Objective: The evidence linking schizophrenia and total and unconjugated bilirubin is primarily from retrospective studies. To overcome this limitation, we conducted a prospective study of total and unconjugated bilirubin levels of patients diagnosed with schizophrenia or bipolar affective disorder.
Methods: Serum total and unconjugated bilirubin levels were compared between patients diagnosed with schizophrenia (n = 50) and bipolar affective disorder (n = 43) (ICD-10 criteria) admitted to an inpatient psychiatric unit of a tertiary hospital in India. The study was conducted between October 2013 and July 2015.
Results: The median serum levels (mg/dL) of total and unconjugated bilirubin were significantly higher (P = .027 and P = .004, respectively) among patients with schizophrenia compared to those with bipolar affective disorder. Analysis of covariance revealed that unconjugated bilirubin was significantly higher (P = .029) in patients with schizophrenia compared to those with bipolar affective disorder, even after controlling for the effects of age, sex, and medications.
Conclusions: In this prospective study, serum levels of unconjugated bilirubin were significantly higher in patients with schizophrenia compared to patients with bipolar affective disorder. The findings suggest that serum unconjugated bilirubin could be a potential marker for schizophrenia. However, the results need to be replicated in a larger sample including patients living in the community.
Elevated Unconjugated Bilirubin in Schizophrenia Compared to Bipolar Affective Disorder
ABSTRACT
Objective: The evidence linking schizophrenia and total and unconjugated bilirubin is primarily from retrospective studies. To overcome this limitation, we conducted a prospective study of total and unconjugated bilirubin levels of patients diagnosed with schizophrenia or bipolar affective disorder.
Methods: Serum total and unconjugated bilirubin levels were compared between patients diagnosed with schizophrenia (n = 50) and bipolar affective disorder (n = 43) (ICD-10 criteria) admitted to an inpatient psychiatric unit of a tertiary hospital in India. The study was conducted between October 2013 and July 2015.
Results: The median serum levels (mg/dL) of total and unconjugated bilirubin were significantly higher (P = .027 and P = .004, respectively) among patients with schizophrenia compared to those with bipolar affective disorder. Analysis of covariance revealed that unconjugated bilirubin was significantly higher (P = .029) in patients with schizophrenia compared to those with bipolar affective disorder, even after controlling for the effects of age, sex, and medications.
Conclusions: In this prospective study, serum levels of unconjugated bilirubin were significantly higher in patients with schizophrenia compared to patients with bipolar affective disorder. The findings suggest that serum unconjugated bilirubin could be a potential marker for schizophrenia. However, the results need to be replicated in a larger sample including patients living in the community.
Prim Care Companion CNS Disord 2019;21(4):19m02448
To cite: Pradeep JR, Acharya MS, Radhakrishnan R, et al. Elevated unconjugated bilirubin in schizophrenia compared to bipolar affective disorder. Prim Care Companion CNS Disord. 2019;21(4):19m02448.
To share: 10.4088/PCC.19m02448
© Copyright 2019 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry, St John’s Medical College Hospital, Bengaluru, India
bDepartment of Psychiatry, Yale University School of Medicine, New Haven, Connecticut
cDivision of Mental Health and Neuroscience, St John’s Research Institute, Bengaluru, India
‘ ¡Joint senior authorship.
*Corresponding author: Krishnamachari Srinivasan, MD, Department of Psychiatry, 1st floor, St John’s Medical College Hospital, Sarjapur Rd, Bengaluru 560034, India ([email protected]).
Higher levels of unconjugated bilirubin have been reported in patients with acute psychotic episodes, and the association is strong in patients with positive psychotic symptoms.1 An increased prevalence of schizophrenia has been reported in patients with idiopathic unconjugated hyperbilirubinemia (Gilbert’s syndrome)2 and in subjects with elevated levels of unconjugated bilirubin in the neonatal period compared to the general population.3 A retrospective study4 of medical records noted that total and unconjugated bilirubin levels were significantly elevated in patients with a diagnosis of schizophrenia compared to those with bipolar disorder. Similarly, another study1 based on medical record review found that the mean levels of unconjugated bilirubin were higher in patients with an acute psychotic episode of schizophrenia and schizoaffective disorders compared to the bipolar disorder group and controls. The presence of idiopathic unconjugated hyperbilirubinemia in patients with schizophrenia confers a poor outcome and is accompanied by neuroimaging findings that include wider frontotemporal sulci, interhemispheric fissure, and lateral ventricular sizes on computed tomography brain scan; increased signal intensity in various areas on flair magnetic resonance imaging brain scan; and reduced metabolism in most areas on 1H-magnetic resonance spectroscopy brain scan.5-9
Some limitations of previous studies1,4 that examined the association between unconjugated bilirubin and schizophrenia include a retrospective design utilizing medical records and a relatively small sample size. To overcome these limitations, we used a prospective design.
