Objective: To describe and compare demographics, outcomes, and comorbidities among schizophrenia patients according to treatment response.
Methods: A cross-sectional survey was conducted in the United States through the Adelphi Schizophrenia Disease Specific Program from January to May 2014. Participating physicians provided information on the first 10 schizophrenia patients aged ≥ 18 years they saw in daily clinical practice; these patients were invited to voluntarily complete a patient self-completion form. Patients were considered partial responders or responders based on the physician-reported Clinical Global Impressions improvement scale. Regression analyses were performed to identify potential drivers of response and the clinical and humanistic outcomes associated with response.
Results: 150 physicians provided data on 433 partial responders and 872 responders; 185 partial responders and 415 responders completed a patient self-completion form. A significant predictor of response was always being adherent with the medication regimen (P < .001). Positive symptoms (P = .006) and moderate (P = .004) or severe (P = .002) illness severity were significant predictors of inadequate response. Responders were more likely to have better EQ-5D (EuroQol 5 Dimensions) visual analog scale, Quality of Life Enjoyment and Satisfaction Questionnaire, and work productivity and impairment scores (all P < .05).
Conclusions: Partial responders were more likely to have significantly poorer clinical and quality of life outcomes compared with responders. Improved therapeutic approaches, either new therapies or optimized treatments, could lead to both better outcomes and improved adherence in this population.
A Real-World Assessment of Outcomes in Schizophrenia Patients According to Treatment Response
ABSTRACT
Objective: To describe and compare demographics, outcomes, and comorbidities among schizophrenia patients according to treatment response.
Methods: A cross-sectional survey was conducted in the United States through the Adelphi Schizophrenia Disease Specific Program from January to May 2014. Participating physicians provided information on the first 10 schizophrenia patients aged ≥ 18 years they saw in daily clinical practice; these patients were invited to voluntarily complete a patient self-completion form. Patients were considered partial responders or responders based on the physician-reported Clinical Global Impressions improvement scale. Regression analyses were performed to identify potential drivers of response and the clinical and humanistic outcomes associated with response.
Results: 150 physicians provided data on 433 partial responders and 872 responders; 185 partial responders and 415 responders completed a patient self-completion form. A significant predictor of response was always being adherent with the medication regimen (P < .001). Positive symptoms (P = .006) and moderate (P = .004) or severe (P = .002) illness severity were significant predictors of inadequate response. Responders were more likely to have better EQ-5D (EuroQol 5 Dimensions) visual analog scale, Quality of Life Enjoyment and Satisfaction Questionnaire, and work productivity and impairment scores (all P < .05).
Conclusions: Partial responders were more likely to have significantly poorer clinical and quality of life outcomes compared with responders. Improved therapeutic approaches, either new therapies or optimized treatments, could lead to both better outcomes and improved adherence in this population.
Prim Care Companion CNS Disord 2020;22(6):20m02681
To cite: Khandker R, Qureshi ZP, Shepherd J, et al. A real-world assessment of outcomes in schizophrenia patients according to treatment response. Prim Care Companion CNS Disord. 2020;22(6):20m02681.
To share: https://doi.org/10.4088/PCC.20m02681
© Copyright 2020 Physicians Postgraduate Press, Inc.
aMerck & Co, Inc, Kenilworth, New Jersey
bAdelphi Real World, Bollington, United Kingdom
*Corresponding author: Farid Chekani, PhD, 351 North Sumneytown Pike (Mailstop: UG-2MW05), North Wales, PA 19454 ([email protected]).
Schizophrenia is the most common psychotic disorder, affecting more than 21 million people worldwide.1 It is a chronic disease associated with increased mortality, morbidity, and high economic costs to society.2 Schizophrenia is one of the leading causes of disability globally,3 with a median point prevalence of 4.6 per 1,000 persons.4 A claims data analysis5 in the United States revealed a 12-month prevalence of 5.1 per 1,000 persons. Despite this low prevalence, the health, social, and economic burden of this disease is substantial.6 The total costs (direct medical, nonmedical, and indirect costs) of schizophrenia across various countries range from $94 million (Puerto Rico) to $102 billion (United States); the total US cost estimates ranged from $25 billion to $102 billion.6 Schizophrenia is often severely disabling if left untreated.7
Individuals with schizophrenia have a range of symptoms, including positive (psychotic symptoms such as delusions and hallucinations),1 negative (such as reduced emotional expression and avolition),1 and cognitive (such as disorganized speech, thought, or attention) symptoms.8,9 The symptoms of schizophrenia usually start in late adolescence or early adulthood.7
Antipsychotic agents are first-line pharmacotherapies for the treatment of schizophrenia, consisting of first-generation (typical) and second-generation (atypical) agents. Guidelines1 recommend prompt initiation of pharmacotherapy after schizophrenia diagnosis. Approximately 10% to 30% of patients have minimal or no response to antipsychotics.9 At least an additional 30% of patients have a partial response, which is characterized as an improvement in psychopathology but not full remission of psychotic symptoms such as hallucinations or delusions.9 Partial treatment response may increase the risk for relapse, placing a burden on not only the patient but also the patient’s family and treating physician.10 Patients with partial response experience more severe depression and treatment side effects and poorer functioning and quality of life compared with full responders.11 Patients with persistent symptoms despite treatment experience a significant burden in terms of mobility and self-care and thus require considerable caregiver support and utilization of health care resources.12 Partial response may also lead to medication discontinuation; a post hoc pooled analysis13 of 4 randomized double-blind clinical trials of atypical antipsychotics revealed that most patients discontinued treatment at an early stage and that poor or worsening psychiatric response was the most frequent reason for discontinuation. Medication adherence is potentially the most challenging aspect of schizophrenia treatment, and nonadherence is a major risk factor for poor treatment outcomes with potentially serious consequences.14-17
To better understand the burden of partial treatment response on schizophrenia patients, their caregivers, and health care resources, we used data from a large, cross-sectional survey of psychiatrists and their consulting schizophrenia patients and compared the demographics, outcomes, and comorbidities among those patients characterized as responding to treatment versus partial responders. An improved understanding of the unmet needs of partial responders may facilitate appropriate patient management, including the need for resource allocation, and highlight the need for alternative treatment options to improve outcomes in this population.
