share: https://doi.org/10.4088/PCC.21m03064
© Copyright 2022 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry, Maharishi Markandeshwar Medical College and Hospital, Kumarhatti, Solan, Himachal Pradesh, India
bDepartment of Psychiatry and De-addiction, Rajindra Hospital, Patiala, Punjab, India
cDepartment of Psychiatry and Behavioral Health, Cooper University Health Care, Cooper Medical School of Rowan University, Camden, New Jersey
dCarle Foundation Hospital, Urbana, Illinois
eAmherst College, Amherst, Massachusetts
fStony Brook University Renaissance School of Medicine, Stony Brook, New York
gAddiction Recovery Treatment Services, Veterans Affairs Northern California Health Care System, University of California, Davis, Sacramento, California
*Corresponding author: Deepam Kundal, MD, Department of Psychiatry, Maharishi Markandeshwar Medical College and Hospital, Kumarhatti, Solan, Himachal Pradesh, India 173229 ([email protected]).
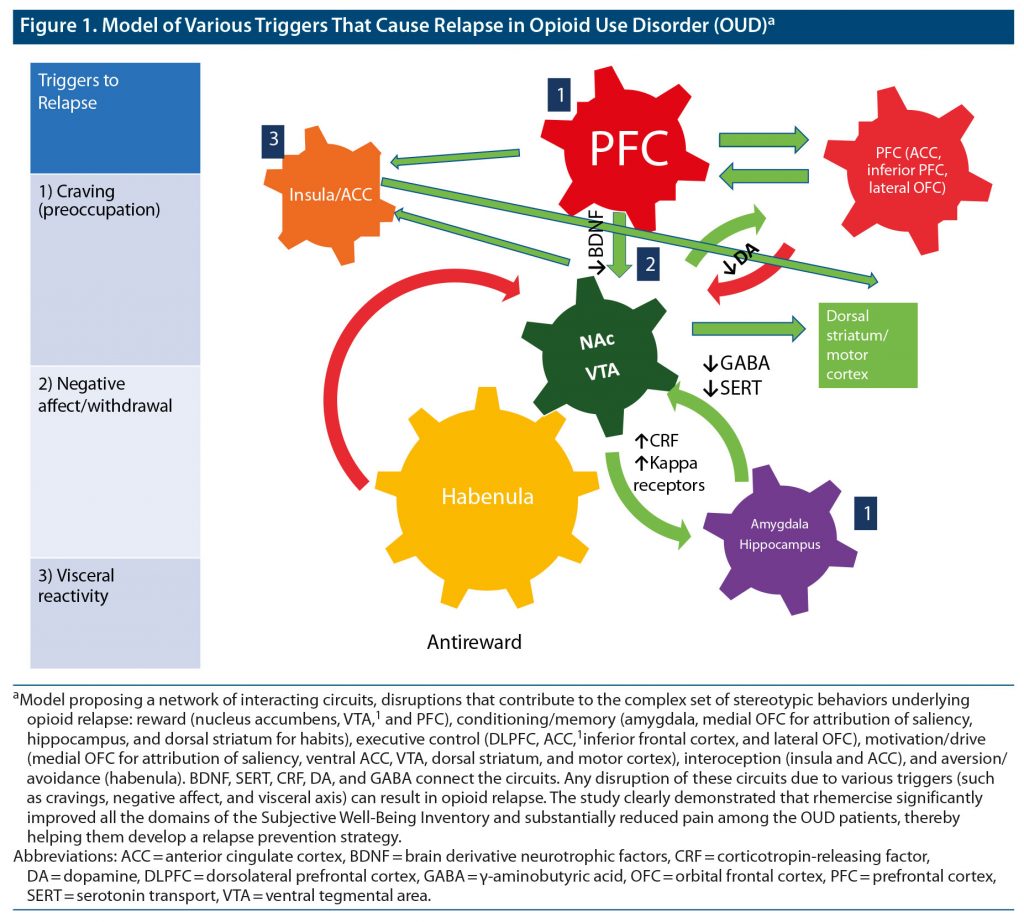
Those with opioid use disorder (OUD) face several challenges while trying to stop using opioids. Craving, negative affect (withdrawal), and visceral reactivity are major reasons for relapse as depicted in Figure 1.1
Pharmacologic management of opioid withdrawal usually consists of using opioid substitutes like methadone, buprenorphine, or clonidine and then tapering them down gradually along with symptomatic treatment. The key to successful management of OUD is not only adherence to treatment, but also a sustained motivation to refrain from using the substance again, and that is where the need for an adjunctive psychosocial therapy arises.