The primary objective of this study was to compare the serum unconjugated bilirubin levels of patients diagnosed with schizophrenia with those of patients diagnosed with bipolar affective disorder. We also examined the association of serum levels of unconjugated bilirubin and severity of psychopathology in patients diagnosed with schizophrenia.
METHODS
In this prospective, cross-sectional study, patients diagnosed with schizophrenia or bipolar affective disorder admitted to an inpatient setting of the psychiatry department in a tertiary care hospital were invited to participate in the study after written informed consent was obtained. The study was conducted between October 2013 and July 2015 and was approved by the Institutional Ethics Review Board at St John’s Medical College, Bengaluru, India.
All patients aged 18-65 years diagnosed with schizophrenia or bipolar affective disorder (based on ICD-10 criteria) on the Mini-International Neuropsychiatric Interview10 were included in the study. Patients with substance dependence disorder and with liver disorders due to any other medical etiology were excluded from the study. Patients were interviewed during 2 sessions on 2 consecutive days, with each session lasting about 30 to 45 minutes. Patients were interviewed with the Family Interview for Genetic Studies11 to obtain family history of psychiatric disorders, and the severity rating of psychotic symptoms was assessed with the Brief Psychiatric Rating Scale (BPRS).12 Bipolar affective disorder patients with mania were assessed with the Young Mania Rating Scale (YMRS),13 and those with depression were assessed with the 21-item Hamilton Depression Rating Scale (HDRS-21).14 Socio-occupational functioning was assessed using the Social Occupational Functioning Scale (SOFS).15
In the morning between 6 am and 7 am, we obtained 5 mL of fasting blood samples from the patients’ antecubital veins using aseptic precautions in a vacutainer. Blood samples were collected before starting the medications in patients who were drug naive and within 2 to 3 days after admission in those who were on medication. Total protein level was assessed using the modified Biuret method, albumin with bromocresol purple, total and conjugated bilirubin with the modified Jendrassik Grof method, aspartate aminotransferase with the International Federation of Clinical Chemistry (IFCC) with pyridoxal-5‘ ²-phosphate (P5P) method, alanine aminotransferase with the modified IFCC with P5P method, alkaline phosphatase with the modified Bowers-McComb method, and γ-glutamyl transferase with the IFCC-recommended method. The serum unconjugated bilirubin level was calculated by subtracting the conjugated bilirubin level from the total bilirubin levels.
Statistical Analysis
Descriptive statistics were reported using frequencies, percentages, means (standard deviations), and medians (interquartile ranges, 25-75). The independent sample t test and Mann Whitney U test were used to assess the difference between schizophrenia and bipolar disorder based on the data distribution. The χ2 test was used to test the differences in the categorical variables between the schizophrenia and bipolar disorder groups. Analysis of covariance (ANCOVA) was used to study the association of liver function test parameters between patients with schizophrenia and bipolar disorder after controlling for the effects of age, sex, and medications. The P value was set at < 5% for statistical significance. Statistical analysis was carried out using SPSS version 16.0 (IBM Corporation, Armonk, New York).
