Antipsychotic Adherence and Its Correlation to Health Outcomes for Chronic Comorbid Conditions

ABSTRACT
Objective: To examine the relationship between adherence to antipsychotics and adherence to medications for cardiometabolic conditions (diabetes, hypertension, and hyperlipidemia) and subsequent health care utilization and expenditures in patients with schizophrenia and preexisting cardiometabolic conditions.
Method: Medstat MarketScan Medicaid databases from 2004 to 2008 were used to identify a retrospective cohort of schizophrenia patients (ICD-9-CM codes 295.1-295.3, 295.6, or 295.9) with preexisting cardiometabolic medication use who had initiated antipsychotic treatments. Patients who initiated a second-generation antipsychotic between July 1, 2004, and December 31, 2006, were identified as the new user cohort. Comorbid conditions were identified if patients had at least 1 inpatient or 2 outpatient claims for hypertension, hyperlipidemia, and/or diabetes (ICD-9-CM codes 401.xx to 405.xx inclusive, 272.xx inclusive, and 250.xx inclusive, respectively) and were using medication to manage these conditions prior to antipsychotic initiation. Adherence to cardiometabolic medications was compared between adherent and nonadherent patients taking antipsychotics using the proportion of days covered over 8 quarters categorized as phases of treatment to reflect initiation (1, days 1-90), continuation (2-4, days 91-360), and maintenance (5-8, days 361-520). Proportion of days covered values ≥ 0.80 were deemed adherent. Prior period antipsychotic adherence was used to predict cardiometabolic medication adherence, health care utilization, and expenditures using generalized estimating equations and negative binomial regressions.
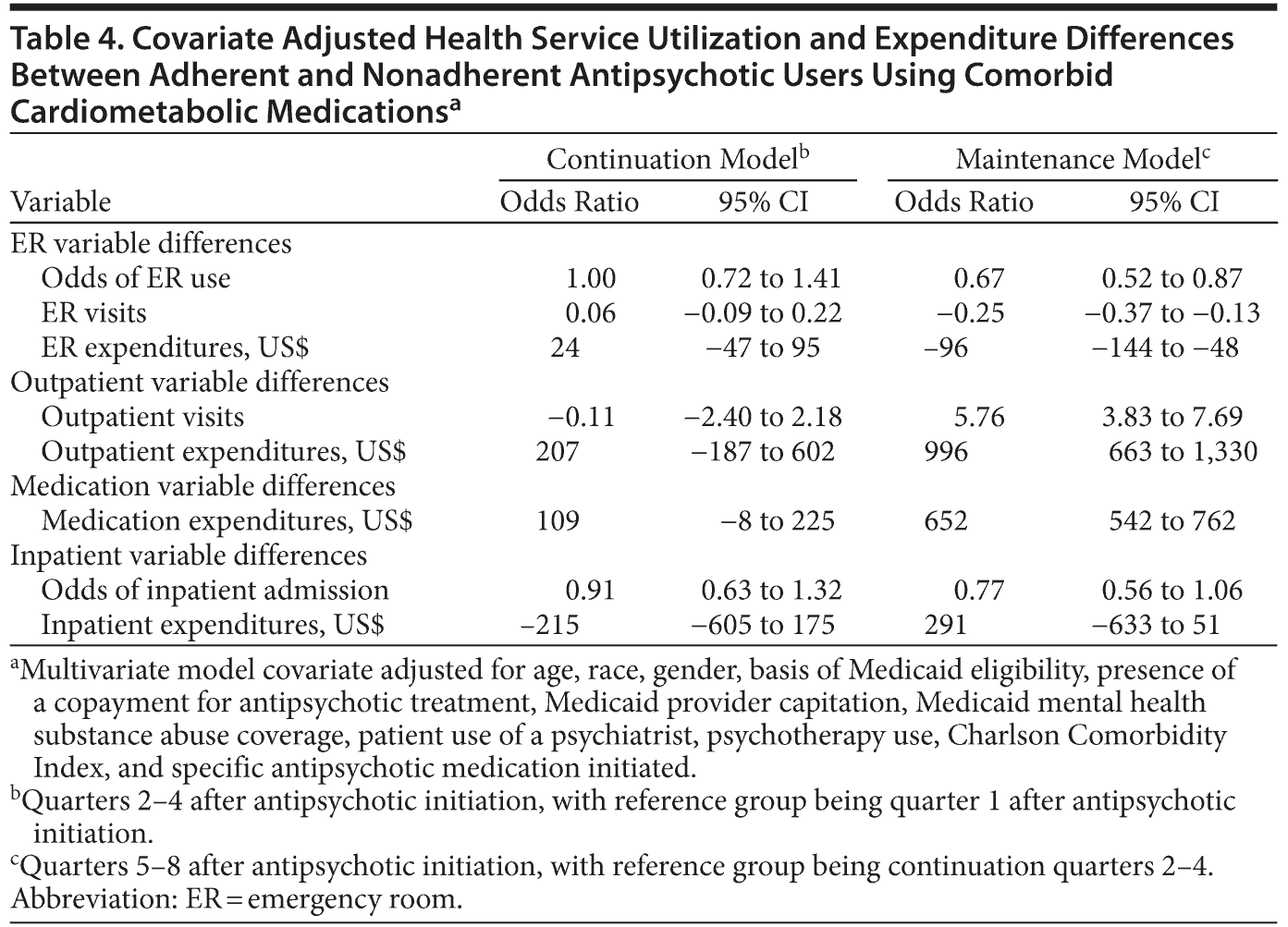
Results: The final population represented 1,006 patients. Antipsychotic adherence during continuation was a significant predictor of medication adherence for hypertension (odds ratio [OR] = 2.40, 95% CI = 1.67-3.44), diabetes (OR = 2.28, 95% CI = 1.43-3.67), and hyperlipidemia (OR = 2.16, 95% CI = 1.11-4.20) during maintenance. Antipsychotic adherence during continuation resulted in significantly lower emergency room use (OR = 0.67, 95% CI = 0.52-0.87), lower inpatient use (OR = 0.77, 95% CI = 0.56-1.06, not significant), and significantly higher outpatient ($996, 95% CI = $663-$1,330), medication ($652, 95% CI = $542-$762), and total health ($1,371, 95% CI = $490-$2,252) expenditures during maintenance.
