Valproic acid stands out as the most widely prescribed antiepileptic drug worldwide, demonstrating its versatility in the treatment of various conditions beyond epilepsy.1 In addition to effectively managing seizures, valproic acid is valuable in treating bipolar disorder, schizoaffective disorder, social phobias, and neuropathic pain. While patients may experience common side effects such as gastrointestinal disturbances, sialoadenitis, tremors, weight gain, symptoms of encephalopathy, platelet disorders, pancreatitis, liver toxicity, and teratogenic effect,2 these challenges can often be managed with appropriate medical guidance. Notably, there are a limited number of documented cases in the literature detailing mucocutaneous side effects from valproic acid,3,4 indicating that further investigation and reporting in this area could enhance our understanding of its side effects. A PubMed search through March 2025 with the keyword search term ‘sodium valproate’ OR ‘valproic acid’ AND hypermelanosis revealed only 3 documented case reports.3–5 We present 2 cases of sodium valproate and valproic acid–induced hypermelanosis, one in a young woman and another in a young man. The case summaries are provided in Table 1.
Case 1
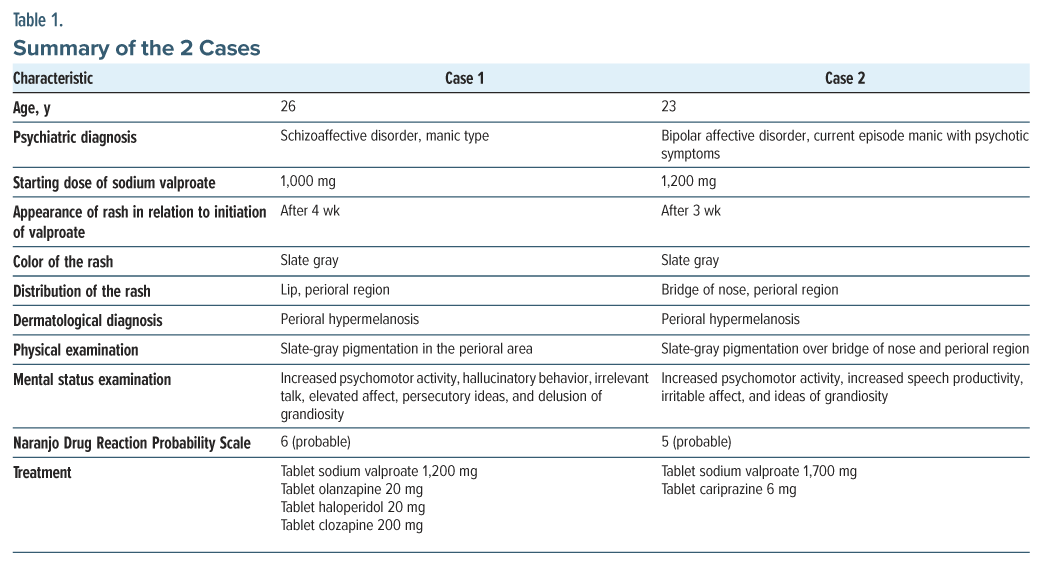
A 26-year-old woman presented to the outpatient department with complaints of aggression without provocation, abusive behavior, increased talk, wandering behavior, disturbed sleep, poor self-care, and smiling to self for the past 4 years. On serial mental status examination, she was found to have increased psychomotor activity, hallucinatory behavior, irrelevant talk, elevated affect, persecutory ideas, and delusion of grandiosity. A diagnosis of schizoaffective disorder, manic type was made according to the International Classification of Diseases, Tenth Revision (ICD-10). She was prescribed olanzapine tablets as an antipsychotic agent and sodium valproate tablets as a mood stabilizer. Due to an inadequate response to olanzapine and haloperidol, she was started on clozapine. Four weeks after starting sodium valproate, hyperpigmentation appeared on her lips and perioral region. Notably, she was not taking any other medications that could have contributed to the hyperpigmentation of the skin or mucosa. A physical examination confirmed slate-gray pigmentation in the perioral area, with no infiltration on her lips (Figure 1). A dermatological referral was promptly made, and she was diagnosed with perioral hypermelanosis. Laboratory testing included liver function test, complete blood cell count, serum urea, and creatinine, which were within physiological range. Her Naranjo Drug Reaction Probability Scale6 score was 6, indicating probable causality.
Case 2
A 23-year-old man from an urban background presented to the outpatient department with chief complaints of increased psychomotor activity, irritability, aggressiveness, increased talk, tall claims, and decreased need for sleep for 2 months. Inpatient management was advised. The mental status examination revealed increased psychomotor activity, increased speech productivity, irritable affect, and ideas of grandiosity. A diagnosis of bipolar affective disorder, manic type was made according to ICD-10 criteria.