RESULTS
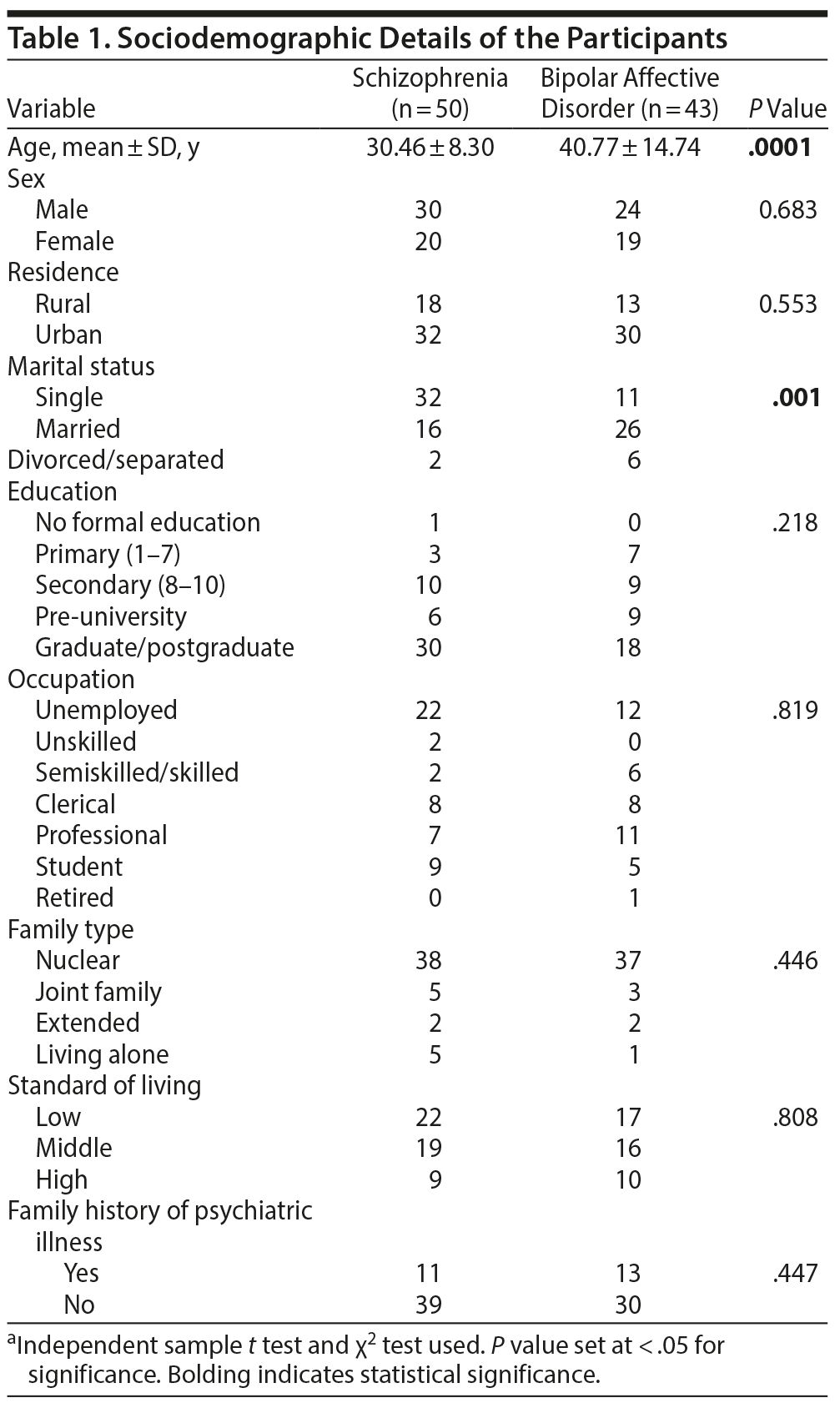
Among the 93 patients recruited in the study, 50 had a diagnosis of schizophrenia and 43 had bipolar affective disorder based on ICD-10 criteria. The mean age was significantly higher in patients diagnosed with bipolar affective disorder (40.77 ± 14.74 years) compared to patients with a diagnosis of schizophrenia (30.46 ± 8.30 years) (P < .0001). The majority of participants were men (58.1%) and from an urban background (66.7%). There were significantly more unmarried patients in the schizophrenia group (n = 32, 34.40%) compared to the bipolar disorder group (n = 11, P = .001). There were no significant differences between the 2 groups of patients in terms of residence, educational status, family type, occupational status, and socioeconomic strata (Table 1).