Conclusion: Antipsychotic adherence was associated with better adherence to cardiometabolic medications and a potential reduction in emergency room and inpatient service utilization. Clinicians should consider adherence to both antipsychotic and cardiometabolic medications when caring for patients with schizophrenia and comorbid conditions.
Prim Care Companion CNS Disord 2012;14(3):doi:10.4088/PCC.11m01324
© Copyright 2012 Physicians Postgraduate Press, Inc.
Submitted: November 28, 2011; accepted February 9, 2012.
Published online: June 21, 2012.
Corresponding author: Joel F. Farley, PhD, UNC Eshelman School of Pharmacy, 2204 Kerr Hall, Campus Box 7573, Chapel Hill, NC 27599 ([email protected]).
Comorbid cardiometabolic health conditions such as diabetes, hypertension, and hyperlipidemia have been shown to be particularly burdensome to patients with schizophrenia.1-3 Despite the burden, studies have shown inadequate rates of treatment for these comorbid conditions. Among patients with confirmed diabetes, hypertension, and dyslipidemia entering the Clinical Antipsychotic Trials of Intervention Effectiveness study, treatment rates were 30.2%, 62.4%, and 88.0%, respectively.4 Low treatment rates may be partly due to inadequate screening. It is estimated that only 25% and 10% of patients initiating second-generation antipsychotics (SGAs) are screened for glucose and lipid abnormalities, respectively.5 However, even in patients who are screened and treated, treatment is only effective if patients remain adherent to therapy.
Studies have shown that fewer than 60% of patients who initiate antipsychotic medications adhere to treatment.6 However, few studies have examined adherence to medications for comorbid conditions in this population, and to our knowledge, no studies have examined the link between nonadherence to antipsychotics and nonadherence to medications for chronic comorbid conditions. The objective of this study was to examine patterns of adherence to both antipsychotics and medications for comorbid cardiometabolic health conditions in patients with schizophrenia newly initiated on antipsychotic treatment. Specifically, we examined the relationship between adherence to antipsychotics among patients with schizophrenia and adherence to medications for diabetes, hypertension, and hyperlipidemia in patients with preexisting comorbidity. In addition, we examined the relationship between adherence to antipsychotic medications and health service utilization and expenditures in patients with these comorbid health conditions.
METHOD
Design
A new user cohort study design was used to examine the relationship between adherence to newly initiated SGA medication and adherence to medications for 3 common comorbid cardiometabolic conditions (diabetes, hypertension, and hyperlipidemia) among patients with preexisting cardiometabolic drug use. This analysis of patients newly initiating antipsychotics enables us to identify unbiased impacts of nonadherence at early stages of treatment for patients with schizophrenia and comorbid cardiometabolic conditions. Specifically, it increases the temporal relationship between adherence to antipsychotic treatments and treatments for metabolic disorders. New users had no SGA claims during a 6-month period preceding SGA initiation. We also examined the relationship between antipsychotic adherence and subsequent all-cause health care utilization and expenditures. The analysis was designed to capture 3 periods of antipsychotic use on the basis of similar phases identified for antidepressant treatment5: (1) an initiation period comprising the first quarter of antipsychotic treatment (days 1-90), (2) a continuation period comprising quarters 2-4 (days 91-360), and (3) a maintenance period comprising quarters 5-8 (days 361-520). The index date that began the 8 quarters was the date of the first antipsychotic fill.
Data
Medstat MarketScan Medicaid databases from 2004 to 2008 were used. These databases contain pooled health care records for some 7 million Medicaid enrollees from multiple state Medicaid programs. Specific files included enrollment, prescription claims, inpatient admissions, inpatient facility records, and outpatient claims including physician visits. Emergency room (ER) encounters were derived from inpatient and outpatient files. Claims data were available for all patients with schizophrenia aged 18 years and older in any year.
Population
Patients initiating an SGA between July 1, 2004, and December 31, 2006, were identified as the new user cohort. Patients with at least 1 inpatient or 2 or more outpatient visits with ICD-9-CM codes 295.1-295.3, 295.6, or 295.9 during the 6-month preindex or 12-month postindex period were identified as having schizophrenia.7 Patients were excluded if they were dually enrolled in Medicare, were less than 18 years or older than 64 years of age, or were not continuously enrolled in Medicaid. Comorbid conditions were identified if patients had at least 1 inpatient or 2 outpatient claims for hypertension, hyperlipidemia, and/or diabetes with ICD-9-CM codes 401.xx to 405.xx inclusive, 272.xx inclusive, and 250.xx inclusive, respectively, and were using medication to manage these conditions prior to antipsychotic initiation. We also constructed a pooled cohort of patients with any of the 3 cardiometabolic conditions. This study was approved by the Western Institutional Review Board (Olympia, Washington).