METHODS
Study Background
Data were extracted from the Adelphi Schizophrenia Disease Specific Program (DSP) conducted in the United States from January to May 2014. DSPs are large, cross-sectional surveys conducted in clinical practice that describe current disease management, disease burden impact, and associated treatment patterns (both clinical and physician perceived). DSPs are point-in-time surveys of physicians and their patients presenting in a real-world clinical practice.
Participating Physicians and Patients
Participating physicians were office- or hospital-based psychiatrists who had been practicing between 2 and 40 years at the time of the study, consulted at least 6 schizophrenia patients per week, and were personally responsible for treatment decisions for schizophrenia patients. A geographically representative sample of physicians was recruited to participate in the DSP. Physician participation was financially incentivized, with reimbursement upon survey completion according to fair market research rates. Participating patients were aged ≥ 18 years, had a diagnosis of schizophrenia, and were not currently participating in a clinical trial. Patients were not compensated for participation.
Data Collection
Participating psychiatrists completed a patient record form (PRF) for the first 10 consecutive patients consulting for schizophrenia. PRFs contain detailed questions on patient demographics, diagnosis, management, clinical status, concomitant conditions, current treatment, and treatment history. Completion of the PRF was undertaken through consultation of existing patient clinical records, as well as the judgement and diagnostic skills of the respondent physician, which is entirely consistent with decisions made in routine clinical practice.
Patients were classified once at the time of the survey as partial responders or responders using the physician-reported Clinical Global Impressions (CGI) scale18 on current treatment. The CGI is a brief 3-item physician-rated scale that assesses illness severity, global improvement or change, and therapeutic response and is validated for ease of use and interpretability with no training required. The improvement score of the CGI has been shown to be a valid proxy measure for remission in schizophrenia.19 In this study, patients rated as “minimally improved” were considered partial responders and patients rated as “very much improved” or “much improved” were considered responders. Nonresponders were analyzed but excluded due to insufficient numbers for statistical comparison. In addition, these patients were more likely to be refractory, and we therefore chose to assess only those with some response to treatment.
The CGI was also used to assess severity of illness.18 Patients rated as “normal, not at all ill,” “borderline mentally ill,” or “mildly ill” were considered to have mild illness severity. Patients rated as “moderately ill” or “markedly ill” were considered to have moderate illness severity, while those rated “severely ill” or “among the most extremely ill patients” were considered to have severe illness severity.
Physicians assessed their patients’ adherence as “not at all adherent,” “rarely adherent,” “sometimes adherent,” “often adherent,” or “always adherent.” Patients were classified as sometimes adherent if the physician reported the patient to be “sometimes adherent” or “often adherent” to current schizophrenia medication. Patients were classified as always adherent if the physician reported the patient to be “always adherent” to current schizophrenia medication.
The list of reported symptoms was derived on the basis of expected schizophrenia-related symptoms, with these categorized under positive (psychotic symptoms such as delusions and hallucinations), negative (such as reduced emotional expression and avolition), cognitive (such as disorganized speech, thought, or attention), anxiety, and other subheadings. These categories then formed the symptom groups for analysis. The severity per symptom was captured on a 5-point scale (from mild to severe), which was defined according to physicians’ subjective assessment.
Number of hospitalizations over the prior 12-month period was collected for each patient and was used to determine whether each patient was grouped as “yes” or “no” to having been hospitalized in the last 12 months.

- A proportion of patients with schizophrenia do not fully respond to antipsychotic treatments, placing them at risk of relapse.
- Partial responders were less likely to be always adherent with their current treatment than responders.
- Partial response was associated with reduced health-related quality of life and quality of life satisfaction, an increase in work absences, and a greater overall work and activity impairment, with partial responders reporting more frequent side effects and poor efficacy than responders.
- Improved therapeutic approaches may improve adherence and response rates, subsequently improving outcomes and reducing burdens not only for patients with schizophrenia but also their families and caregivers.
Each patient for whom the physician completed a PRF was then invited to complete a voluntary patient self-completion form (PSC) and upon agreement provided their informed consent to participate. PSCs contain questions on demographics and current health condition. The following validated instruments were included in the PSC: self-rated health as assessed by the EuroQol 5 Dimensions visual analog scale (EQ-5D VAS),20,21 rated from 0 (worst imaginable health state) to 100 (best imaginable health state); overall life satisfaction assessed by the Quality of Life Enjoyment and Satisfaction Questionnaire (Q-LES-Q),22 rated from 1 (very poor) to 5 (very good); and impairment assessed by the work productivity and activity impairment (WPAI) measure,23 with higher scores indicating greater impairment. PSCs were completed by the patient independent of the physician immediately after consultation and were returned in a sealed envelope to ensure confidentiality.
Ethics
The DSP methodology has been described and validated in detail previously.24-26 Patients provided informed consent to complete a questionnaire. Physicians provided consent to participate and provided patient information during screening into the study. Data were collected such that patients and physicians could not be identified; all data were aggregated and deidentified before analysis. Data collection was performed in accordance with the European Pharmaceutical Marketing Research Association guidelines, and, as such, ethics committee approval was not required. The survey was performed in full accordance with relevant legislation at the time of data collection, including the US Health Insurance Portability and Accountability Act 199627 and Health Information Technology for Economic and Clinical Health Act legislation.28
Statistical Methods
Analyses were performed using Stata 15.1 (StataCorp LLC, College Station, Texas). Descriptive statistics were numeric (expressed as count, mean, and standard deviation) or categorical (expressed as count and percentage of patients falling into each response). Bivariate statistical tests used to compare outcomes between groups included t tests or analysis of variance for numeric variables, Mann-Whitney U (nonparametric) tests for ordered categorical variables, and Fisher exact test or χ2 test for nonordered categorical variables.