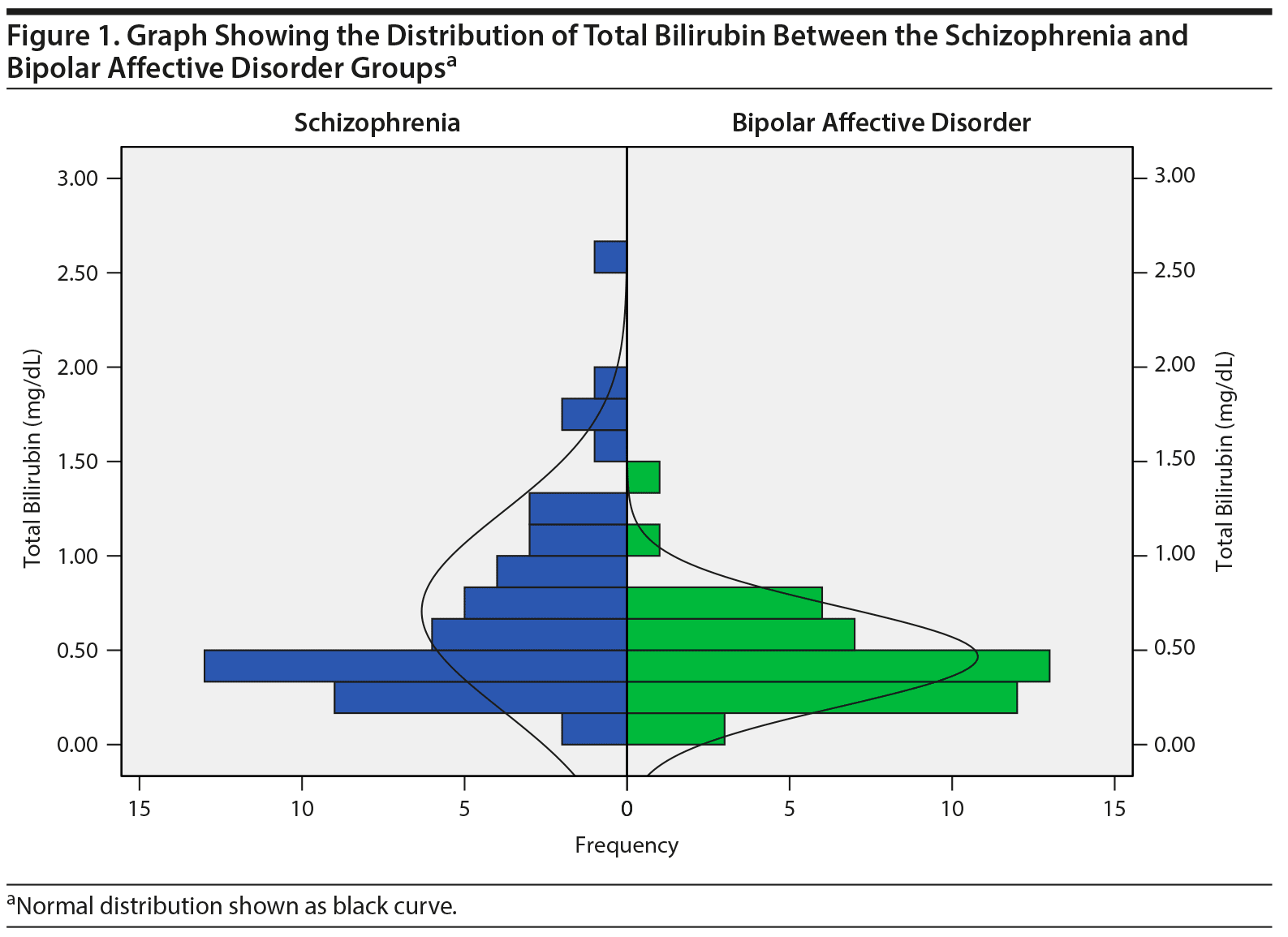
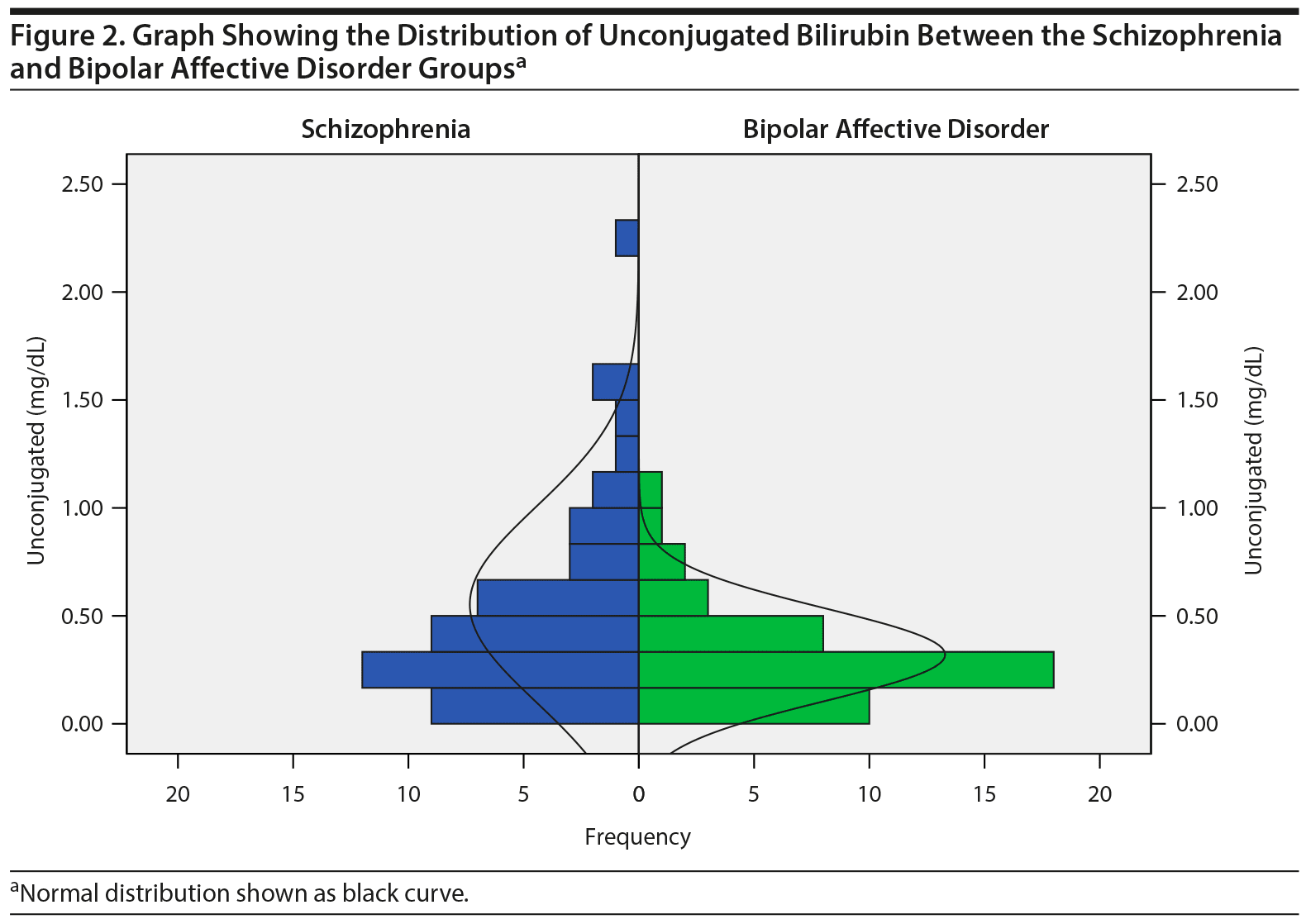
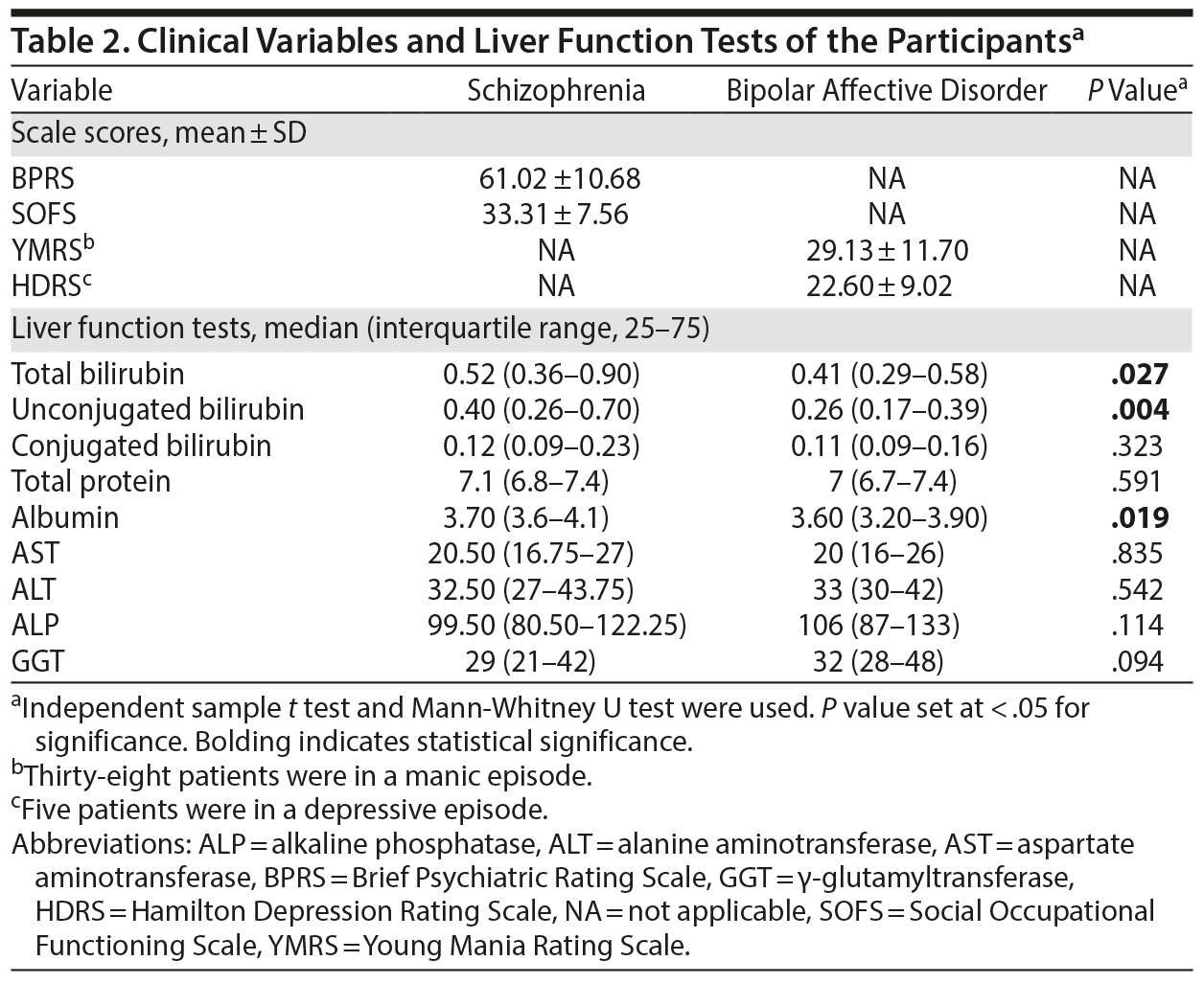
The mean ± SD BPRS score of the schizophrenia group was 61.02 ± 10.68, suggesting that they were markedly ill, and the SOFS score was 33.31 ± 7.56, suggesting an inability to function in almost all areas of social and occupational functioning. Of 43 bipolar patients, 38 were in a manic episode and 5 were in a depressive episode. The mean ± SD YMRS score of those in a manic episode was 29.13 ± 11.70, and the mean ± SD HDRS-21 score of those in a depressive episode was 22.60 ± 9.02, which was suggestive of severe depression. Of 93 patients, 15 (schizophrenia = 6, bipolar affective disorder = 9) were on medications at the time of admission. The total and unconjugated