- Patients with schizophrenia frequently have non-mental health comorbidities that should also be managed.
- Clinicians should monitor adherence to antipsychotic medications as well as medications for non-mental health conditions to improve health outcomes in patients with schizophrenia.
Outcomes
The primary dependent variables included medication adherence, health service utilization, and health expenditures. Adherence was measured as the proportion of days covered, which captured the number of days without gaps in medication coverage in each quarter over the number of days observed in each quarter and ranged from 0 to 1, with 0 representing no medication use and 1 representing perfect adherence.8 We adjusted the proportion of days covered to allow for patient switching and up to 30 days of medication carryover from the previous quarter. Patients were dichotomized as adherent or nonadherent using a proportion of days covered cutpoint of ≥ 0.80.9 Adherence for the pooled cardiometabolic cohort was calculated as an unweighted mean of the 3 cardiometabolic classes. Specific health service utilization outcomes included quarterly counts of all-cause outpatient visits and ER visits and a dichotomous measure of inpatient admission. Specific health expenditure outcomes included prescription, inpatient, outpatient, ER, and total quarterly payments.
Explanatory Variables
The explanatory variable of interest was adherence to newly initiated antipsychotics. We controlled for several demographic covariates that may influence a patient’s likelihood of receiving treatment and his/her health outcomes including age (18-34 years, 35-54 years, and 55 years and older), race (white non-Hispanic, black non-Hispanic, and other/missing), and male gender. We also corrected for basis of Medicaid eligibility, which could predict the need for health care resources, using a dichotomous variable to capture patients with eligibility on the basis of blindness or disability. Medicaid plan characteristics, which might influence a patient’s ability to obtain prescription and other health care services, were controlled for and included a dichotomous indicator for the presence of antipsychotic copayment, enrollment in a Medicaid plan with provider capitation, and state mental health substance abuse coverage. In addition, we controlled for the presence of baseline psychiatrist and psychotherapy use as potential proxy measures of schizophrenia severity. We calculated a Charlson Index score using ICD-9 coding for each patient as specified by Quan and colleagues10 to control for additional comorbidities. Finally, we constructed indicators for type of atypical antipsychotic initiated to examine the association of the specific treatment initiated on outcomes.
Statistical Analyses
In each of the 3 study intervals, we compared patient characteristics, adherence, health care utilization, and health expenditures of adherent and nonadherent patients via t tests for continuous variables and χ2 tests for categorical variables. To examine the relationship between adherence to antipsychotics and adherence to cardiometabolic medications, health care utilization, and health outcomes, we ran 2 separate regression models. Antipsychotic adherence was lagged in all regressions to reduce simultaneity bias. First, antipsychotic adherence in the first quarter of initiation was regressed against each outcome variable during quarters 2-4 of the continuation period. Second, antipsychotic adherence during quarters 2-4 of the continuation phase was regressed against each outcome variable during quarters 5-8 of the maintenance period. For the adherence outcomes, generalized estimating equations (GEEs) with a binomial distribution and logit link function were applied to account for the repeated measures on patients over multiple quarters.
To examine the relationship between lagged antipsychotic adherence and outpatient and ER visits, we estimated random effects negative binomial regression to account for the overdispersion of these outcomes. To examine the relationship between lagged antipsychotic adherence and outpatient, ER, medication, inpatient, and overall expenditures, we estimated GEEs that accounted for the proportion of nonusers and outcome distribution among users. Outpatient, medication, and overall expenditures were estimated using 1-part GEEs with γ distributions and log link functions to account for kurtosis. Inpatient and ER expenditures were estimated using 2-part GEEs to account for the high proportion of nonusers. For the first-part model that estimated the probability of being admitted or using the ER, we estimated GEEs with a binomial distribution and logit link function. For the second-part model that estimated the level of expenditures among users, we estimated GEEs with γ distributions and log link functions.
Each adjusted regression included previously defined demographic characteristics, Medicaid eligibility status, Medicaid plan characteristics, psychiatrist use, psychotherapy use, comorbidity, type of antipsychotic initiated, and the number of cohorts a patient falls in for patients with hypertension, hyperlipidemia, and/or diabetes. In addition, to control for the association of lagged antipsychotic adherence on adherence to medications for cardiometabolic indications during the period of interest, we included mean adherence to antipsychotic medication during the prior period in the adjusted adherence models. From these regressions, we estimated predicted differences in adherence, utilization, or expenditures using the method of recycled predictions for all 1-part models. For the 2-part models for inpatient and ER expenditures, we generated 95% confidence intervals via 1,000 bootstrapped iterations.
RESULTS
Analytic Sample
We identified 24,435 patients who were newly initiated on an atypical antipsychotic. Of these, 9,805 had no schizophrenia diagnosis 6 months preceding or 12 months following antipsychotic initiation. We eliminated 5,515 patients dually enrolled in Medicare; 130 patients younger than 18 years or older than 64 years; and 5,401 patients not continuously eligible for Medicaid benefits. Of the remaining 3,584 patients, we excluded 2,578 patients with no diagnosis of the 3 comorbid conditions of interest who were not previously using medication to manage these comorbid conditions. The final population represented 1,006 patients with schizophrenia who were newly initiated on antipsychotic treatments and who also used cardiometabolic medication.