Regression analysis was used to determine the effect associated with being a responder after adjusting for age, sex, body mass index (BMI), severity of disease, and number of comorbid conditions. Regression type was dependent on the outcome being modeled. We employed negative binomial for count outcomes, logistic for binary outcomes, and linear regression for other continuous outcomes, with each outcome variable run as a separate regression with a set of covariates. Individual regressions were run using each outcome as dependent variables, with responder as the main independent variable of interest. In all regressions age, sex, BMI, disease severity, and comorbidities were adjusted for as covariates. Additionally, a regression was run with responder as the dependent variable, with each variable adjusted for as a covariate.
RESULTS
Physicians and Patients
A total of 150 physicians participated in this survey, with 72 (48%) physicians being office based, 75 (50%) both office and hospital based, and 3 (2%) only hospital based. In the hospital setting, 28 were public hospitals, 23 were regional hospitals, 19 were private clinics, 7 were university hospitals, and 2 were other types of settings. In the office setting, 61 were private offices and 16 were community mental health centers. Some hospitals/offices fell under more than 1 type. Physicians completed PRFs for 1,489 patients; 433 (29.1%) patients were classified as partial responders and 872 (58.6%) as responders. A total of 115 (7.7%) patients were considered nonresponders and were not analyzed further due to insufficient numbers. Response status was not available for the remaining 69 (4.6%) patients. A total of 680 patients completed PSCs, of which 185 (27.2%) were partial responders and 415 (61.0%) were responders. Of the remaining PSCs, 53 (7.8%) were nonresponders, and response status was not available from 27 (4.0%) PSCs.
Demographics and Clinical Characteristics
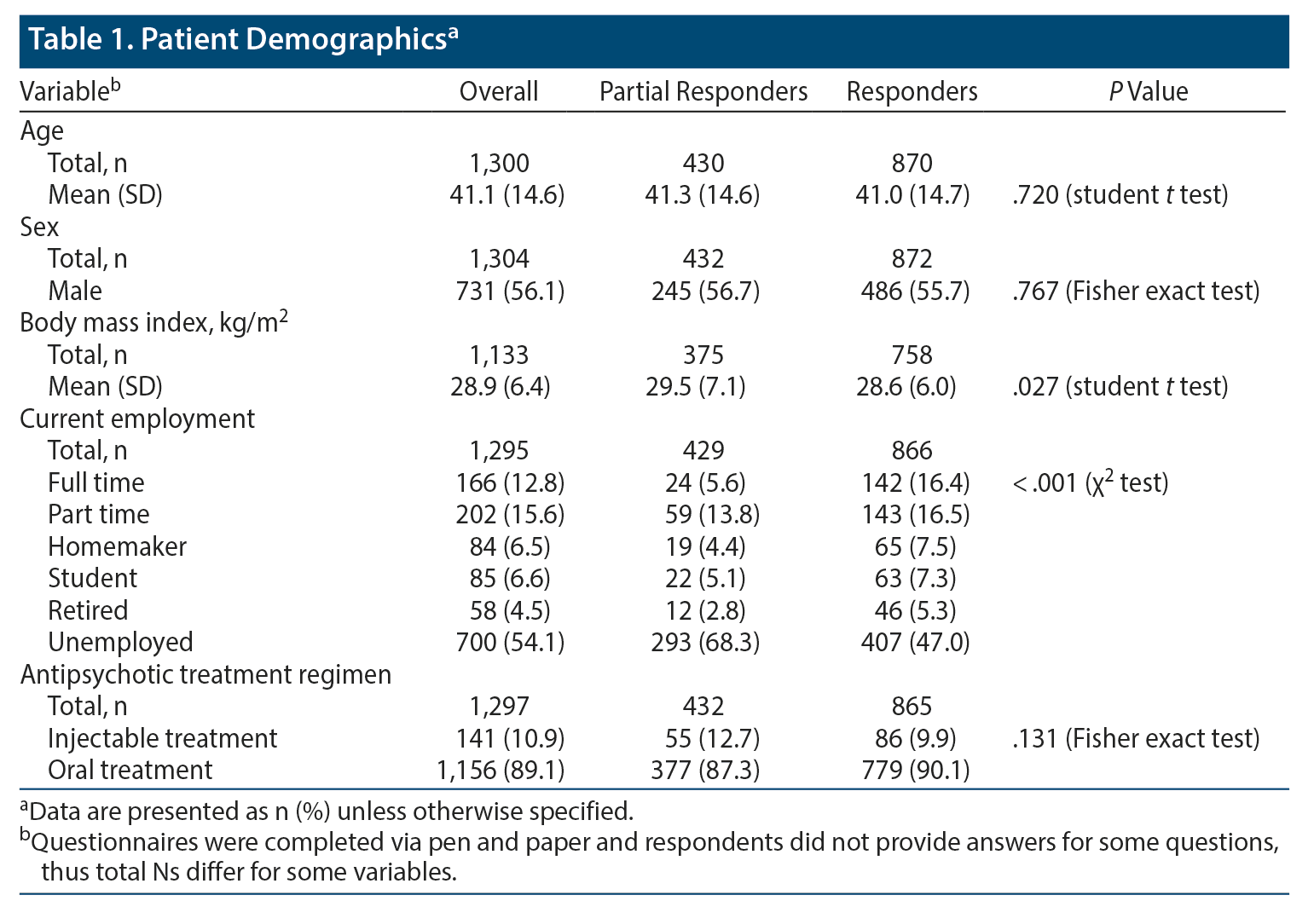