In this article, we propose a novel mindfulness intervention, rhemercise, to prevent relapse in OUD. Rhemercise is a quantifiable and intentional slow-breathing technique intended for use in mindfulness-based intervention (MBI). Rhema is a Greek term that means “spoken word.” This mindfulness technique begins by slowly breathing out while smiling a Duchenne smile and speaking positive words, followed by slowly breathing in and yawning with the mouth closed as one meditates on what was just spoken. A Duchenne smile, also referred to as a “genuine” smile, is when both the zygomaticus major and orbicularis oculus muscles are activated bilaterally. Smiling, both with and without orbicularis oculus muscle activation, has been found to facilitate stress recovery, but a Duchenne smile has been found to be more advantageous.2 Yawning has been shown to be associated with a change in mental state, from being in a state of drowsiness to increased wakefulness and increased brain activity.3 Rhemercise is a mindfulness technique to alleviate the symptoms of various types of stress.4 The objective of this study was to assess the efficacy of rhemercise as an adjunctive therapy in OUD patients undergoing detoxification.
METHODS
Study Design
This was a hospital-based, open-label, prospective, and exploratory study conducted between June 2018 and June 2019 with male inpatients admitted for OUD at Saket Hospital, Patiala, Punjab, India. A total of 197 psychiatric inpatients were screened for the study, of which only 147 fulfilled the eligibility criteria. Six of 147 patients were referred to a tertiary center due to various complications, eg, seizures (n = 2), delirium (n = 1), high-grade fever (n = 1), and aggression (n = 2). Fifteen patients (9 in the treatment as usual [pharmacotherapy; TAU] + rhemercise [R] group and 6 in the TAU only group) did not complete the full 4 weeks of treatment. A total of 126 patients completed the study. Written informed consent was obtained from all participants. Institute ethics committee clearance was obtained before the study was initiated. Subjects were not paid for participation in the study.
Inclusion and Exclusion Criteria
Patients aged 18–65 years with OUD diagnosed according to ICD-10 criteria were included in the study. Patients with other psychiatric disorders such as psychosis, delirium, intellectual disability, serious medical comorbidities, and any other substance use disorders (SUDs) (other than tobacco use disorder) were excluded. Participants were divided into 2 groups by using computer-generated random tables. Group A (n = 63) comprised patients receiving TAU + R, and group B (n = 63) received TAU only.
Patient Intervention
The following drugs were used in the TAU regimen: tramadol (50–400 mg/d), loperamide (2–6 mg/d), levocetirizine (10–30 mg/d), clonazepam (0.5–1.5 mg/d), ibuprofen (400–2,400 mg/d), clonidine (0.1–0.4 mg/d), pantoprazole (20–80 mg/d), and multivitamins dispensed as needed. No active intervention was done to induce withdrawal. Patients were admitted to the hospital under withdrawal, or we waited until the effects of the last intake of opioid had worn off and withdrawal symptoms were severe enough to start treatment. Baseline assessments were conducted before starting treatment at the time of admission when the patient was suffering from withdrawal. Follow-up assessments were carried out at 2 weeks and 4 weeks. Rhemercise was initiated 48 hours after admission. Rhemercise was carried out daily at the Saket Deaddiction Center in group sessions with a maximum of 30 patients per session. Each session lasted an hour, usually between 10 am and 11 am, and patients were taught and supervised by a team of 3 trained counselors. Videos and roleplay were used to train patients in the technique. A self-rated 2-item questionnaire was developed and distributed among patients for feedback regarding understanding and ability to perform rhemercise independently. Both the items were rated from 1 to 10. It took 10 days for users to become adequately proficient in the technique. If any patient found it difficult to learn, individual sessions were conducted until the patient became proficient. Patients were advised to practice rhemercise 1 hour daily under supervision of a trained counselor, and the sessions were intended to be carried out throughout the patient’s stay. Sixty-three patients became proficient in the technique, completed a minimum of 30 sessions, and were included in the final analysis.
Rhemercise Technique
Rhemercise uses a simple hand fan on which a clock face is pasted with numerical hours from 1 to 12 and a big black dot in the center.
First breath. As one breathes out, one smiles and speaks “12 o’clock, 2 o’clock,” and so on with the nose pointing to these numbers rotating clockwise, while the eyes are fixed on the center black dot. As one breathes in, the nose is pointed to the numbers 10 o’clock, 8 o’clock, and so on and rotates anticlockwise as one reflects, muses, and meditates the same numbers. The facial expression as one breathes in is one of “awe and wonder,” similar to one yawning with the mouth closed.
Second breath. The nose swings as a pendulum from the right shoulder to the left shoulder with the nose pointing to 3 o’clock, 4 o’clock, 5 o’clock, and so on until 9 o’clock is reached, as one breathes out, smiles, and speaks the numbers aloud. As one breathes in, the nose swings from the left shoulder to the right shoulder, while one reflects, muses, and meditates the same words. The facial expression as one breathes in is one of awe and wonder similar to one yawning with the mouth closed.
The numbers are replaced by positive valence words such as “love now” said repeatedly as one rotates the nose clockwise while breathing out. As one breathes in, the same words are reflected, mused, and meditated on as one rotates the nose counterclockwise. Other positive valence words that may be used are “joy now,” “peace now,” “calm now,” “rest now,” and “relax now.”