bilirubin levels (in mg/dL) were not normally distributed based on the Kolmogorov-Smirnov test; hence, the data are presented as median and interquartile ranges. The distribution of the serum values of total and unconjugated bilirubin between the 2 diagnostic groups is represented in Figures 1 and 2. The median serum levels of total and unconjugated bilirubin (in mg/dL) were significantly higher among patients diagnosed with schizophrenia compared to those with bipolar affective disorder (total = 0.52 [0.36-0.90] vs 0.41 [0.29-0.58], P = .027; unconjugated = 0.40 [0.26-0.70] vs 0.26 [0.17-0.39], P = .004). The median serum albumin levels were also higher in patients with schizophrenia compared to those with bipolar affective disorder (3.70 [3.6-4.1] vs 3.60 [3.20-3.90], P = .019) (Table 2). None of the patients had total bilirubin values > 1.2 mg/dL, which is suggestive of Gilbert’s syndrome.
We further analyzed the effect of age, sex, and medications on total and unconjugated bilirubin levels between the groups. The total and unconjugated values were log transformed since they were not distributed normally. ANCOVA was conducted to compare total and unconjugated levels between the 2 groups, while controlling for the effects of age, sex, and medications. We found no significant difference in the mean total bilirubin levels (F1,88 = 3.371, P = .070) between the groups; however, the mean serum unconjugated levels continued to be significantly higher in patients with schizophrenia compared to those with bipolar affective disorder (F1,88 = 4.900, P = .029).