Data extracted from PRFs revealed that both partial responders and responders were more likely to be male (56.7% and 55.7%, respectively) and were a similar mean age (41.3 years and 41.0 years, respectively). Partial responders were more likely than responders to be unemployed (68.3% vs 47.0%, P < .001) and to have a higher mean BMI (29.5 kg/m2 vs 28.6 kg/m2, P = .027) (Table 1). Use of injectable treatments (responders: 9.9% vs partial responders: 12.7%) and oral treatments (responders; 90.1% vs partial responders: 87.3%) was similar between the 2 patient groups (P = .131)
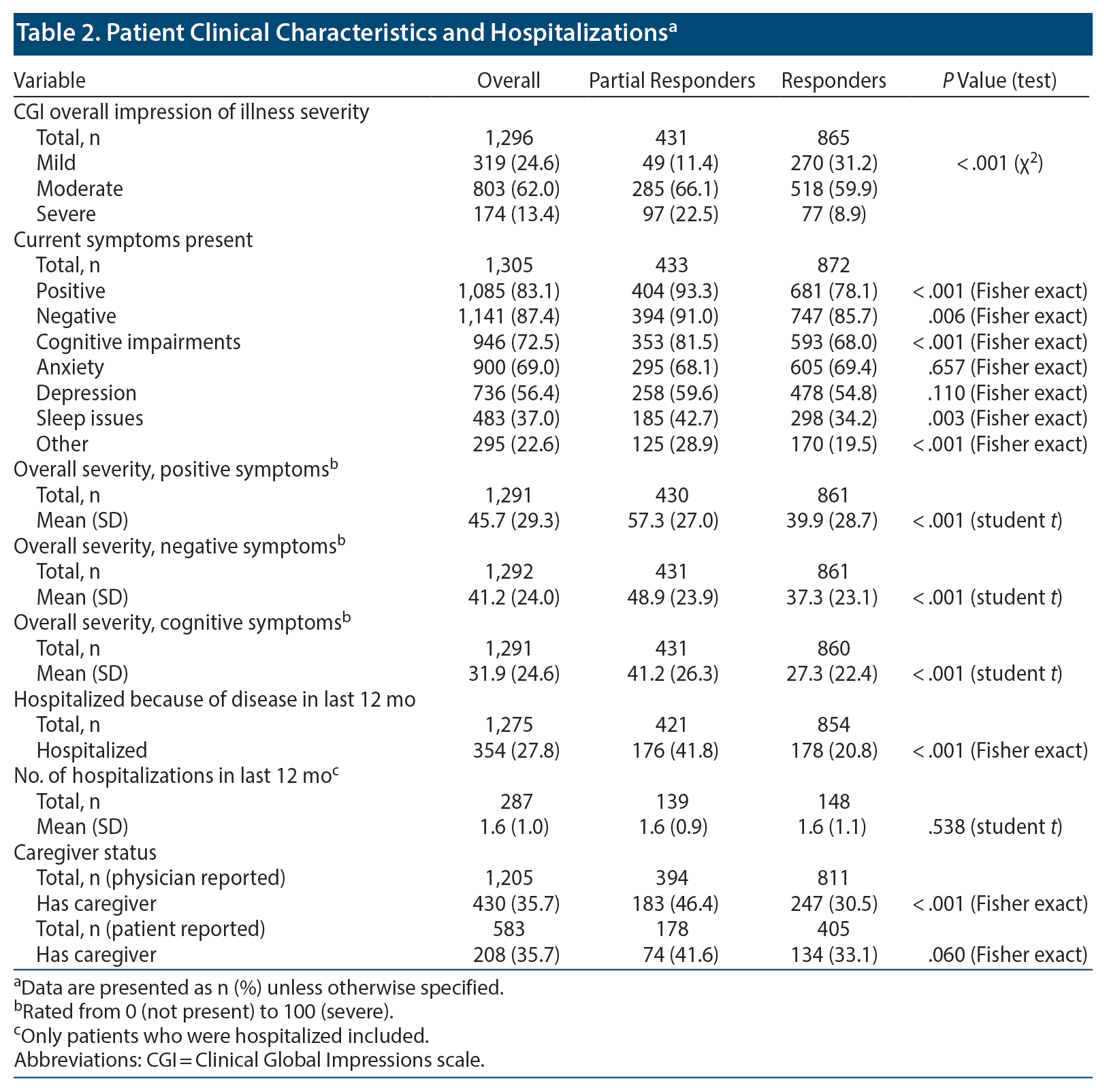
Responders were more likely than partial responders to have mild CGI illness (31.2% vs 11.4%, P < .001) and were less likely to have severe CGI illness (8.9% vs 22.5%, P < .001). A greater proportion of partial responder patients had current positive symptoms (93.3% vs 78.1%, P < .001), negative symptoms (91.0% vs 85.7%, P = .006), cognitive impairments (81.5% vs 68.0%, P < .001), and sleep issues (42.7% vs 34.2%, P = .003). Partial responder patients reported greater mean severity of positive (57.3 vs 39.9, P < .001), negative (48.9 vs 37.3, P < .001), and cognitive symptoms (41.2 vs 27.3, P < .001) compared with responders. Partial responders were more likely to have been hospitalized in the last 12 months compared with responders (41.8% vs 20.8%, P < .001). Physicians reported that a greater proportion of partial responders compared with responders had a caregiver (46.4% vs 30.5%, P < .001). Although partial responders also more often reported having a caregiver, the difference compared with responders was not significant (41.6% vs 33.1%, P = .060) (Table 2). Partial responders had a higher mean number of comorbidities compared with responders (2.4 vs 2.0, P = .006); although not significant, partial responders more often had comorbidities such as hypertension (28.4% vs 23.8%, P = .078), dyslipidemia (18.7% vs 15.9%, P = .209), and obesity (18.9% vs 16.4%; P = .275) compared with responders.