Rhemercise also employs a biofeedback method of feeling one’s own pulse in both the wrists. Clasping the hands, the left thumb feels the pulse on the right wrist, while the right thumb sticks up in front of the eyes, acting as the center of an imaginary clock face. The patient then rotates his/her nose on the virtual clock face around the thumb. One breathes in with a facial expression of awe and wonder and thinking “calm, cool, quiet, and serene now” with 6 pulse counts. One breathes out with the expression of a progressive smile and audible self-talk repeating “calm, cool, quiet, and serene now” with 6 pulse counts. Thus, one calms down in 1 breath lasting less than 15 seconds.
Tools for Data Collection
A semistructured proforma was used to gather the sociodemographic and clinical history of the patients including age, marital status, education level, employment status, living arrangement, type of opioid used, other substances used, route of administration of opioids, and family history of OUD.
Clinical Opiate Withdrawal Scale
The Clinical Opiate Withdrawal Scale (COWS)5 is an 11-item scale with a total score of 48 designed to be administered by a clinician. The COWS has satisfactory validity, reliability (interrater and test-retest), sensitivity, and specificity indices. Scores are categorized as 5–12 (mild withdrawal syndrome), 13–24 (moderate withdrawal syndrome), 25–36 (moderate to severe withdrawal syndrome), and > 36 (severe withdrawal syndrome).
Brief Pain Inventory
The Brief Pain Inventory (BPI)6 is a short, self-administered questionnaire. The BPI provides a pain severity score and pain interference in daily activities score. Test-retest reliability has been assessed for malignant pain and shows good reliability for pain intensity (r = 0.8) and pain interference (r = 0.8). Internal consistency of the BPI is high for the severity scale (0.81 < α < 0.89) and interference scale (0.88 < α < 0.95).
Subjective Well-Being Inventory
The Subjective Well-Being Inventory (SUBI)7 measures feeling of well-being as experienced by an individual or a group of individuals in various day-to-day life concerns. It consists of 40 items with a score range of 0–3 under 11 factors. Minimum and maximum scores are 40 and 120, respectively. The higher the score, the better the well-being.
Statistical Analysis
Frequency, mean, mean percentage, and standard deviation were calculated. A comparison of variables between the groups was performed using the independent t test for numeric variables and χ2 test for categorical data. The observations were statistically analyzed using Statistical Package for Social Sciences software version 25.0 for Windows. Paired t test, unpaired t test, and χ2 tests were used wherever applicable. P values < .05 and < .01 were considered significant and highly significant, respectively.
RESULTS
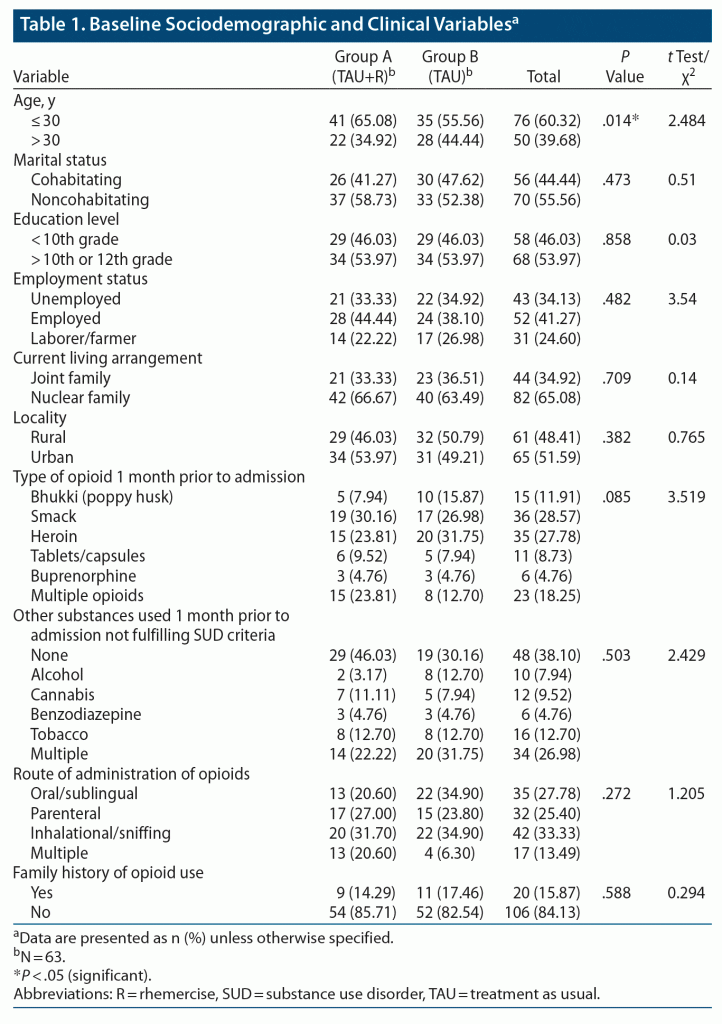
Most patients in both groups were < 30 years of age, single, and belonged to nuclear families; 34 (54%) patients in both groups had education above 10th grade. Also, 21 (33%) and 22 (35%) patients in groups A and B, respectively, were currently unemployed; 34 (54%) patients in group A were residing in an urban area, whereas only 31 (49%) in group B lived in an urban area. Smack was the most used opioid, with inhalation being the most preferred route of administration. These findings are summarized in Table 1.