Unadjusted Patient Characteristics
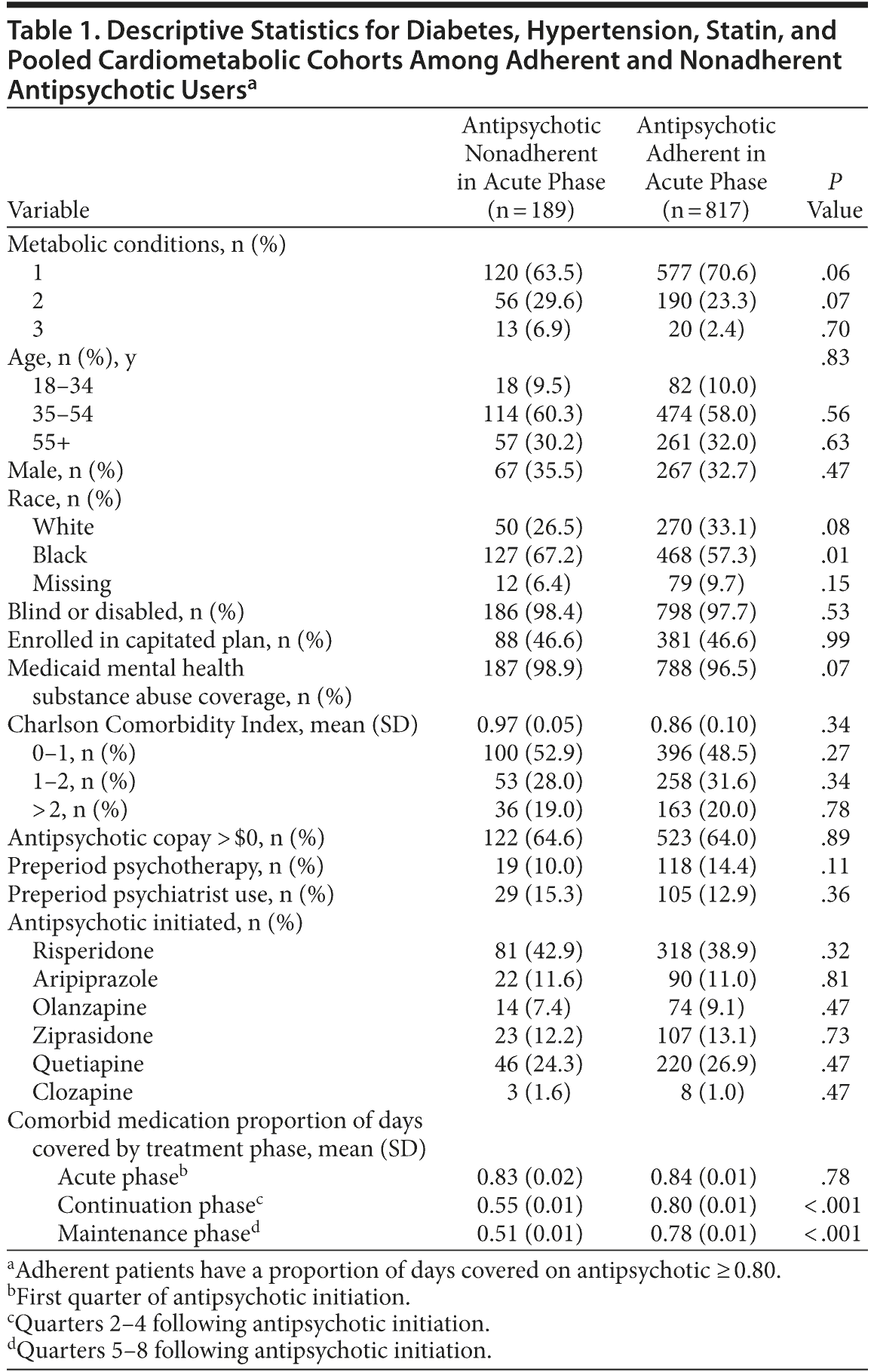
Of the 1,006 patients initiating SGAs, 817 were categorized as antipsychotic adherent (as categorized by a proportion of days covered ≥ 80%) and 189 were nonadherent during the first 90 days of treatment (Table 1). During the first quarter of treatment, adherent patients were similar in many respects to nonadherent patients. Nonadherent patients were statistically more likely to report their race as black compared to adherent patients (67.2% vs 57.3%, respectively). Unadjusted cardiometabolic medication adherence appeared similar between patients who were adherent and nonadherent to antipsychotics during the acute phase (0.84 vs 0.83, respectively). However, nonadherent SGA users were less likely than adherent SGA users to be adherent to comorbid medications during the continuation (0.55 vs 0.80, respectively, P < .001) and maintenance (0.51 vs 0.78, respectively, P < .001) phases.