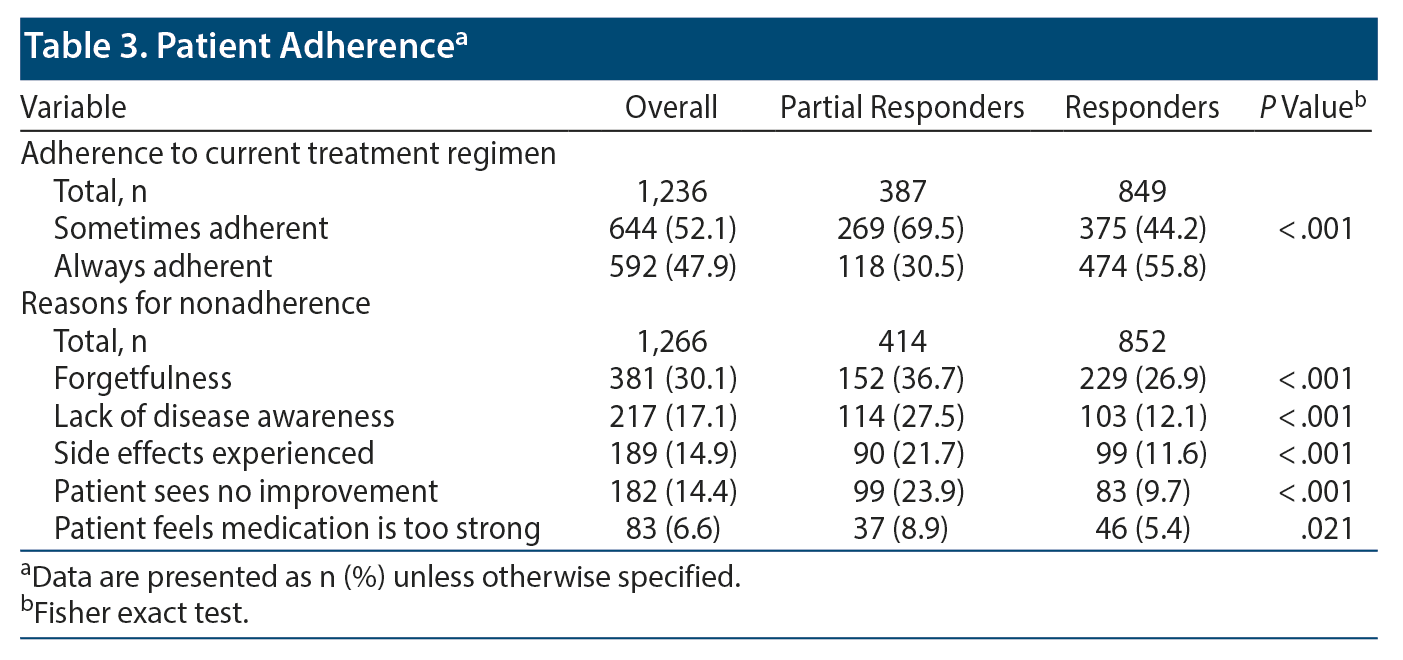
A lower proportion of partial responders were always adherent with their current treatment regimen compared with responders (30.5% vs 55.8%, P < .001). Among the top 5 physician-reported reasons for nonadherence, a greater proportion of partial responders compared with responders experienced side effects (21.7% vs 11.6%, P < .001) and did not see improvement (23.9% vs 9.7%, P < .001). Forgetfulness was the most common (30.1%) reason for nonadherence followed by lack of disease awareness (17.1%). Forgetfulness (36.7% vs 26.9%, P < .001) and lack of disease awareness (27.5% vs 12.1%, P < .001) were also reported by a greater proportion of partial responders than responders (Table 3).
Drivers of Patient Response
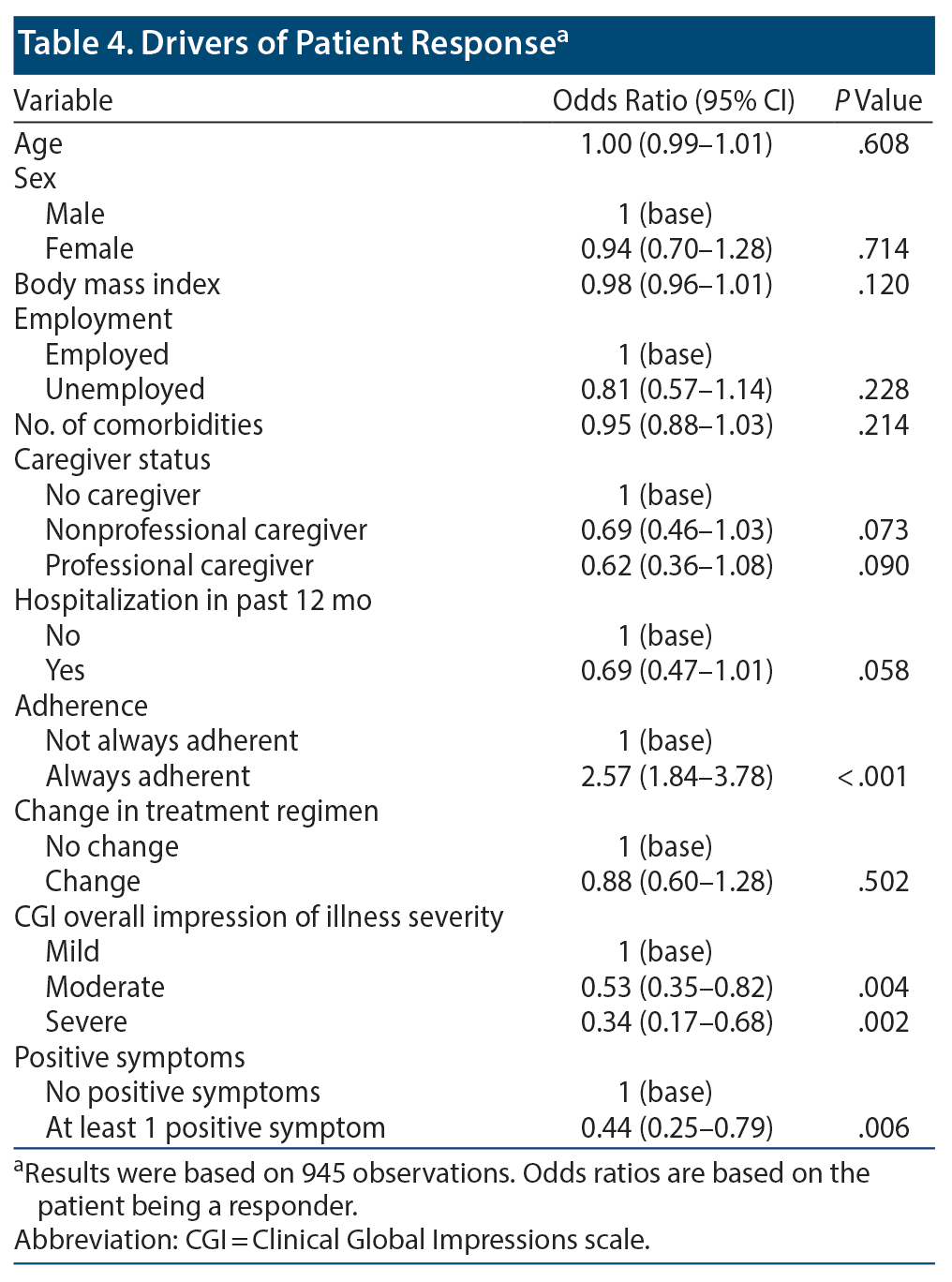
A logistic regression analysis was performed to identify potential drivers of response. We observed that always adherent patients were more likely to be responders compared with those who were not always adherent (odds ratio [OR]: 2.57; 95% CI, 1.84-3.78; P < .001). Compared to those with mild CGI severity of illness, patients with a moderate (OR: 0.53; 95% CI, 0.35-0.82; P = .004) or severe (OR: 0.34; 95% CI, 0.17-0.68; P = .002) CGI severity of illness score were less likely to be responders. Patients with at least 1 positive symptom were also more likely to be responders compared with those without such symptoms (OR: 0.44; 95% CI, 0.25-0.79; P = .006) (Table 4).