In patients diagnosed with schizophrenia (n = 50), we found no association between serum unconjugated bilirubin levels and severity of psychopathology, as measured on the BPRS, or socio-occupational dysfunction.
DISCUSSION
In this prospective study, we found significantly higher levels of unconjugated bilirubin in patients diagnosed with schizophrenia compared to bipolar affective disorder, and this difference was independent of age, sex, or medication. Findings from the studies on the association between bilirubin and schizophrenia have yielded discrepant findings. While several studies have found an association between schizophrenia and increased serum unconjugated bilirubin levels,1,2,4,8 others have found decreased total bilirubin levels16 or no difference.17 Most of the earlier investigations on the association between unconjugated bilirubin and schizophrenia were retrospective in nature and did not control for the effects of antipsychotic medications1,4 or were conducted on small sample sizes.9
Some of the important confounders that may influence total, unconjugated, and conjugated bilirubin levels include fasting status, body mass index, smoking and alcohol intake (these induce UGT [uridine 5‘ ²-diphospho-glucuronosyltransferase]), increased red blood cell membrane fragility (which may be intrinsic to schizophrenia and thus may result in hemolyzed samples), comorbid drug or alcohol use, medication-induced changes, sex (with increased total and unconjugated bilirubin levels in men compared to women), and oxidative stress.2,8,9 In the present study, after controlling for age, sex, and medication use, serum levels of unconjugated bilirubin continued to be elevated in patients with schizophrenia compared to those with bipolar disorder.

- In this prospective study, unconjugated bilirubin was significantly higher in patients with schizophrenia compared to patients with bipolar affective disorder after controlling for the effects of age, sex, and medications.
- Unconjugated bilirubin may be a potential peripheral marker for schizophrenia.
- The findings of elevated levels of unconjugated bilirubin in patients with schizophrenia should be replicated using larger patient samples that include community-dwelling patients with schizophrenia.
Some earlier studies noted an association between bilirubin levels and severity of psychosis. Widschwendter et al18 conducted a retrospective data review of total bilirubin levels serially assessed at baseline (N = 52), 2 weeks (n = 40), 4 weeks (n = 46), and 12 weeks (n = 30) in patients with schizophrenia on monotherapy with a new-generation antipsychotic. A significant correlation was observed between total bilirubin levels with PANSS positive (r = 0.371, P = .007) and excitement (r = 0.322, P = .020) subscores at baseline. The total bilirubin level also correlated with PANSS positive subscore changes from baseline to weeks 2, 4, and 12.18 In another study by Pae et al,16 among patients with a diagnosis of schizophrenia, total bilirubin levels were found to be significantly lower in a subgroup of patients with negative symptoms. We found no association between levels of total or unconjugated bilirubin and severity of psychotic symptoms. This lack of association in the present study could be because all participants were from a psychiatric facility and severely ill. Future studies examining the association between unconjugated bilirubin and severity of psychosis must include community-dwelling patients with varying degrees of illness severity.
One of the proposed mechanisms of the role of unconjugated bilirubin in schizophrenia includes glial cell injury, which leads to glutamate secretion and may trigger an inflammatory response.3 This response, in turn, could lead to release of proinflammatory cytokines, which may influence gliogenesis, neurogenesis, and deterioration of neuronal development in the form of decrease in the dendrite extension and ramification, leading to deficits in learning and memory, especially if this exposure occurs early in life. This exposure could result in an increased risk for later development of psychiatric illnesses.19 Therefore, effects of unconjugated bilirubin on glutamate metabolism dysregulation, overexpression of tumor necrosis factor-α, and interleukin-1β may have a role in the neuropathology of schizophrenia.3
Limitations of the study include that it was conducted with severely ill patients admitted to a psychiatric inpatient facility. Also, we did not assess the body mass index and smoking status of participants, which could influence the levels of unconjugated bilirubin.
Our findings of elevated unconjugated bilirubin levels in patients with a diagnosis of schizophrenia are preliminary in nature based on severely ill patients admitted to a psychiatric facility. These findings need to be replicated in a larger patient sample including community-dwelling residents.
Submitted: February 23, 2019; accepted April 24, 2019.
Published online: July 25, 2019.
Potential conflicts of interest: None.
Funding/support: None.