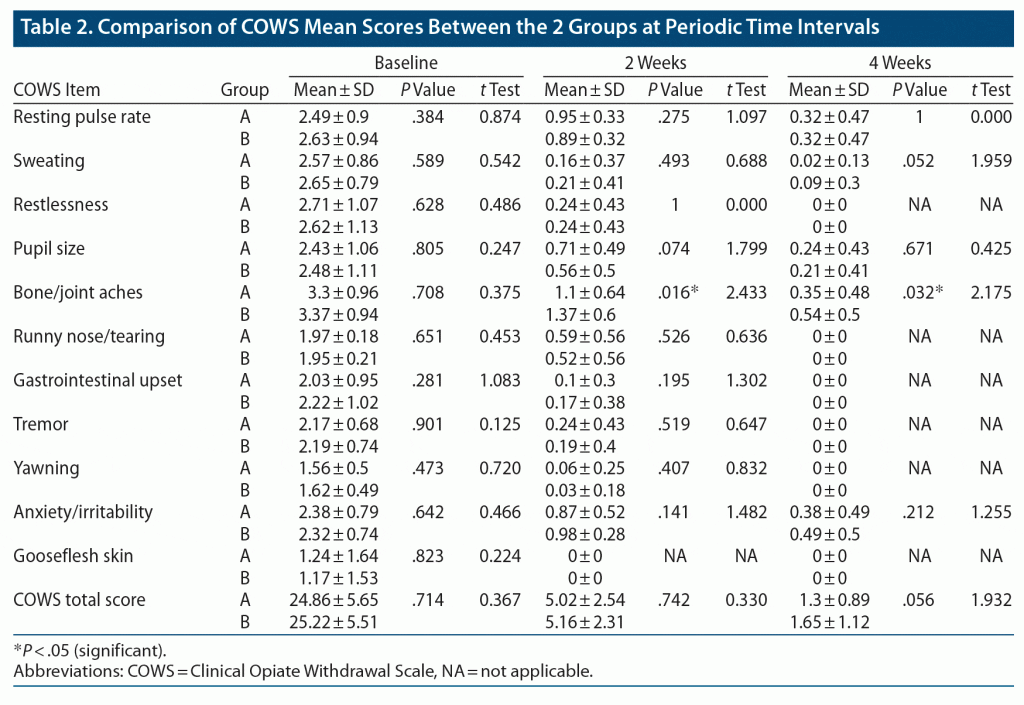
Table 2 shows significant reduction in the severity of opioid withdrawal on all items and total mean ± SD COWS scores in groups A and B from baseline to 2 weeks and from 2 to 4 weeks. No statistically significant difference was seen between the 2 groups on the items or total scores of the COWS at baseline, 2 weeks, or 4 weeks except for the item “bone and joint aches,” which showed 19% and 35% lower scores in group A compared to group B at 2 weeks and 4 weeks, respectively. At 2 weeks, the mean ± SD bone and joint aches COWS score of group A (1.1 ± 0.64) was significantly lower compared to group B (1.37 ± 0.60, P = .016, t = 2.433). Also, at 4 weeks, the mean ± SD bone and joint aches score of group A (0.35 ± 0.48) was significantly lower compared to group B (0.54 ± 0.50, P = .032, t = 2.175).
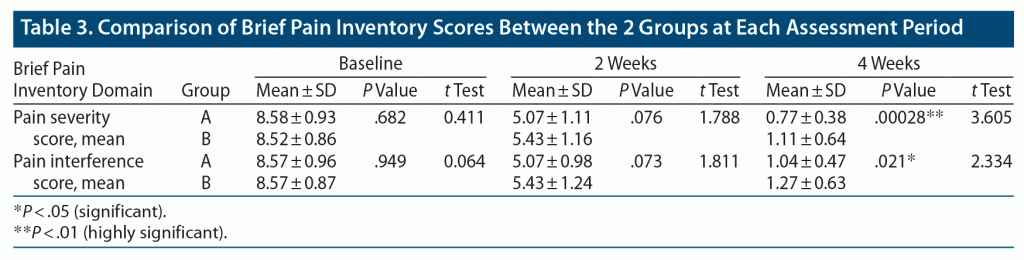
Table 3 shows that in both groups, significant reductions in pain severity and interference in daily activities scores were found on the BPI at follow-up at 2 and 4 weeks compared to baseline. There was no statistically significant difference in pain severity and interference scores between the groups at baseline or at 2-week follow-up assessment. However, at 4 weeks, a 31% and 18% significantly greater reduction in pain severity and interference score, respectively, was seen in group A compared to group B. The mean ± SD pain severity score in group A (0.77 ± 0.38) was lower than that of group B (1.11 ± 0.64, P = .00028, t = 3.605), and the mean ± SD pain interference score in group A (1.04 ± 0.47) was significantly lower than that of group B (1.27 ± 0.63, P = .021, t = 2.334) at 4 weeks postintervention.