Clinical and Humanistic Outcomes Associated With Response
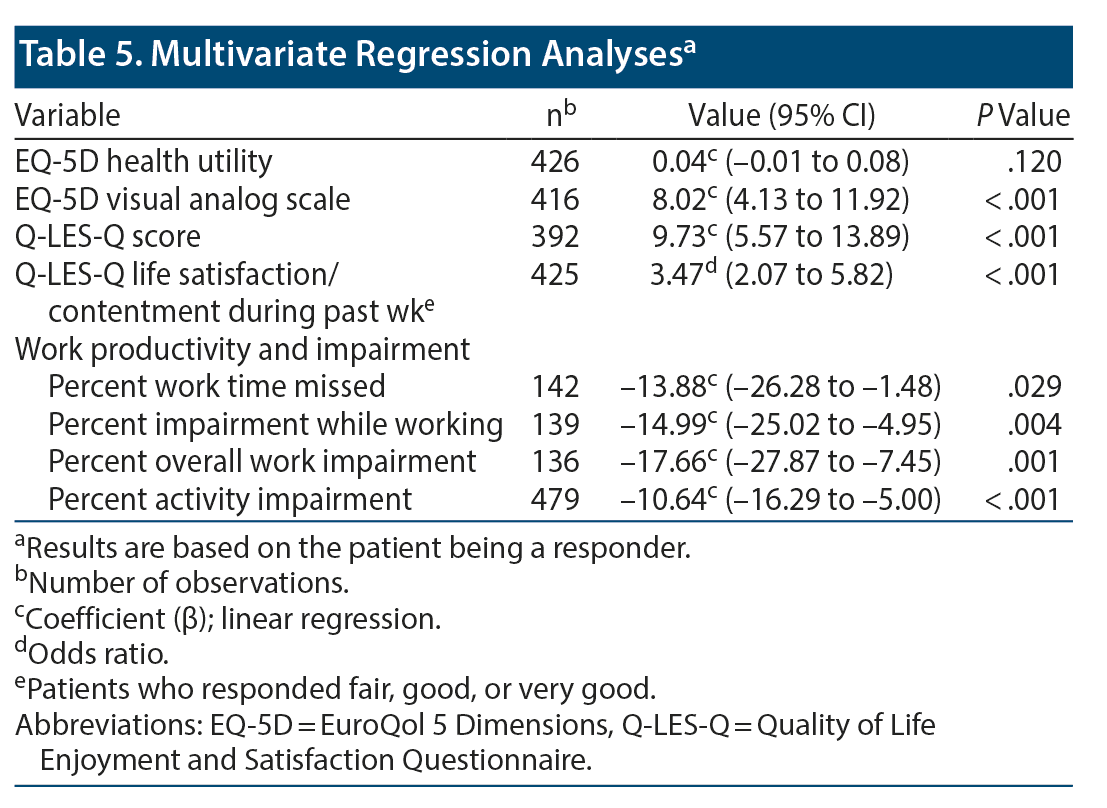
Multivariate regression analyses were performed to identify outcomes associated with response status. Responders had better EQ-5D VAS (β = 8.02; 95% CI, 4.13-11.92; P < .001) and Q-LES-Q (β = 9.73; 95% CI, 5.57-13.89; P < .001) scores and were more likely to have fair to very good Q-LES-Q life satisfaction during the past week (OR = 3.47; 95% CI, 2.07-5.82; P < .001). According to WPAI scores, responders had less work time missed (β = –13.88; 95% CI, –26.28 to –1.48; P = .029) and had less percentage impairment while working (β = –14.99; 95% CI, –25.02 to –4.95; P = .004), overall work impairment (β = –17.66; 95% CI, –27.87 to –7.45; P = .001), and activity impairment (β = –10.64; 95% CI, –16.29 to –5.00; P < .001) (Table 5).
DISCUSSION
This analysis of data from the Adelphi Schizophrenia DSP revealed that schizophrenia partial responder patients experience poorer clinical and humanistic outcomes than do responders. These observed associations between real-world treatment response and outcomes thus provide an important addition to the schizophrenia literature.
Partial responders were less likely than responders to be always adherent with their current treatment, with greater proportions of partial responders reporting side effects and poor efficacy. It is uncertain whether this lack of response drives reduced adherence or whether poor adherence leads to reduced response; however, it is well recognized that these 2 factors are associated. An analysis of clinical trials of second-generation antipsychotics revealed that poor response followed by poor tolerability were the most common reasons for treatment discontinuation.13 Two reviews14,16 also identified poor response and poor tolerability as risk factors for poor adherence. An interview study29 on 22 patients with schizophrenia revealed that patients felt that medication was useful only if side effects were minimal and well-controlled. Once this control of side effects was achieved, medication could then facilitate attainment of short-term humanistic goals, such as social interaction and meaningful activity.29 Given the association between poor adherence and the increased likelihood of side effects and poor efficacy, improving antipsychotic therapy, either by the introduction of new therapeutic approaches or optimizing current treatment regimens such that side effects are minimal or manageable, may improve both patient response and adherence.