Acknowledgments: The authors thank Sumithra Selvan, MSc, from the St John’s Research Institute for assisting in the statistical analysis and Anitha Devanath, MD, from St John’s Medical College Hospital for providing technical support in the biochemical tests. Mrs Selvan and Dr Devanath have no conflicts of interest to declare.
REFERENCES
1. Gama-Marques J, Tinoco I, Pedro I, et al. Unconjugated bilirubin and acute schizophrenia: a probable correlation? Actas Esp Psiquiatr. 2017;45(2):79-88. PubMed
2. Miyaoka T, Seno H, Itoga M, et al. Schizophrenia-associated idiopathic unconjugated hyperbilirubinemia (Gilbert’s syndrome). J Clin Psychiatry. 2000;61(11):868-871. PubMed CrossRef
3. Brites D, Fernandes A, Falc×£o AS, et al. Biological risks for neurological abnormalities associated with hyperbilirubinemia. J Perinatol. 2009;29(suppl 1):S8-S13. PubMed CrossRef
4. Radhakrishnan R, Kanigere M, Menon J, et al. Association between unconjugated bilirubin and schizophrenia. Psychiatry Res. 2011;189(3):480-482. PubMed CrossRef
5. Miyaoka T. Clinical features of schizophrenia associated with idiopathic unconjugated hyperbilirubinemia (Gilbert’s syndrome). Int J Psychiatry Clin Pract. 2003;7(3):199-203. CrossRef
6. Miyaoka T, Seno H, Itoga M, et al. Structural brain changes in schizophrenia associated with idiopathic unconjugated hyperbilirubinemia (Gilbert’s syndrome): a planimetric CT study. Schizophr Res. 2001;52(3):291-293. PubMed CrossRef
7. Miyaoka T, Yasukawa R, Mihara T, et al. Fluid-attenuated inversion-recovery MR imaging in schizophrenia-associated with idiopathic unconjugated hyperbilirubinemia (Gilbert’s syndrome). Eur Psychiatry. 2005;20(4):327-331. PubMed CrossRef
8. Miyaoka T, Yasukawa R, Mizuno S, et al. Proton magnetic resonance spectroscopy (1H-MRS) of hippocampus, basal ganglia, and vermis of cerebellum in schizophrenia associated with idiopathic unconjugated hyperbilirubinemia (Gilbert’s syndrome). J Psychiatr Res. 2005;39(1):29-34. PubMed CrossRef
9. Yasukawa R, Miyaoka T, Mizuno S, et al. Proton magnetic resonance spectroscopy of the anterior cingulate gyrus, insular cortex and thalamus in schizophrenia associated with idiopathic unconjugated hyperbilirubinemia (Gilbert’s syndrome). J Psychiatry Neurosci. 2005;30(6):416-422. PubMed
10. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22-33, quiz 34-57. PubMed
11. Maxwell ME. Manual for the FIGS. Bethesda, MD: Clinical Neurogenetics Branch, National Institute of Mental Health; 1992.
12. Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10(3):799-812. CrossRef
13. Young RC, Biggs JT, Ziegler VE, et al. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133(5):429-435. PubMed CrossRef
14. Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6(4):278-296. PubMed CrossRef
15. Saraswat N, Rao K, Subbakrishna DK, et al. The Social Occupational Functioning Scale (SOFS): a brief measure of functional status in persons with schizophrenia. Schizophr Res. 2006;81(2-3):301-309. PubMed CrossRef
16. Pae CU, Paik IH, Lee C, et al. Decreased plasma antioxidants in schizophrenia. Neuropsychobiology. 2004;50(1):54-56. PubMed CrossRef
17. Bach DR, Kindler J, Strik WK. Elevated bilirubin in acute and transient psychotic disorder. Pharmacopsychiatry. 2010;43(1):12-16. PubMed CrossRef
18. Widschwendter CG, Rettenbacher MA, Kemmler G, et al. Bilirubin concentration correlates with positive symptoms in patients with schizophrenia. J Clin Psychiatry. 2016;77(4):512-516. PubMed CrossRef
19. Falc×£o AS, Silva RF, Pancadas S, et al. Apoptosis and impairment of neurite network by short exposure of immature rat cortical neurons to unconjugated bilirubin increase with cell differentiation and are additionally enhanced by an inflammatory stimulus. J Neurosci Res. 2007;85(6):1229-1239. PubMed CrossRef
Enjoy this premium PDF as part of your membership benefits!