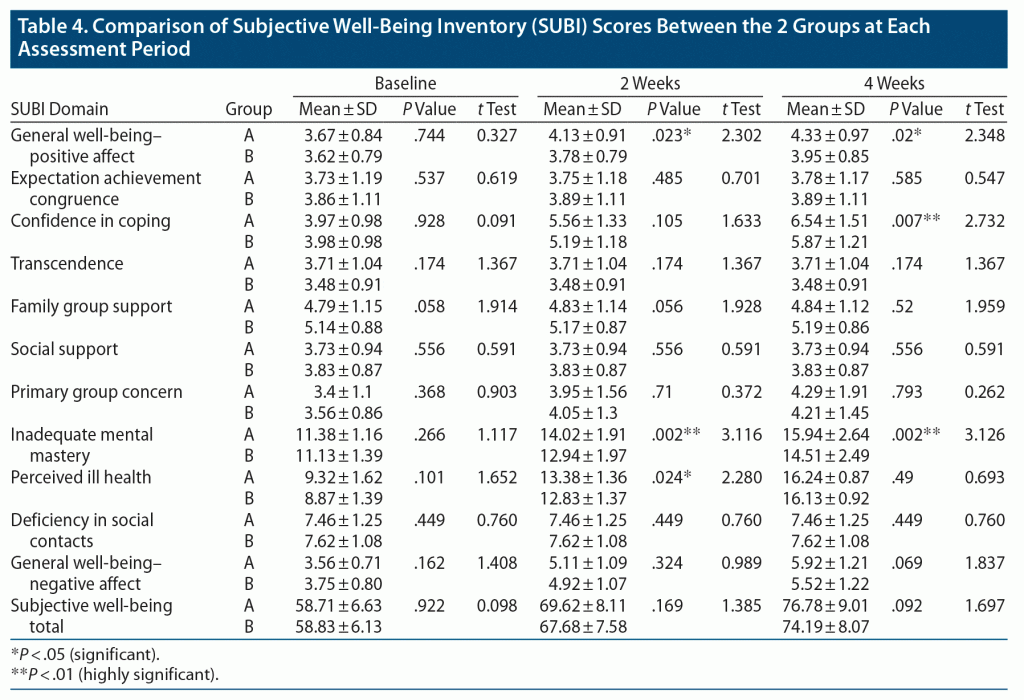
Table 4 shows that there were significant improvements in general well-being–positive affect, confidence in coping, primary group concern, inadequate mental mastery, perceived ill health, and general well-being–negative affect as well as in total SUBI score from baseline to 2 weeks and 4 weeks in both groups. The rest of the domains showed no significant improvement. At baseline, there was no significant difference between the groups in any domain or in general well-being. At 2-week follow-up, the mean ± SD score of general well-being–positive affect was 9% significantly higher in group A (4.13 ± 0.91) compared to group B (3.78 ± 0.79, P = .023, t = 2.302). The mean ± SD score in the domain of inadequate mental mastery in group A was 14.02 ± 1.91, which was 8% significantly greater compared to group B (12.94 ± 1.97, P = .002, t = 3.116). A 4% significantly greater score was seen in group A in the domain of perceived ill health when the mean ± SD score of 13.38 ± 1.36 in group A was compared with the score of 12.83 ± 1.37 in group B (P = .024, t = 2.280). At 4-week assessment, the mean ± SD score of general well-being–positive affect was significantly higher in group A (4.33 ± 0.97), indicating a 10% greater score compared to group B (3.95 ± 0.85, P = .02, t = 2.348). The mean ± SD score of confidence in coping for group A was 6.54 ± 1.51, which was 11% higher compared to group B (5.87 ± 1.21, P = .007, t = 2.732). A 10% significantly greater score was seen in group A in the domain of inadequate mental mastery when the mean ± SD score of 15.94 ± 2.64 in group A was compared with the score of 14.51 ± 2.49 in group B (P = .002, t = 3.126).
DISCUSSION
Substantial alleviation of the severity of opioid withdrawal symptoms was seen in both groups (group A: TAU + R and group B: TAU), but a statistically significant difference between groups was seen only in the bone and joint aches item of the COWS at 2- and 4-week follow-up. The score of perceived ill health that measured subjective feelings of anxiety, giddiness, pain, palpitations, and general well-being reflected a state at baseline. However, after rhemercise therapy, there was a significant reduction in all domains of the SUBI (general well-being–positive affect, confidence in coping, inadequate mental mastery). Significant reductions were seen in pain severity and interference in both groups. However, group A (TAU + R) showed a greater statistically significant reduction in pain severity and interference compared to group B at 4-week follow-up.