Unadjusted Medication Adherence
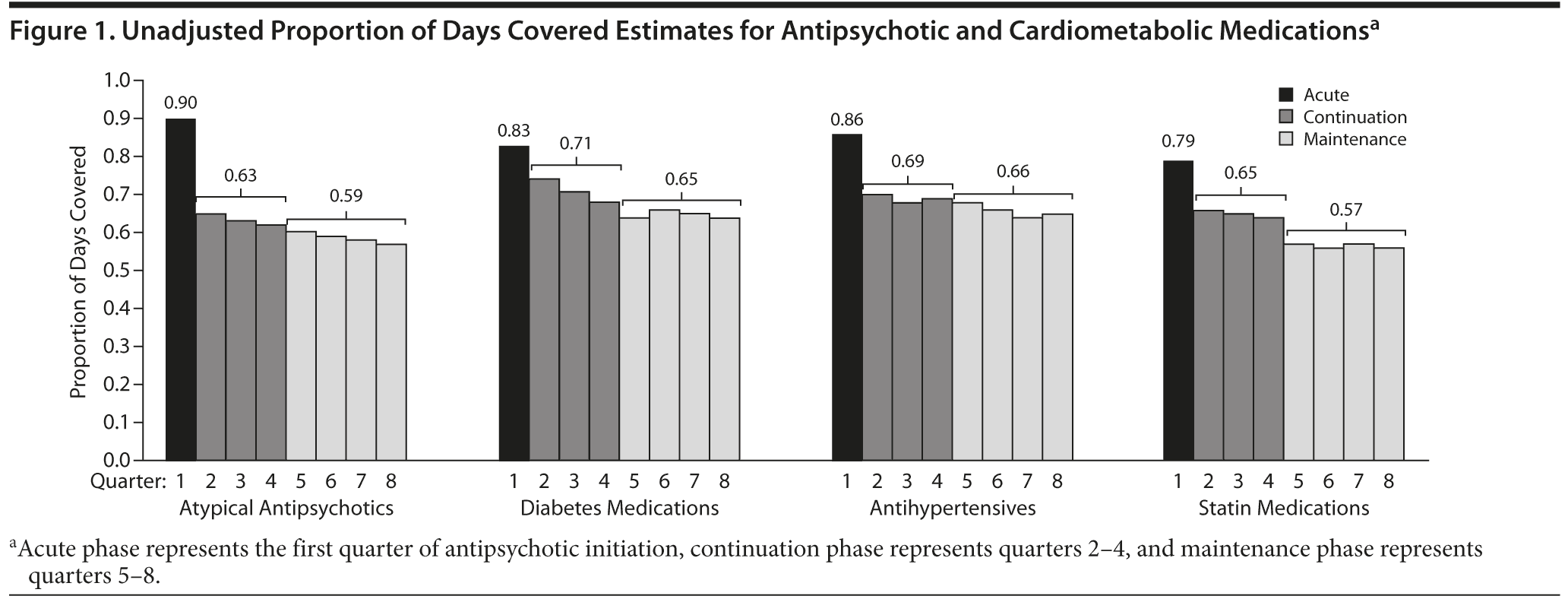
Figure 1 describes patterns of adherence for antipsychotics and cardiometabolic medications across each cohort. Antipsychotic adherence among patients newly initiating treatment during the first quarter averaged 0.90 and subsequently declined to 0.63 and 0.59 during continuation and maintenance phases, respectively. Among each of the 3 cardiometabolic cohorts, similar patterns of adherence were noted. During the acute phase of antipsychotic treatment, mean proportion of days covered values ranged from 0.86 for antihypertensives to 0.83 and 0.79 for diabetes medications and statins, respectively. Mean proportion of days covered values ranged from 0.65 for statins, 0.69 for antihypertensives, and 0.71 for diabetes medications during the continuation phase. Similarly, proportion of days covered values for diabetes medications, antihypertensives, and statins during the maintenance phase of antipsychotic treatment were estimated at 0.65, 0.66, and 0.57, respectively.
Unadjusted Health Care Utilization and Expenditures
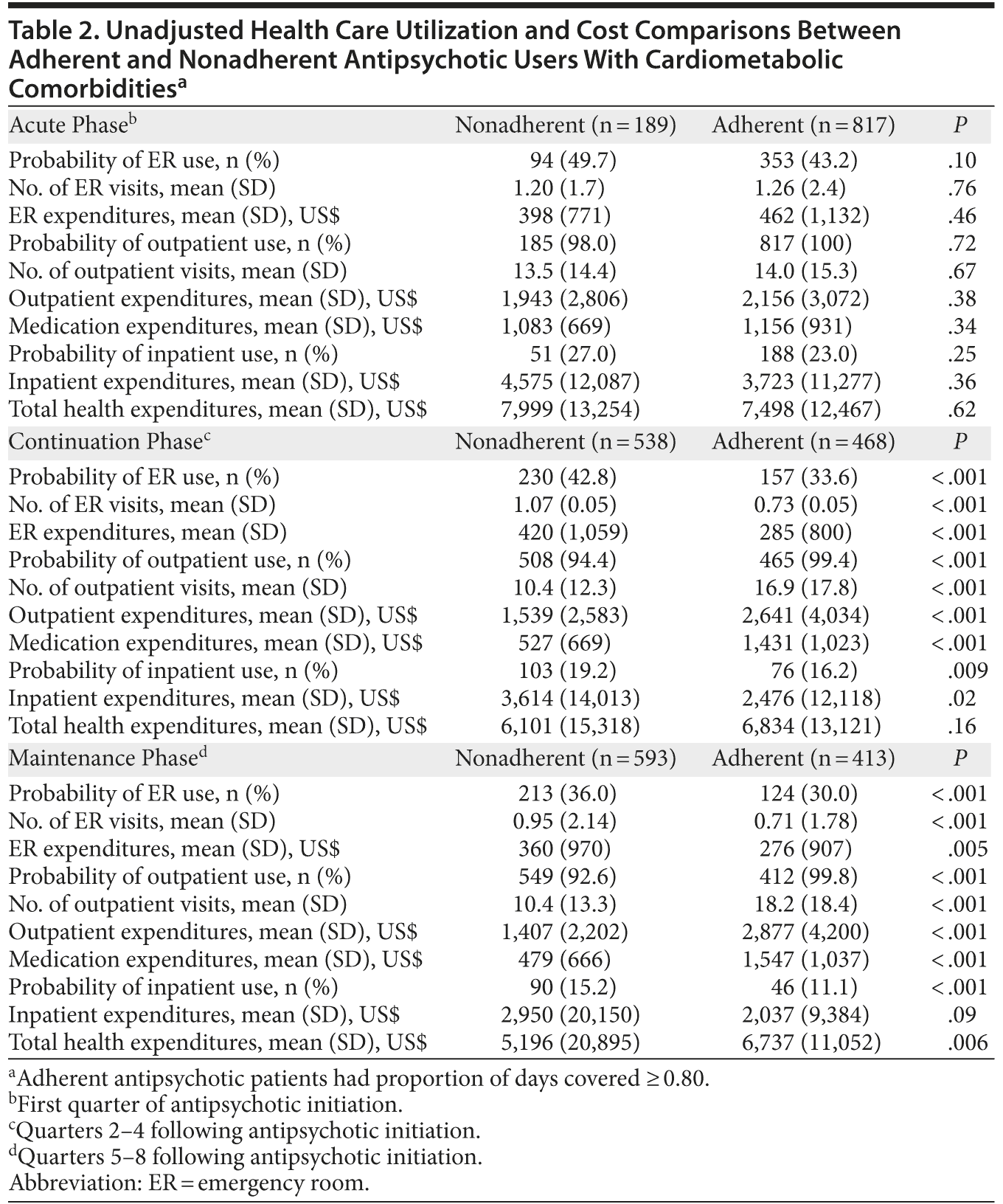
Although minimal differences between adherence groups are noted during initiation, noticeable differences appear during continuation and maintenance periods in unadjusted models (Table 2). Compared to nonadherent patients, adherent patients appeared less likely to use ER services during continuation (33.6% vs 42.7%, respectively) and maintenance periods (30.0% vs 36.0%, respectively), had fewer ER visits during continuation (0.73 vs 1.07 visits, respectively) and maintenance periods (0.71 vs 0.95, respectively), and had lower ER expenditures during continuation ($285 vs $420, respectively) and maintenance periods ($276 vs $360, respectively). Adherent antipsychotic users had more frequent