Furthermore, medication regimens need to consider possible comorbidities in schizophrenia. Although not statistically significant, greater proportions of partial responder patients had comorbidities such as hypertension, dyslipidemia, and obesity. Antipsychotic use is associated with significant weight gain,30 which would most likely further exacerbate these cardiometabolic comorbidities. Weight gain may also be a cause of nonadherence.31 Accordingly, weight management may be an important unmet need in this patient population.
Improvement of humanistic (as opposed to only clinical) outcomes may be of greater relevance to patients and their caregivers.29,32 A study on patients with schizophrenia, their family members, and psychiatrists revealed that in daily clinical practice, good subjective well-being was more important to patients and family members than symptomatic remission according to standard criteria.33 The same interview study33 of 22 patients with schizophrenia revealed that in addition to symptom reduction, achieving employment, a positive sense of self, social connectedness, and psychosocial, functional, and physical health (including comorbidity management) improvement are important long-term outcomes for patients.29 On the basis of the better clinical and humanistic outcomes observed in responders, improving response (such as through improved adherence and medication optimization as described above) in partial responders would be expected to lead to not only better clinical outcomes but also better humanistic outcomes, which may ultimately be of greater importance to patients.
A logistic regression analysis revealed that always adherent patients were more likely to be responders than partial responders. It is important to note that the relationship between adherence and response may be bidirectional. Although one interpretation could be that improved adherence appears to drive response, it is also possible that a patient who achieves sufficient response is motivated to remain adherent (ie, that response drives compliance, rather than compliance drives response). This relationship may also exist for the other variables examined in this study.
There are some limitations to this study. The DSP is not based on a true random sample of physicians or patients. Physician participation was influenced by willingness to complete the survey. Although there were no formal patient selection procedures, physicians were asked to provide information on the next 10 patients they consulted. The cross-sectional nature of the study data limits the ability to define response and adherence, which would have required a cohort/pre-post design to be robust. Furthermore, the subjective assessments used to define response or adherence may not be capturing the true response or adherence. Levels of adherence were included in this study so that differences in adherence by response status could be investigated; however, as this was a point-in-time study, we were unable to investigate whether changes in adherence rates had an impact on response. While the point-in-time study design prevents any conclusions about causal relationships, identification of significant associations is possible. Recall bias may also have affected patient and physician responses to the questionnaires, which is a common limitation of surveys. However, the data were collected at the time of each patient’s consultation and physicians had access to the patient’s medical history, which are both expected to reduce the likelihood of recall bias. A total of 1,489 PRFs were completed; in contrast, only 680 patients completed PSCs. Of those who did complete PSCs, 27.2% and 61.0% were partial responders and responders, respectively, which is comparable to the level of response reported by physicians (29.1% and 58.6%, respectively).
Nonresponders and patients without response status (n = 115 patients or 7.7% of the cohort) were excluded from this analysis due to insufficient numbers for analysis. Nonresponding patients are likely to be underrepresented in this cohort and in psychiatric research in general and are less likely to be engaged with health care physicians, particularly in a voluntary setting. Nonresponding patients were more likely to be refractory, and in any case, the focus of this article was to investigate the differences between partial responders and responder to try and highlight drivers that may allow the response of partial responders to be more successful. A comparison of responders versus nonresponders would have shown even greater differences than those observed in this study between partial responders and responders, so in that respect, the results presented here are more conservative in terms of the impact of response on outcomes.
Despite these limitations, analyses of real-world data are necessary to address concerns that are not explored in clinical trials. Patients included in clinical trials are not fully representative of the schizophrenia consulting population. This DSP collected data on adherence, treatment response, health-related quality of life and work productivity and employment, thus providing valuable insights into the implications of schizophrenia and its treatment, reflecting real-world practice without preselection of patients. Evidence from this DSP may enhance disease understanding and provide insights that reflect the realities of current treatment practices and can be used to augment findings from other data sources such as registries and administrative databases.
This real-world study on schizophrenia patients revealed that response to treatment may be driven by disease severity, adherence, and the presence of positive symptoms, with partial responders experiencing poorer clinical and humanistic outcomes than responders. In particular, partial response was associated with reduced health-related quality of life and quality of life satisfaction, an increase in work absences, and a greater overall work and activity impairment compared with responders. While it was not possible to determine causality in this study, there is a clear need for improved therapeutic approaches and medication optimization in schizophrenia, which may improve both adherence and response rates and subsequently improve outcomes in partial responders.
Submitted: May 18, 2020; accepted August 5, 2020.
Published online: December 23, 2020.
Author contributions: All authors were involved in (1) conception or design or analysis and interpretation of data, (2) drafting and revising the article, (3) providing intellectual content of critical importance to the work described, and (4) final approval of the version to be published.
Potential conflicts of interest: Drs Khandker, Qureshi, and Shepherd are employees of Merck Sharp & Dohme Corp, a subsidiary of Merck & Co, Inc, who may own stock and/or hold stock options in Merck & Co, Inc. Drs Samant and Chekani and Ms Bailey report no conflicts of interest related to the subject of this article.
Funding/support: This study was funded by Merck Sharp & Dohme Corp, a subsidiary of Merck & Co, Inc.
Role of the sponsor: Data collection was undertaken by Adelphi Real World as part of an independent survey; all data supporting the study are the intellectual property of Adelphi Real World. Merck Sharp & Dohme Corp did not influence the original survey through either contribution to the design of questionnaires or data collection.
Previous presentation: Parts of this study were previously presented at ISPOR 2019; May 18-22, 2019; New Orleans, LA.
Acknowledgments: The authors thank Derek Ho, PhD (ScriboMedica Ltd, Helsinki, Finland), supported by Merck & Co, Inc, for medical writing support with the manuscript. Dr Ho has no conflicts of interest to declare. Additional medical writing and administrative support was provided by Gary Sidgwick, PhD, of Adelphi Real World, under the guidance of the authors. Dr Sidgwick has no conflicts of interest to declare.