Mindfulness has been defined as paying attention in a particular way, on purpose, in the present moment, and nonjudgmentally.8 The prefrontal cortex (PFC) plays an important role in craving (preoccupation). The PFC modulates the relationship between brain-derived neurotrophic factor (BDNF) and the autonomous nervous system (ANS). Emerging evidence suggests that lower BDNF levels and poor PFC function modulate the ANS so as to maintain physiologic homeostasis in the central nervous system.9 Serum levels of BDNF have also been associated with craving in OUD.10 A positive correlation also exists between serum and cortical BDNF levels.11 Increased BDNF mRNA is associated with increased histone acetylation,12 and global cellular DNA methylation is linked to opioid tolerance.13 Studies have shown that early life stress can also influence the prevalence of substance-induced mood disorder.14 People with active OUD display abnormal basal and induced levels of adrenocorticotropic hormone and cortisol, which become normalized during methadone treatment.15 It has also been shown that mice with an overexpression of corticotropin-releasing factor (CRF) are more easily sensitized to opioid administration and have worse withdrawal symptoms in comparison to wild mice. This upregulation is mainly due to the maladaptation of CRF receptors 1 and 2,16 implying that a similar disposition toward opioids caused by CRF receptor abnormalities could occur in humans as well.
MBI alleviates anxiety, possibly through manipulation of autonomic variables that reduce sympathetic activity and increase vagal tone.17 Top-down control is crucial in opposing the urges associated with cravings and is dependent on proper functioning of the PFC.1 Psychological disturbances, such as depression and anxiety, are very strong factors leading to relapse in SUD, and a poorly functioning PFC and low BDNF are associated with serotonergic and ANS compensation related to the pathologic onset of depression and anxiety disorders.9 MBI training may alleviate chronic pain by enhancing nonreactivity toward distressing thoughts and emotions. MBI promotes a shift from affective to sensory processing of pain sensations.7 Mindfulness-oriented recovery enhancement (MORE) has therapeutic effects on ecological momentary assessment indices of pain mediated by 2 processes, (1) disengaging from negative emotional appraisals of somatic sensations and (2) reorientation of attention to interoceptive experience, and thus has less affective bias.18
Rhemercise is a form of alternative mindfulness-based therapy that includes humming, whistling, singing, or a combination of our favorite tunes as we move and dance during our activities of daily living.19 MBIs are known to enhance cognitive control capacities, are inversely associated with substance use and cravings, and are positively associated with the ability to disengage attention, facilitate pain attenuation, and increase the ability to return to more positive mental states, thereby improving overall subjective well-being.20,21 Thus, the space between stimulus and response might be developed through practices such as MBI.
Rhemercise helps patients shift their attention from pain by repetitively focusing on and vocalizing positive thoughts and emotions. The relaxing effect of rhemercise could be due to the deep, slow abdominal breathing exercise known as rhemercise yoha.4 It could modulate the ANS (decreases sympathetic tone and increases parasympathetic activity), which further decreases the heart rate by increasing baroreflex sensitivity and thus improves cardiovascular function by causing a widespread vasodilatation. This physiologic change can improve blood circulation in tissues, increase exercise tolerance, and provide improvement in the feeling of personal well-being.22,23
There is only 1 other similar study,24 which was conducted in China, that assessed the effectiveness of qigong therapy (a mindfulness-based therapy) in detoxification for chronic OUD patients over a 10-day intervention period. However, it was of short duration, and the effects on individual opioid withdrawal symptoms were not evaluated.25 Other studies26,27 have assessed the role of MORE in reducing chronic body aches as well as opioid cravings among habitual opioid abusers.
In a previous study25 conducted to understand the mechanisms by which mindfulness attenuates pain, of a total of 34 participants, 17 practiced mindfulness and 17 were placed in a control group. None of the subjects had any psychological illness. Transcutaneous electrical stimuli of moderate intensity were administered, and brain images were acquired using 1.5-T symphony scanner. Visual rating scales were used to rate the stimulus intensity, unpleasantness, and anticipatory anxiety. Results showed that mindfulness reduced pain and anticipatory anxiety by 22% and 29%, respectively, which is similar to the findings of our study. The neural mechanism was an increase in activation of anterior cingulate cortex, ventromedial PFC, and posterior insula but a decrease in activation of the lateral PFC.25 Moreover, MBI increases activation in the anterior cingulate cortex, anterior insula (nociceptive pain regulation), and orbitofrontal cortex, while causing deactivation in the thalamic region for reducing unpleasant pain.26
The baseline subjective well-being scores on the SUBI in the present study were lower compared to those of the general Indian population. In a cross-sectional survey conducted in 881 Australian injection drug users using the Personal Well-Being Index consisting of 7 domains,28 injection drug users scored lower in all domains and in overall mean well-being compared to the general population. In contrast, our study showed improvement in 6 of 11 domains as well as overall SUBI scores. However, similar to the Australian study,28 all domains of personal satisfaction on the SUBI showed improvement, whereas domains pertaining to social satisfaction did not show statistically significant improvement.