outpatient visits during continuation (16.9 vs 10.4) and maintenance (18.2 vs 10.4) periods and higher outpatient expenditures during continuation ($2,641 vs $1,539) and maintenance ($2,877 vs $1,407) periods than nonadherent users. Adherent patients were less likely to be admitted to a hospital during continuation (16.2% vs 19.2%) and maintenance (11.1% vs 15.2%) periods, and inpatient expenditures were lower among adherent compared with nonadherent patients during continuation ($2,476 vs $3,614) and maintenance ($2,037 vs $2,950) periods. Not surprisingly, adherent patients had higher medication costs than nonadherent patients, averaging $1,156, $1,431, and $1,547 compared to $1,083, $527, and $479, respectively, during acute, continuation, and maintenance periods. Overall, treatment expenditures for adherent patients were higher during the maintenance period than they were for nonadherent patients ($6,737 vs $5,196, respectively).
Adjusted Medication Adherence
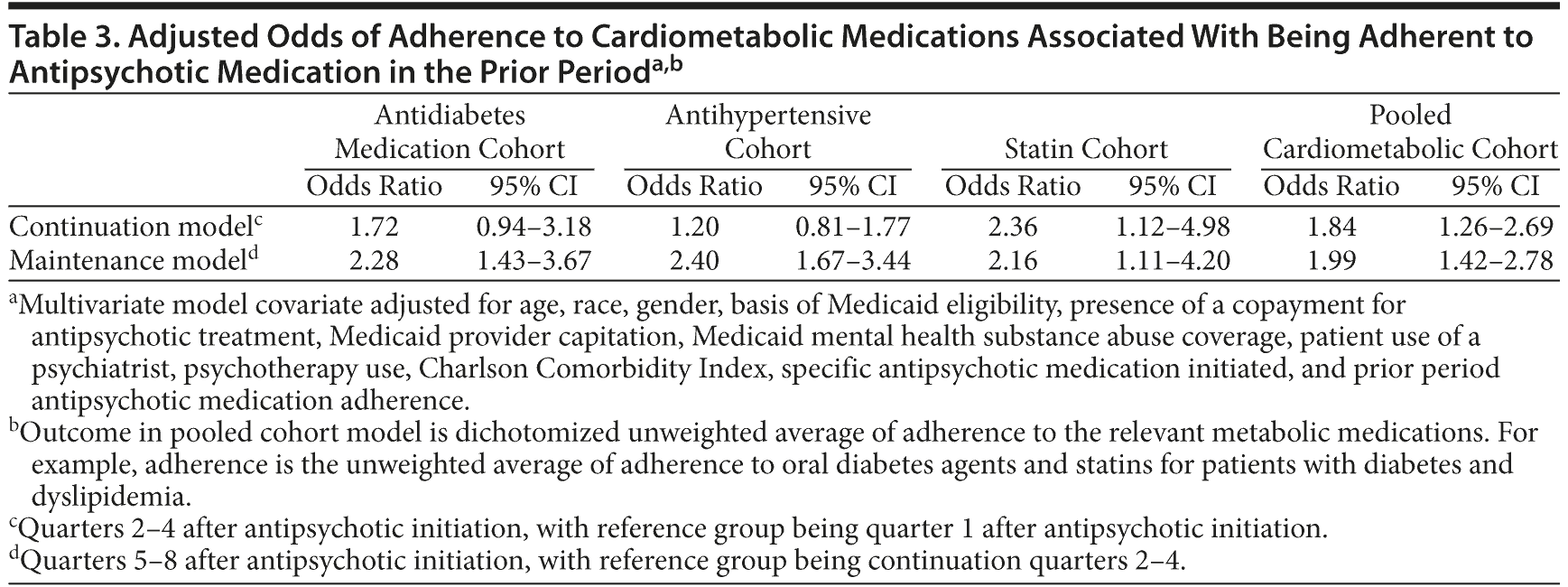
In covariate adjusted analyses (Table 3), patients who were adherent to their antipsychotic medications in the acute phase were more likely to be adherent to their cardiometabolic medications in the continuation phase (odds ratio [OR] = 1.84, 95% confidence interval [95% CI] = 1.26-2.69). The results appear to be driven by adherence to statins (OR = 2.36, 95% CI = 1.12-4.98). We also found that patients who were adherent to their antipsychotic medications in the continuation phase were more likely to be adherent to cardiometabolic medications across all cohorts including antidiabetic medications (OR = 2.28, 95% CI = 1.43-3.67), antihypertensives (OR = 2.40, 95% CI = 1.67-3.44), and statins (OR = 2.16, 95% CI = 1.11-4.20) and for the pooled cohort (OR = 1.99, 95% CI = 1.42-2.78) during the maintenance phase of treatment.