REFERENCES
1.Holder SD, Wayhs A. Schizophrenia. Am Fam Physician. 2014;90(11):775-782. PubMed
2.Haddad PM, Correll CU. The acute efficacy of antipsychotics in schizophrenia: a review of recent meta-analyses. Ther Adv Psychopharmacol. 2018;8(11):303-318. PubMed CrossRef
3.GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1545-1602. PubMed CrossRef
4.Saha S, Chant D, Welham J, et al. A systematic review of the prevalence of schizophrenia. PLoS Med. 2005;2(5):e141. PubMed CrossRef
5.Wu EQ, Shi L, Birnbaum H, et al. Annual prevalence of diagnosed schizophrenia in the USA: a claims data analysis approach. Psychol Med. 2006;36(11):1535-1540. PubMed CrossRef
6.Chong HY, Teoh SL, Wu DB, et al. Global economic burden of schizophrenia: a systematic review. Neuropsychiatr Dis Treat. 2016;12:357-373. PubMed
7.Jones PB, Barnes TR, Davies L, et al. Randomized controlled trial of the effect on quality of life of second- vs first-generation antipsychotic drugs in schizophrenia: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS 1). Arch Gen Psychiatry. 2006;63(10):1079-1087. PubMed CrossRef
8.American Psychiatric Association. Diagnostic and Statistical Manual for Mental Disorders. Fifth Edition. Washington, DC: American Psychiatric Association; 2013.
9.Lehman AF, Lieberman JA, Dixon LB, et al; American Psychiatric Association; Steering Committee on Practice Guidelines. Practice guideline for the Treatment of Patients with Schizophrenia, Second Edition. Am J Psychiatry. 2004;161(2 suppl):1-56. PubMed
10.Emsley RA. Partial response to antipsychotic treatment: the patient with enduring symptoms. J Clin Psychiatry. 1999;60(suppl 23):10-13. PubMed
11.Millier A, Azorin JM, Angermeyer MC, et al. PMD3 partial responders in schizophrenia. Value Health. 2011;14(3):A79. CrossRef
12.Nordstroem AL, Talbot D, Bernasconi C, et al. Burden of illness of people with persistent symptoms of schizophrenia: a multinational cross-sectional study. Int J Soc Psychiatry. 2017;63(2):139-150. PubMed CrossRef
13.Liu-Seifert H, Adams DH, Kinon BJ. Discontinuation of treatment of schizophrenic patients is driven by poor symptom response: a pooled post-hoc analysis of four atypical antipsychotic drugs. BMC Med. 2005;3(1):21. PubMed CrossRef
14.Higashi K, Medic G, Littlewood KJ, et al. Medication adherence in schizophrenia: factors influencing adherence and consequences of nonadherence, a systematic literature review. Ther Adv Psychopharmacol. 2013;3(4):200-218. PubMed CrossRef
15.Phan SV. Medication adherence in patients with schizophrenia. Int J Psychiatry Med. 2016;51(2):211-219. PubMed CrossRef
16.Acosta FJ, Hernández JL, Pereira J, et al. Medication adherence in schizophrenia. World J Psychiatry. 2012;2(5):74-82. PubMed CrossRef
17.Bitter I, Fehér L, Tényi T, et al. Treatment adherence and insight in schizophrenia. Psychiatr Hung. 2015;30(1):18-26. PubMed
18.Guy W. ECDEU Assessment Manual for Psychopharmacology. Revised Edition. Washington, DC: US Department of Health, Education, and Welfare; 1976.
19.Masand P, O’ Gorman C, Mandel FS. Clinical Global Impression of Improvement (CGI-I) as a valid proxy measure for remission in schizophrenia: analyses of ziprasidone clinical study data. Schizophr Res. 2011;126(1-3):174-183. PubMed CrossRef
20.EuroQol Group. EuroQol—a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199-208. PubMed CrossRef
21.Brooks R. EuroQol: the current state of play. Health Policy. 1996;37(1):53-72. PubMed CrossRef
22.Endicott J, Nee J, Harrison W, et al. Quality of Life Enjoyment and Satisfaction Questionnaire: a new measure. Psychopharmacol Bull. 1993;29(2):321-326. PubMed
23.Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4(5):353-365. PubMed CrossRef
24.Anderson P, Benford M, Harris N, et al. Real-world physician and patient behaviour across countries: Disease-Specific Programmes—a means to understand. Curr Med Res Opin. 2008;24(11):3063-3072. PubMed CrossRef
25.Babineaux SM, Curtis B, Holbrook T, et al. Evidence for validity of a national physician and patient-reported, cross-sectional survey in China and UK: the Disease Specific Programme. BMJ Open. 2016;6(8):e010352. PubMed CrossRef
26.Higgins V, Piercy J, Roughley A, et al. Trends in medication use in patients with type 2 diabetes mellitus: a long-term view of real-world treatment between 2000 and 2015. Diabetes Metab Syndr Obes. 2016;9:371-380. PubMed CrossRef
27.Summary of the HIPAA security rule. US Department of Health & Human Services website. https://www.hhs.gov/hipaa/for-professionals/security/laws-regulations/index.html. Accessed March 5, 2019.
28.HITECH Act Enforcement Interim Final Rule. US Department of Health & Human Services website. https://www.hhs.gov/hipaa/for-professionals/special-topics/hitech-act-enforcement-interim-final-rule/index.html. Accessed March 5, 2019.
29.Lloyd H, Lloyd J, Fitzpatrick R, et al. The role of life context and self-defined well-being in the outcomes that matter to people with a diagnosis of schizophrenia. Health Expect. 2017;20(5):1061-1072. PubMed CrossRef
30.Allison DB, Mentore JL, Heo M, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry. 1999;156(11):1686-1696. PubMed
31.Dayabandara M, Hanwella R, Ratnatunga S, et al. Antipsychotic-associated weight gain: management strategies and impact on treatment adherence. Neuropsychiatr Dis Treat. 2017;13:2231-2241. PubMed CrossRef
32.Lloyd J, Lloyd H, Fitzpatrick R, et al. Treatment outcomes in schizophrenia: qualitative study of the views of family carers. BMC Psychiatry. 2017;17(1):266. PubMed CrossRef
33.Karow A, Naber D, Lambert M, et al; EGOFORS Initiative. Remission as perceived by people with schizophrenia, family members and psychiatrists. Eur Psychiatry. 2012;27(6):426-431. PubMed CrossRef
Enjoy this premium PDF as part of your membership benefits!