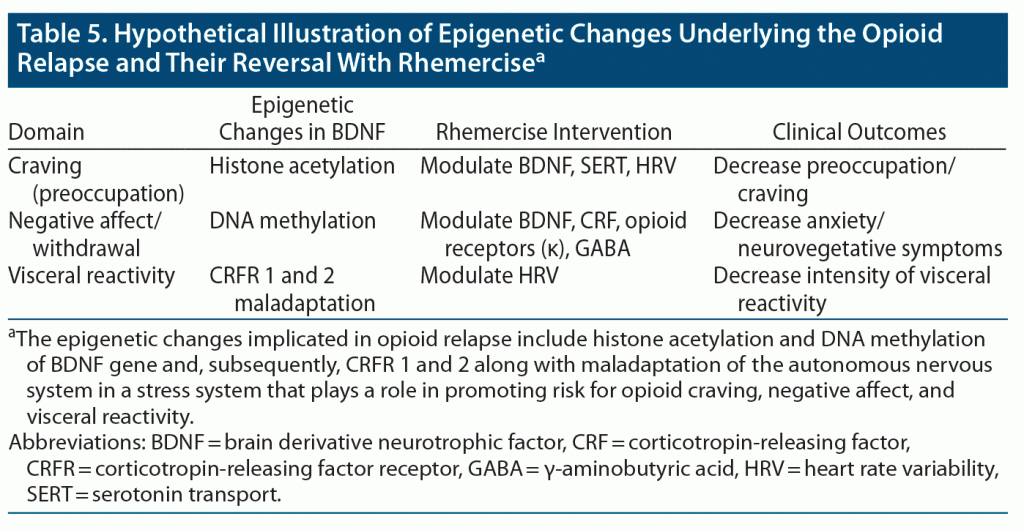
Another study29 reported a significantly greater improvement in momentary pain and positive affect in opioid-treated chronic pain patients participating in MORE compared to those in a support group. Additionally, positive affect regulation was more likely to occur in patients participating in MORE (odds ratio = 2.75) than in support group patients.29 Treatment with MBI + TAU has a positive affect regulation and develops a capacity to shift/maintain affect from moment to moment.30 Our study also showed improvement in general well-being–positive affect and decreases in opioid withdrawal symptoms by achieving feelings of joy, mastery of control, and improved confidence. Expectation achievement congruence was also reported. We hypothesize that rhemercise could modulate BDNF activity in the nucleus accumbens, PFC, and amygdala. Through this process, histone acetylation and DNA methylation could be regulated by rhemercise (Table 5).
A strength of the present study is that we were able to investigate the role of MBI in the detoxification phase of opioid withdrawal. Another strength is the large sample size (N = 126). A limitation is that the study comprised male inpatients only, so the effects of rhemercise cannot be extrapolated to women and the general population. This study was conducted in OUD patients undergoing detoxification. Some confounding factors, which include (1) duration of opioid use, (2) past abstinent attempts, and (3) family support, were missed during data collection. Furthermore, we did not include the data of dropout patients in the final analysis.
CONCLUSION
This is the first study, to our knowledge, to assess the efficacy of MBI in reducing opioid withdrawal symptoms and pain and in improving subjective well-being in the detoxification phase of OUD. Longer follow-up studies are required to assess the effect of rhemercise in rehabilitation and relapse prevention including among women.
Submitted: July 2, 2021; accepted September 17, 2021.
Published online: February 10, 2022.
Author contributions: Dr Kundal designed the study, performed the analysis (verified by Drs Raj and Garg and Mr Paul), and prepared the first draft of the manuscript. Dr Zachariah coined the term rhemercise and developed a novel procedure. All authors edited the manuscript with intellectual contribution and approved the final version of the manuscript.
Potential conflicts of interest: None.
Funding/support: None.
Previous presentation: This material was presented virtually at the 75th Annual Scientific Meeting of the Society of Biological Psychiatry; April 30–May 2, 2020; New York, New York.
Acknowledgments: The authors thank Parminder Kaur, MPhil, Project Director, Red Cross Integrated Rehabilitation Centre for Addict IRCA, Saket Hospital, Patiala, Punjab, India) for her assistance with the study.
References (30)

- Volkow ND, Baler RD. Addiction science: uncovering neurobiological complexity. Neuropharmacology. 2014;76(Pt B(0 0):235–249. PubMed CrossRef
- Kraft TL, Pressman SD. Grin and bear it: the influence of manipulated facial expression on the stress response. Psychol Sci. 2012;23(11):1372–1378. PubMed CrossRef
- Gallup AC, Eldakar OT. The thermoregulatory theory of yawning: what we know from over 5 years of research. Front Neurosci. 2013;6:188. PubMed
- Varghese S, Varghese S, Chackochan A, et al. 652. novel complementary and alternative mindfulness-based intervention: therapeutic approach to manage substance use disorders and posttraumatic stress disorder. Biol Psychiatry. 2017;81(10):S264. CrossRef
- Wesson DR, Ling W. The Clinical Opiate Withdrawal Scale (COWS). J Psychoactive Drugs. 2003;35(2):253–259. PubMed CrossRef
- Poquet N, Lin C. The Brief Pain Inventory (BPI). J Physiother. 2016;62(1):52. PubMed CrossRef
- World Health Organization. Regional Office for South-East, A. Assessment of Subjective Well-Being, the Subjective Well-Being Inventory (SUBI). New Delhi: WHO Regional Office for South-East Asia; 1992.