Adjusted Health Care Utilization and Expenditures
In covariate adjusted analyses (Table 4), patients who were adherent to their antipsychotic medications in the continuation phase were less likely to have ER visits during the maintenance phase (OR = 0.67, 95% CI = 0.52-0.87). This result translates into a reduction of 0.25 ER visits (95% CI = −0.37 to −0.13) per adherent patient and a $96 reduction (95% CI = -$144 to -$48) in expenditures for ER services. The majority of the reduction in ER service use appears to have occurred in patients in the hypertension cohort, while differences in the diabetes and statin cohorts were not statistically significant.
A more consistent relationship is seen when examining the effect of adherence on outpatient service utilization and medication expenditures. In the pooled sample, patients who adhered to antipsychotic treatments during the continuation phase of treatment had 5.76 (95% CI = 3.83-7.69) more outpatient visits, $996 (95% CI = $663-$1,330) more in outpatient expenditures, and $652 (95% CI = $542-$762) more in medication expenditures during the maintenance phase of treatment. There was not a significant difference in inpatient service utilization or expenditures among patients adherent to antipsychotic treatments in the pooled cohort or any of the individual cohorts examined. Overall, total health care expenditures were $1,371 (95% CI = $490-$2,252) higher in adherent patients during the maintenance phase of antipsychotic treatment compared to nonadherent patients among patients in the pooled cohort. This finding was consistent across the disease cohorts examined, with slightly higher total health care spending among patients taking statins ($2,460; 95% CI = $613-$4,306) or antidiabetic medications ($2,690; 95% CI = $743-$4,637).
DISCUSSION
Despite the prevalence of cardiometabolic conditions in patients with schizophrenia, these comorbid conditions are poorly managed and undertreated in this population. We hypothesized that adherent antipsychotic users would have better adherence to cardiometabolic medications and lower health care utilization and expenditures. Our results suggest that adherence to cardiometabolic medications is suboptimal, declining (26%-34%) within 180 days of starting an antipsychotic. In addition, adherence to cardiometabolic medications is lower among nonadherent antipsychotic users. This result suggests that adherence to antipsychotic treatments may influence adherence to medications for comorbid health conditions. However, caution should be used in interpreting these findings as causal given the observational study design selected and the limitations that this design presents to our findings.
We further sought to examine the effect of antipsychotic adherence on all-cause health expenditures and utilization in patients with schizophrenia and comorbid cardiometabolic conditions. Our results suggest a significant reduction in ER visits and a marginal, albeit not statistically significant, reduction in inpatient utilization among adherent antipsychotic users. This finding suggests the potential for antipsychotic adherence to significantly reduce the need for aggressive treatments to control symptoms of both schizophrenia and cardiometabolic conditions. Paradoxically, we saw an increase in the frequency of outpatient service use, outpatient expenditures, and medication expenditures, which led to increases in overall health care expenditures. Adherent patients use more medication, which increases medication expenditures. In addition, adherent patients must utilize physician services to obtain prescriptions, which can contribute to additional expenditures. Of note, the majority of the increase in overall health care expenditures among patients adherent to antipsychotic treatment resulted from expenditures for these 2 services.
Increasing attention has been given to improving continuity of care for patients with severe mental illnesses.11 This coordination of care is particularly important for patients with both mental health and comorbid cardiometabolic health conditions. Psychiatrists managing mental health conditions may be reluctant to address comorbid health concerns if they believe they are being screened in the primary care setting, while primary care physicians may be reluctant to treat mental health disorders.12 Coordination between these providers could improve monitoring of comorbid health conditions and ensure attention is given to antipsychotic adherence as well as cardiometabolic medication adherence in patients with schizophrenia.
Coordination of care is particularly important given the potential for antipsychotic treatments to increase obesity and cardiometabolic health problems.13 Our study examined patterns of adherence to antipsychotic treatment among patients with preexisting cardiometabolic conditions using cardiometabolic medications. The new user design was thought the best way to establish a temporal relationship between antipsychotic adherence and adherence to cardiometabolic medications.14 Future studies may wish to examine the relationship of antipsychotic adherence and adherence to medications for comorbid health conditions among prevalent users of antipsychotic medications.