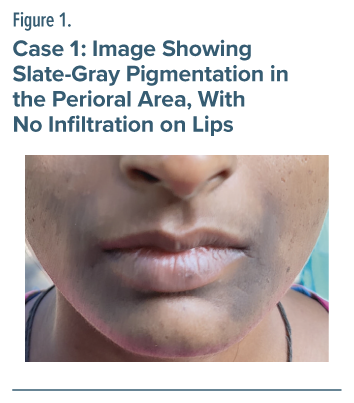
He was started on tablet sodium valproate 1,200 mg as a mood-stabilizing agent and tablet cariprazine. Three weeks after sodium valproate was initiated, he developed hyperpigmentation on his lips and perioral region. He was not taking concomitant medications that could cause skin or mucosa hyperpigmentation. The physical examination showed slate-gray pigmentation of the perioral region with infiltration on his lips (Figure 2). A dermatological referral was made, and he was diagnosed with perioral hypermelanosis. Laboratory testing included liver function test, complete blood cell count, serum urea, and creatinine, which were within physiological range. His Naranjo Drug Reaction Probability Scale6 score was 5, indicating probable causality.
Discussion
Drug-induced hyperpigmentation often occurs as post-inflammatory changes in response to a resolving drug-induced rash.7 Cutaneous hyperpigmentation can arise from various factors, including physiological changes, genetic disorders of the skin, inflammatory conditions, hormonal imbalances, medications, benign and malignant tumors, and nonmelanotic conditions. Drug-induced hyperpigmentation is common, often occurring after the resolution of drug-induced rashes or fixed-drug eruption.4 It can also result from direct stimulation of melanin production, deposition of iron following vessel damage, or the accumulation of a drug (or its metabolites) within the skin. Various classes of drugs are associated with pigmentation changes in the skin or mucous membranes. These include nonsteroidal anti-inflammatory drugs, antimalarials, amiodarone, antineoplastic agents, tetracyclines, heavy metals, clofazimine, oral contraceptives, and psychotropic drugs. Additionally, anticonvulsants such as hydantoin, phenytoin, and barbiturates are known to cause hyperpigmentation. Some antihypertensive medications, including diltiazem, telmisartan, and amlodipine, have also been reported to induce skin hyperpigmentation.8
Article Information
Published Online: September 30, 2025. https://doi.org/10.4088/PCC.25cr03987
© 2025 Physicians Postgraduate Press, Inc.
Prim Care Companion CNS Disord 2025;27(5):25cr03987
Submitted: April 21, 2025; accepted June 13, 2025.
To Cite: Sarkar M, Kamila AS, Mukherjee A, et al. Two cases of sodium valproate and valproic acid–induced hypermelanosis. Prim Care Companion CNS Disord. 2025;27(5):25cr03987.
Author Affiliations: Central Institute of Psychiatry, Ranchi, India (all authors).
Corresponding Author: Sourav Khanra, MD, Central Institute of Psychiatry, Ranchi, 834006, India ([email protected]).
Funding/Support: None.
Relevant Financial Relationships: None.
Patient Consent: Consent was received from the patient to publish the case report and images, and information has been de-identified to protect anonymity.
ORCID: Sourav Khanra: https://orcid.org/0000-0002-8684-6773
References (8)

- Rahman M, Awosika AO, Nguyen H. Valproic Acid. [Updated 2024 Mar 19]. In: StatPearls [Internet]. StatPearls Publishing; 2025. https://www.ncbi.nlm.nih.gov/books/NBK559112/. Accessed 21 March 2025.
- Boland RJ, Verduin ML, eds. Kaplan & Sadock’s Synopsis of Psychiatry. 12th ed. Wolters Kluwer; 2020.
- Giménez-García R, Carrasco-Molina S, Zambrano-Centeno B. Val-proic acid-induced hyperpigmentation. J Craniofac Surg. 2017;28(2):e127–e129. PubMed
- Gondak RO, da Silva-Jorge R, Jorge J, et al. Oral pigmented lesions: clinicopathologic features and review of the literature. Med Oral Patol Oral Cir Bucal. 2012;17(6):e919–e924. PubMed CrossRef
- Elwadhi A, Neha KC, Gupta D, et al. Sodium valproate-induced hyperpigmentation. J Pediatr. 2023;263:113660. PubMed CrossRef
- Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239–245. PubMed CrossRef
- Dereure O. Drug-induced skin pigmentation: epidemiology, diagnosis and treatment. Am J Clin Dermatol. 2001;2(4):253–262. PubMed CrossRef
- Lerner EA, Sober AJ. Chemical and pharmacologic agents that cause hyperpigmentation or hypopigmentation of the skin. Dermatol Clin. 1988;6(2):327–337. PubMed CrossRef
Enjoy this premium PDF as part of your membership benefits!