- Varghese SP, Koola MM, Eiger RI, et al. Opioid use remits, depression remains. Curr Psychiatr. 2014;13(18):45–50. PubMed
- Chang WH, Lee IH, Chi MH, et al. Prefrontal cortex modulates the correlations between brain-derived neurotrophic factor level, serotonin, and the autonomic nervous system. Sci Rep. 2018;8(1):2558. PubMed CrossRef
- Heberlein A, Dürsteler-MacFarland KM, Lenz B, et al. Serum levels of BDNF are associated with craving in opiate-dependent patients. J Psychopharmacol. 2011;25(11):1480–1484. PubMed CrossRef
- Karege F, Schwald M, Cisse M. Postnatal developmental profile of brain-derived neurotrophic factor in rat brain and platelets. Neurosci Lett. 2002;328(3):261–264. PubMed CrossRef
- Schmidt HD, Sangrey GR, Darnell SB, et al. Increased brain-derived neurotrophic factor (BDNF) expression in the ventral tegmental area during cocaine abstinence is associated with increased histone acetylation at BDNF exon I-containing promoters. J Neurochem. 2012;120(2):202–209. PubMed CrossRef
- Trivedi MS, Deth R. Redox-based epigenetic status in drug addiction: a potential contributor to gene priming and a mechanistic rationale for metabolic intervention. Front Neurosci. 2015;8:444. PubMed
- Koola MM, Qualls C, Kelly DL, et al. Prevalence of childhood physical and sexual abuse in veterans with psychiatric diagnoses. J Nerv Ment Dis. 2013;201(4):348–352. PubMed CrossRef
- Kreek MJ, Ragunath J, Plevy S, et al. ACTH, cortisol and beta-endorphin response to metyrapone testing during chronic methadone maintenance treatment in humans. Neuropeptides. 1984;5(1–3):277–278. PubMed CrossRef
- Varghese SP, Montalvo-Ortiz JL, Csernansky JG, et al. Early life stress as a risk factor for substance use disorders: clinical and neurobiological substrates. Indian J Psychol Med. 2015;37(1):36–41. PubMed CrossRef
- Upadhyay Dhungel K, Malhotra V, Sarkar D, et al. Effect of alternate nostril breathing exercise on cardiorespiratory functions. Nepal Med Coll J. 2008;10(1):25–27. PubMed
- Garland EL, Manusov EG, Froeliger B, et al. Mindfulness-oriented recovery enhancement for chronic pain and prescription opioid misuse: results from an early-stage randomized controlled trial. J Consult Clin Psychol. 2014;82(3):448–459. PubMed CrossRef
- Zachariah AR. Let’s Move Mindfully. Illinois: Compassion Now Network for All International; 2017.
- Garland EL, Boettiger CA, Gaylord S, et al. Mindfulness is inversely associated with alcohol attentional bias among recovering alcohol-dependent adults. Cognit Ther Res. 2012;36(5):441–450. PubMed CrossRef
- Garland EL, Roberts-Lewis A, Kelley K, et al. Cognitive and affective mechanisms linking trait mindfulness to craving among individuals in addiction recovery. Subst Use Misuse. 2014;49(5):525–535. PubMed CrossRef
- Joseph CN, Porta C, Casucci G, et al. Slow breathing improves arterial baroreflex sensitivity and decreases blood pressure in essential hypertension. Hypertension. 2005;46(4):714–718. PubMed CrossRef
- Pal GK, Velkumary S, Madanmohan. Effect of short-term practice of breathing exercises on autonomic functions in normal human volunteers. Indian J Med Res. 2004;120(2):115–121. PubMed
- Li M, Chen K, Mo Z. Use of qigong therapy in the detoxification of heroin addicts. Altern Ther Health Med. 2002;8(1):50–54, 56–59. PubMed
- Gard T, Hölzel BK, Sack AT, et al. Pain attenuation through mindfulness is associated with decreased cognitive control and increased sensory processing in the brain. Cereb Cortex. 2012;22(11):2692–2702. PubMed CrossRef
- Zeidan F, Martucci KT, Kraft RA, et al. Brain mechanisms supporting the modulation of pain by mindfulness meditation. J Neurosci. 2011;31(14):5540–5548. PubMed CrossRef
- Garland EL. Pain processing in the human nervous system: a selective review of nociceptive and biobehavioral pathways. Prim Care. 2012;39(3):561–571. PubMed CrossRef
- Dietze P, Stoové M, Miller P, et al. The self-reported personal wellbeing of a sample of Australian injecting drug users. Addiction. 2010;105(12):2141–2148. PubMed CrossRef
- Garland EL, Bryan CJ, Finan PH, et al. Pain, hedonic regulation, and opioid misuse: modulation of momentary experience by mindfulness-oriented recovery enhancement in opioid-treated chronic pain patients. Drug Alcohol Depend. 2017;173(suppl 1):S65–S72. PubMed CrossRef
- Keune PM, Forintos DP. Mindfulness meditation: a preliminary study on meditation practice during everyday life activities and its association with well-being. J Psychological Topics. 2010;19(2):373–386.
Enjoy this premium PDF as part of your membership benefits!