Our results should be interpreted in light of several limitations. We restricted our study to incident antipsychotic users with previous comorbidity. This restriction was done to improve temporal precedence between the relationship of adherence to antipsychotics and cardiometabolic medications. However, caution should be used when generalizing beyond incident antipsychotic users to patients with other comorbidities and to patients who attained comorbid conditions following antipsychotic initiation. A primary outcome from this study was medication adherence, which was measured using pharmacy claims data. This measure assumes medication consumption by patients who obtain refills, which is not always true. Similarly, health utilization and expenditures were not differentiated between mental health and comorbid diagnoses. Future studies may wish to differentiate reasons for health care utilization resulting from nonadherence. In addition, unobservable factors such as self-efficacy (a patient’s belief that adherence will actually improve health) or social support could also influence our study findings. Primary data collection could be used in future studies to capture the influence of other variables on the relationships examined.
CONCLUSION
Despite these limitations, our study suggests that adherence to antipsychotic medication is significantly related to adherence to medications for comorbid cardiometabolic conditions and a reduced likelihood of emergency department utilization. Clinicians should assess adherence to both antipsychotic medications and cardiometabolic medications to improve the overall quality of care for patients with schizophrenia.
Drug names: aripiprazole (Abilify), clozapine (Clozaril, FazaClo, and others), olanzapine (Zyprexa), quetiapine (Seroquel), risperidone (Risperdal and others), ziprasidone (Geodon).
Author affiliations: UNC Eshelman School of Pharmacy, University of North Carolina at Chapel Hill, Chapel Hill (Dr Farley); Harrison School of Pharmacy, Auburn University, Auburn, Alabama (Dr Hansen); Global Health Economics and Outcomes Research, GlaxoSmithKline, Durham, North Carolina (Dr Yu-Isenberg); and Division of General Internal Medicine, Durham VA Medical Center and Duke University, Durham, North Carolina (Dr Maciejewski).
Potential conflicts of interest: Dr Farley has received grant/research support from Agency for Healthcare Research and Quality, Novartis, Pfizer, and Robert Wood Johnson Foundation and consulting support from Novartis and Takeda. Dr Hansen has received grant/research support from Agency for Healthcare Research and Quality, National Institutes of Health, Novartis, and Takeda and consulting support from Novartis and Takeda. Dr Yu-Isenberg was an employee of Novartis at the time of the study and is currently an employee of GlaxoSmithKline. Dr Maciejewski has received grant/research support from Agency for Healthcare Research and Quality and Robert Wood Johnson Foundation and consulting support from Novartis and Takeda.
Funding/support: This study was funded through a contract with Novartis Pharmaceuticals Corporation.
Previous presentation: A portion of this work was previously presented in poster format at the Annual International Meeting of the International Society for Pharmacoeconomics and Outcomes Research; May 25, 2011; Baltimore, Maryland.
Acknowledgments: The authors acknowledge helpful comments during the preparation of this manuscript from Christopher Zacker, PhD, and Edward Kim, MD, MBA. Drs Zacker and Kim are employees of Novartis Pharmaceuticals Corporation (Emmaus, Pennsylvania, and East Hanover, New Jersey, respectively).
Additional information: The Medstat MarketScan databases are available from Thomson Reuters (Healthcare) Inc at http://marketscan.thomsonreuters.com/marketscanportal/.
REFERENCES
1. Correll CU, Druss BG, Lombardo I, et al. Findings of a US national cardiometabolic screening program among 10,084 psychiatric outpatients. Psychiatr Serv. 2010;61(9):892-898. PubMed
2. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22. PubMed doi:10.1016/j.schres.2006.06.026
3. Hennekens CH, Hennekens AR, Hollar D, et al. Schizophrenia and increased risks of cardiovascular disease. Am Heart J. 2005;150(6):1115-1121. PubMed doi:10.1016/j.ahj.2005.02.007
4. Morrato EH, Newcomer JW, Kamat S, et al. Metabolic screening after the American Diabetes Association’s consensus statement on antipsychotic drugs and diabetes. Diabetes Care. 2009;32(6):1037-1042. PubMed doi:10.2337/dc08-1720
5. Mann JJ. The medical management of depression. N Engl J Med. 2005;353(17):1819-1834. PubMed doi:10.1056/NEJMra050730
6. Valenstein M, Blow FC, Copeland LA, et al. Poor antipsychotic adherence among patients with schizophrenia: medication and patient factors. Schizophr Bull. 2004;30(2):255-264. PubMed doi:10.1093/oxfordjournals.schbul.a007076
7. Lurie N, Popkin M, Dysken M, et al. Accuracy of diagnoses of schizophrenia in Medicaid claims. Hosp Community Psychiatry. 1992;43(1):69-71. PubMed
8. Benner JS, Glynn RJ, Mogun H, et al. Long-term persistence in use of statin therapy in elderly patients. JAMA. 2002;288(4):455-461. PubMed doi:10.1001/jama.288.4.455
9. Karve S, Cleves MA, Helm M, et al. Good and poor adherence: optimal cut-point for adherence measures using administrative claims data. Curr Med Res Opin. 2009;25(9):2303-2310. PubMed doi:10.1185/03007990903126833
10. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139. PubMed doi:10.1097/01.mlr.0000182534.19832.83
11. Dombrovski A, Rosenstock J. Bridging general medicine and psychiatry: providing general medical and preventative care for the severely mentally ill. Curr Opin Psychiatry. 2004;17(6):523-529. doi:10.1097/00001504-200411000-00018
12. Goldman LS. Medical illness in patients with schizophrenia. J Clin Psychiatry. 1999;60(suppl 21):10-15. PubMed
13. Lieberman JA, Stroup TS, McEvoy JP, et al; Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209-1223. PubMed doi:10.1056/NEJMoa051688
14. Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158(9):915-920. PubMed doi:10.1093/aje/kwg231